Radiation-induced graft copolymerization (RIGC) is a powerful technique enabling permanent tunable and desired surface modifications imparting antimicrobial properties to polymer substrates to prevent disease transmission and provide safer biomaterials and healthcare products.
- antimicrobial polymer surfaces
- radiation induced graft copolymerization
- protective fabrics
- biomedical devices
- tissue engineering materials
- food packaging films
1. Introduction
The development of various materials with antimicrobial properties has enabled medical progress in the past several decades to take place [1]. The key function of these materials relies on their surface properties such as adhesion, biocompatibility, nontoxicity biomimicry, and microbial retention/extinction [2]. Particularly, the antimicrobial properties are of utmost significance in preventing/reducing the spreading of infectious diseases and combating the continuous emergence of new microbes with drug resistance. On the other hand, antimicrobial properties are not only needed to reduce the risk posed to human health by the contamination of medical tools, implants, and other healthcare products by bacterial colonization forming biofilm, but also to enhance the performance of many industrial processes including food processing and packing, marine transport, and water treatment [3][4][3,4]. Despite the immense research efforts aiming at elimination of microbial proliferation and biofouling formation, a comprehensive solution to microbial surface colonization has not been reached and the quest to design and fabricate new antimicrobial surfaces remains a high research priority [5]. Thus, the development of new antimicrobial materials to eliminate/substantially reduce the extent of bacterial attachment and biofilm formation to surfaces remains of utmost significance and continues to receive intensive efforts.
Surface modification of existing polymers is one of the most effective means to impart desired functionality to polymer substrates for a wide range of applications [6]. Various surface modification methods varying from physical to chemical approaches with each approach has own pros and cons, but chemical methods are favored for efficiency and property endurance. Therefore, the selection of a particular modification method always determines the level of enhanced properties and their stability in the functionalized polymers. Chemical-induced graft copolymerization is a well-established method for surface modification of polymeric surfaces to endow covalent functionalities that have been practiced for many years. However, this method is marred by its detrimental residues and the difficulty in controlling the level of grafting, and overall, it poses environmental concerns because of the use of hazardous chemical initiators and solvents [7]. Alternatively, treatment of surfaces with radiation-induced graft copolymerization (RIGC) using low-energy radiation (UV and plasma) or high-energy radiation (γ-rays or EB) as initiators leads to activation of polymer surfaces allowing reaction with specific polar monomers (under varying conditions relevant to each initiator) to yield polymers with chemically (covalently) bonded functionalities. The level of modification (grafting yield) can be easily controlled by the variation of reaction parameters. The versatility of RIGC made it possible to modify almost all polymer surfaces, especially with the presence of various polar monomers allowing the creation of desired functional properties including physicochemical and biological characteristics such as antimicrobial activity and biocompatibility [8].
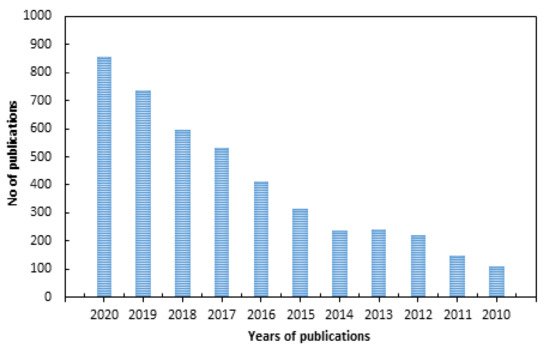
There have been many research articles addressing surface modifications of polymer surfaces to impart antimicrobial properties using various physical and chemical techniques, as indicated by the large number of publications in the past 10 years shown in Figure 1 (according to a Science Direct search up to the year 2020). The classification of antimicrobial polymers and their interaction mechanisms were reviewed extensively [9][10][11][12][9,10,11,12]. Reviews with limited scopes, such as those addressing individual surface modification techniques such as photo-grafting for surface modification [13], plasma treatment [14], modification of specific polymers such as silicon [15], fabrics (cotton and wool) [16], antimicrobial biomaterials containing quaternary ammonium [17], modification of biomaterials (polymer, ceramics, and metals) to impart biocompatibility and cellular interaction [18] have been published. Other articles reviewed strategies pertaining to bio-fouling prevention on silicon and silicon-based materials [15] and the use of nanometals to impart antimicrobial properties to polymers and textile fibers [19]. On the other hand, general strategies for designing antimicrobial surfaces and material selection [20] and the role of surface protection mechanisms in affecting future developments of advanced antibacterial and antiviral materials were also reported [21]. However, a review dedicated to the use of RIGC strategies to engineer surface modifications for imparting antimicrobial properties to a variety of polymeric materials of different physical forms used in developing protective fabrics, health care products, medical devices, biomedical materials, and food packaging films can be barely found in the literature.
2. Strategies for Imparting Antimicrobial Properties to Polymers
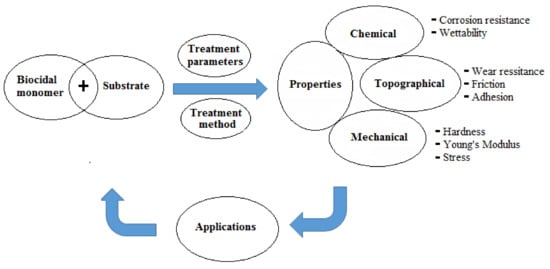
The basic principles for the development of surfaces with antimicrobial properties and manipulation of microbial stability in biological environments rely mainly on (i) surface treatment parameters for the applied technique and (ii) the induced surface chemistry (e.g., corrosion resistance). The former affects the topographical properties (e.g., wear resistance, friction, and adhesion) whereas the latter modulates the wettability characteristics. Both sets of parameters ultimately control the biological/microbiological cell response on the modified surfaces in addition to their mechanical properties (e.g., hardness, Young’s modulus, and stress), which maintain the stability of the bulk of the modified substrates [22][23][24,26]. To prevent cell bacterial colonization, the surface modification should induce either super-hydrophilicity (θ ≤ 10°) or super-hydrophobicity (θ ≥ 150°) [23][26]. A schematic diagram showing the critical properties which should be considered for designing antimicrobial surfaces is presented in Figure 2 .
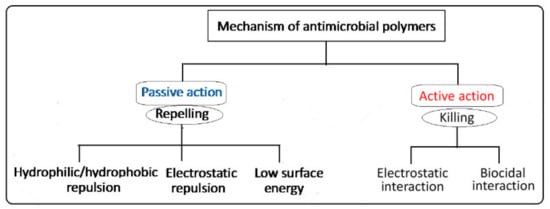
The design of antimicrobial surfaces has been focusing on the prevention of bacterial adhesion, proliferation, and biofilm formation. This can be carried out using two main strategies classified based on two action methods: (i) passive defense (bio-barrier formation) and (ii) active attack (controlled release) mechanisms [24][27]. The passive defense involves the direct incorporation of antimicrobial agents (e.g., polyethylene glycol, albumin, polyphenols, and zwitterionic polymers) to form a barrier preventing the initial bacterial adhesion. This strategy is based on hydrophilic/hydrophobic repulsion and electrostatic repulsion, or it can be driven by low surface energy, as depicted in the schematic diagram shown in Figure 3. On the other hand, the active attack strategy involves the incorporation of synthetic biocides such as quaternary ammonium and tertiary amine working by electrostatic interactions, or natural agents (e.g., nisin and essential oils) working by biocidal interactions to kill bacteria [11][25][26][11,28,29]. A variety of other antimicrobial agents such as phosphonium salts, triclosan (2,4,4-hydroxyphenyl trichloro (II) ether), and N-halamine have been employed for inhibiting bacterial growth and fouling prevention. The mechanism of the bactericidal action of such biocides is known to involve destructive interactions with the cell wall and/or cytoplasmic membranes [27][30]. Nevertheless, most of these biocides are toxic or carcinogenic and pose an environmental concern when applied to preformed surfaces such as fabrics. Furthermore, the alternative natural-based biocides such as essential oils, chitosan, and peptides are likely to be degradable and difficult to withstand sterilization conditions. Thus, imparting covalent immobilization of antimicrobial properties to polymeric surfaces by graft copolymerization of monomers having biocidal activities or alternatives that can be activated in a post-grafting treatment is highly effective and demanded.
Various studies have been carried out using both strategies to induce antimicrobial properties, which were proven to be effective but challenged by significant issues such as durability and loss of function by the accumulation of dead bacteria [28][31]. Thus, the criteria for the selection of antimicrobial agents for surfaces’ treatments include not only high efficiency against a wide spectrum of microorganisms and non-toxicity to the users and environment but also substrate durability and absence of impacts on functional properties [29][22]. Moreover, the design of an antimicrobial surface must satisfy additional requirements such as a facile and inexpensive modification method, water insolubility, resistance to the emission of toxic products, and having a non-irritating nature [30][32].
3. Challenges and Future Directions
RIGC is an old process but is making inroads into the chemical design of polymeric materials for a vast number of applications. The interesting part is that the process is applicable to almost all the polymer–monomer combinations, which is otherwise a very difficult task in chemical initiation approaches. The most interesting aspect of the process over chemical ones is that the substrates may be functionalized across the bulk irrespective of their shape and size. This could be realized by the ability of RIGC to modify surfaces of various physical forms including nonwoven fabrics, filaments, nanofibrous scaffolds, nanocomposites catheters, contact lenses, and cell culture dishes. Such substrates need careful designing in a way that allows the sought bioactivity to be efficiently imparted. Thus, the proper selection of monomer(s) and antimicrobial agent(s) are key components to incur specific features to such substrates using RIGC.
Radiation grafting leads to materials with very high purity, as the initiator (except in grafting with UV) is not used during the polymerization and the radiation itself acts as the initiator for the grafting. These are likely the features of radiation processing that made this process so interesting for biomaterials and other technologically interesting domains. One must look for further advancements in this direction. One of the aspects is this regard is to pursue the development of the many researched surfaces with engineered bioactivity made by RIGC from the laboratory scale to commercial products. Drug delivery and antimicrobial implants have been in use for several decades. However, more attention should be given to growing materials such as nanogels and nanocomposites, which can be designed by radiation processing with high-energy radiation and may be used for hydrogels in tissue contacting materials for human organs reconstruction. The biodegradable gel may be a better approach for radiation processing. It seems that RIGC will remain the subject of immense interest in the future as well due to its novel nature in functionalizing polymeric materials.
4. Conclusions
In this review, it has been shown that RIGC is an appealing and feasible method to impart durable bioactive functionalities to polymer surfaces regardless of polymer shape and size. The method allows engineering polymeric surfaces with inherently antifouling and antimicrobial properties, which are highly needed to control the ever-growing microbial infections and promote healthy lifestyles. The use of high-energy radiation (γ-rays and EB) for grafting initiation is superior to low-energy radiation (UV and plasma) initiation in terms of speed, flexibility, the ability to control the level of incorporated grafted functionality, and its industrial-scale applications. RIGC allows facile endowment of stable antimicrobial properties to fabrics, which can be utilized in applications promoting hygiene and health care such as protective clothing and face masks. Moreover, this method enables the preparation of thermo-responsive properties and pH-sensitive sutures without compromising their mechanical properties and cytocompatibility. The effectiveness of RIGC in imparting antimicrobial activities to catheters and wound dressing polymers is also realized but achievements in the latter application are mostly limited to crosslinking by irradiation with γ-rays and EB. Grafting of bioactive agents for modification of contact lenses is mostly performed with low-energy radiation sources such as UV and plasma. RIGP with EB irradiation was found to be the most widely applied method to graft cell culture substrates with PNIPAAm, allowing the development of thermo-responsive cell culture dish for thermally modulated initial cell adhesion and detachment for tissue engineering.
