The provision and adoption of technological solutions and the sharing of information globally has the potential to drive knowledge acquisition and positively affect healthcare worldwide. Digital solutions offer great promise in delivering increasingly individualised, easily accessible, and effective healthcare, with the capacity to evolve with time and adapt to the changing needs of people living with MS (PLwMS) and health care providers (HCPs). The impact of the COVID-19 pandemic has given additional proof of such versatility and usefulness, highlighting how barriers can be overcome through the adoption of digital tools, where capturing digital data remotely may mean that symptom tracking can be maintained even when clinic visits are not possible.
- multiple sclerosis
- software as a medical device
- digital health
- participatory health
- monitoring
- smartphone-based assessments
- clinical validation
- technical validation
- MS apps
- digital health solution development
1. Introduction
Multiple sclerosis (MS) is an inflammatory demyelinating and degenerative disease [1] characterised by a wide clinical variability in disease trajectory between individuals [2]. Clinical monitoring is intermittently, and often inconsistently [3[3][4],4], applied via in-clinic measures, such as the Expanded Disability Status Scale (EDSS) [5] and magnetic resonance imaging; detecting early disease progression is thus challenging [3,4][3][4]. Progressive worsening in specific domains (e.g., cognition [6]) can be subtle or subclinical, especially in the early stages of the disease, but tends to increase in frequency and severity over time. The worsening of disability is a multidimensional process and difficult to detect [7,8][7][8]. At present, the diagnosis of progression in MS is typically retrospective with a heavy reliance on clinical history, requiring progressive worsening for more than 6 months based on EDSS score, without evidence of relapses [3,4][3][4]. Monitoring in MS relies on infrequent outpatient assessments (typically occurring once or twice annually) with a lack of objective assessments of progression available to healthcare professionals (HCPs). New clinical and research tools are therefore needed to address the unmet need of early detection and ongoing assessment of progressive worsening, rendering this an inviting area for innovation in the digital health space.
Remote digital solutions such as smartphone-based apps, wearables, and decision support algorithms are increasingly utilised in research and clinical trial settings [9] and are beginning to emerge in routine medical care. This paper will focus on smartphone technology, which is ubiquitous and broadly accessible [10[10][11],11], making it a viable approach for facilitating remote assessment [12,13,14][12][13][14]. Smartphones can be used in a patients’ home environment as frequently as required and their use is increasingly familiar and unobtrusive. Further, most off-the-shelf smartphones contain sensors with the capacity to gather objective data unaffected by inter- and intra-rater variability. Measurements and patient-reported information captured with smartphone technology have the potential to enable more frequent, decentralised, and home-based care to supplement the infrequent in-clinic assessments typically offered to patients. Mutually sharing this information with patients can help focus the clinical conversation or empower shared decision making. Smartphone-based digital solutions are thus ideally placed to contribute to improving clinical care management for people living with MS (PLwMS) [15,16][15][16] and providing personalised healthcare [17].
Today, smartphone applications, performing a variety of functions, are available to support PLwMS. Many of these tools are non-regulated wellness applications designed to support day-to-day disease management, for example through symptom or medication intake tracking, visit-scheduling, provision of disease education, and connectivity to supportive care facilities or patient social media networks [18,19,20,21,22,23][18][19][20][21][22][23]. Other smartphone-based solutions enable assessment of functional parameters affected by the disease, such as mobility and cognition, or therapeutic benefit, such as for fatigue or depression [24]. Data and digital biomarkers collected by patient-facing apps may provide clinical value by generating new insights into the MS disease course, ultimately improving the understanding of individual disease trajectories and response to intervention. Despite their promise, however, smartphone-based solutions have not yet been fully integrated into routine medical practice.
The development journey of a smartphone-based solution for remote assessment of PLwMS will be presented here as a case study to illustrate the design and development process, validation, regulatory and clinical requirements, as well as deployment in the emergent digital health landscape. The process will be discussed from the perspective of industry developers and academic collaborators, from ideation through to technical solution development and version iteration, certification, and deployment. The Floodlight programme is a Roche-led initiative that aims to create digital solutions to facilitate functional assessment in MS. The first Floodlight app was an assessment suite for clinical research that required provisioned smartphones. More recent versions have been developed under design control to ensure that they meet the regulatory standards of reliability and meaningfulness associated with software as a medical device (SaMD)—standalone software that can perform medical functions without being part of a specific medical device hardware [25]—and to enable access for use on personal smartphones in a variety of integrated MS care settings.
2. Desirability: Challenges in Developing a Digital Solution That PLwMS and HCPs Need and Use
Identifying and balancing the needs and desires of different users when creating a digital solution can be challenging. Prior to initiating design control, the Jobs-to-be-Done (JTBD) framework [38][26] was used to define concrete user need statements for Floodlight MS. JTBD is an outcome-driven innovation strategy used to provide an in-depth understanding of user goals in a structured manner. Core functional desired outcomes (e.g., “Minimize the time it takes to determine how the patient’s past symptoms have changed since their last consultation”), as well as emotional and related jobs that might impact the ability to achieve an outcome (e.g., “Avoid feeling guilty for not spending enough time with a patient”), were collected for each user type. For Floodlight MS solution design, users were defined as (1) individuals with MS who are trying to live their lives while managing their MS, and (2) neurologists who are maintaining MS patients’ quality of life.
JTBD analysis also clarified factors that might limit the ability of PLwMS to interact with an app, such as comorbidities and disability status. The findings indicated that assessments within the solution must be convenient, with a reasonable duration and frequency. Different levels of user ability in terms of digital skills, as well as aspects such as dexterity and cognitive and visual impairments, would be likely to impact engagement. For many commercial applications, engagement is a key performance indicator and revenue driver. In digital health, however, solutions should only strive for sufficient engagement to support successful outcomes, in order to strike the balance between benefit and burden to the users.
Insufficient adherence to remote digital health solutions often presents a challenge to long-term engagement [39][27]. This is a significant obstacle for developers of apps intended for users with MS, where engagement may be required throughout the user’s lifetime. Adherence to the use of a digital health solution over time may be regarded as a behaviour, determined by factors such as the user’s motivation, ability, and other aspects such as forgetfulness. Behavioural design is based on insights from behavioural science, which can be implemented to aid in evoking desired user behaviour, and is recognised as a key element for development of digital health solutions to increase the likelihood of achieving the desired outcomes [40,41,42][28][29][30]. For example, the concept of the “neurological loop” has been used to explain how habits are formed via a three-step loop composed of cue, routine, and reward; solutions can thus be designed to provide users with strategic rewards to elicit repeated behaviour, based on specific cues [43][31]. A behavioural design approach was adopted to identify features that might enable users of Floodlight MS to achieve the outcome of improving clinical conversations. Fogg’s behaviour model (Behaviour = Motivation × Ability × Prompt [44][32]) was used as a framework to audit the design to identify facilitators and barriers to engagement in terms of motivation, ability, and prompts [44,45][32][33]. Feedback architecture was then designed to ensure appropriate communication with users and rewards (motivational prompts) for short-, medium-, and long-term outcomes. For example, the PLwMS interface home screen was designed to incorporate prompts for action, a progress indicator, and an appointment calendar to orient use of Floodlight MS around the care conversation. Further, notification and content architecture were also devised to sustain motivation across different use cases.
As there is great variability in symptomatology and disease course between individuals living with MS, solutions designed for these users need to accommodate diverse characteristics and varied needs, preferences, and behaviours when utilising smartphones. The complexity of addressing individual preferences and needs in a “one-size-fits-all” approach is typified in the end-user reaction to app gamification. The utility of gamification (the use of game design elements in other contexts) is widely discussed in relation to digital health solution development, as it may aid in increasing motivation and sustaining usage (i.e., increasing adherence [46][34]); however, any elements need to be applied cautiously in the context of healthcare and must support the desired outcome, which, for Floodlight MS, is the use of data for a care conversation. The topic of gamification—where, in the context of the Floodlight programme experience, some users considered it an inappropriate approach to disease assessment—may represent an example of possible divergent perspectives from different users and user types, which further illustrates the importance of behavioural strategies in studying use patterns. Even after final solution design, regular testing of the applied concepts should be conducted to ensure the usability of the features for all users, aligning with the specific needs of PLwMS. Moreover, careful consideration must be given to how the solution is implemented to ensure effective use. The application of behavioural science, for example through built-in analytics, whilst continuing to develop, test, and iterate on a periodic release cycle, will be important to enable iterative development throughout the solution lifecycle.
3. Regulatory Standards: Data Security, Verification and Validation
To satisfy regulatory requirements, each of the assessments provided by Floodlight MS were subject to technical verification, as well as clinical and analytical validation ( Figure 31 ). Individual assessments and data features were selected for SaMD certification based upon evidence obtained from the Floodlight PoC study and insights from DD.
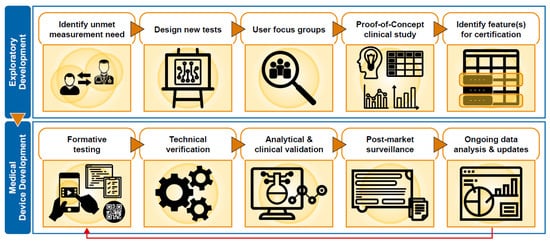
Whereas analytical validation establishes reliability, a process of clinical evaluation establishes clinical association and validation and is used to determine the sufficiency of evidence and the requirement for further clinical investigation. Clinical evaluation is a systematic and methodologically sound process used to continuously generate, collect, analyse, and assess the clinical data pertaining to a device in order to verify and validate the safety and performance of the device, including any clinical benefits, in the target user population and when used as intended [49][35]. Each assessment in the Floodlight MS solution was subject to evaluation, supported by multiple evidence sources, including the Floodlight PoC study and an observational study with PLwMS that provided an assessment by clinical content area experts that the process of, and results from, the Floodlight MS assessments achieved their intended purpose.
Post-marketing surveillance of SaMD is also required to provide ongoing monitoring of any defects and/or safety concerns, in order to ensure that solutions are safe and effective during real-world use. The post-market clinical follow-up plan, which is part of the clinical evaluation, specifies methods and procedures for collection and evaluation of clinical data from on-market use to confirm safety and performance. In addition to implementing subsequent clinical trials and real-world evidence generation initiatives, customer support should be present to capture reportable SaMD events for investigation, such as technical defects and safety issues, in order to fully comply with regulatory requirements. For Floodlight MS, customer support was tailored to respond to users’ needs (HCPs and PLwMS) in a specific geography, for example by offering local language support, in addition to addressing technical and medical questions.
Two key aspects of this dialogue are data safety and cybersecurity, as it is crucial to not only establish and maintain robust data privacy and security, but also to adapt them to comply with local requirements across geographies (e.g., General Data Protection Regulation in the EU, the Health Insurance Portability and Accountability Act in the USA, etc.). Data security can be divided into technical (obtaining and storage of data), methodological (the software application and infrastructure used to deliver it), and procedural aspects (data usage, data access, and security breaches), and each of these must be carefully considered at each stage of design and development [51][36]. Demonstrating a robust approach to personal data security is also a means to build users’ trust: 45% of users worry about the unwanted use of their data when using mobile devices for health-related activities [52[37][38],53], and there are legitimate concerns over user identification, data sharing with third parties, or accidental data leakage [52][37]. As data privacy and security provisions must be placed at the fore from the start of a user’s interaction with a digital solution, Floodlight MS users are presented with a data privacy notice during the sign-up process. This contains detailed information on the treatment of their personal information, which is in line with the applicable regulatory frameworks. Different types of security measures are in place for Floodlight MS, including password-protected access with automatic logout after a period of inactivity, and data encryption in transit and at rest. Moreover, the legal manufacturer of the device ensures that appropriate training and processes for data management operators are set up and that action plans are ready in case of any incidents.
4. Taking an Adaptive Approach
Agility is key when developing digital solutions, requiring a fluid approach to facilitate an iterative developmental process that aligns with the design control and requisite regulatory requirements. The rapidly changing technological environment contrasts markedly with classical drug development with its careful, largely linear and standardised processes [54,55][39][40]. Once a new solution is developed and deployed, post-market data can serve to further validate clinical effectiveness, evolve technical capabilities, and refine the user experience, and may even support subsequent regulatory engagement and reassessment.
Real-world evidence generation and non-interventional studies are often more efficient than, and can be complementary to, interventional trials. For the Floodlight programme, non-interventional studies and real-world evidence generated with Floodlight MS serves to complement assessment of Floodlight test technology in more formal clinical trial settings. Research is also being conducted to improve the clinical utility of the test suite, support clinical analysis, develop quality control features, and advance the understanding of sensor data [56,57,58,59][41][42][43][44].
The development path outlined here culminated in the release of Floodlight MS, which contains the five assessments registered as SaMD. Additional features, such as the Patient Journal, designed to help users reach the outcome of improved care conversations, are grouped separately and are thus able to be more frequently and flexibly iterated and improved upon based on user feedback, without necessitating resubmission to regulatory authorities. This continual iteration is enabled by recurring development cycles, which allow improvements to be implemented frequently, generating updated versions several times throughout the year. Floodlight MS is set to continue evolving, and thus certain topics covered in this paper may be revisited as knowledge advances.
The use of different technical deployment models, tailoring the means by which the solution is provided, may also provide the flexibility needed to meet local regulatory requirements and enable interoperability and integration in a complex and fragmented electronic health records landscape [60,61][45][46]. Three deployment models were designed for Floodlight MS: (i) a standalone solution with an HCP-facing web-based portal for data access, (ii) a standalone solution that can be integrated with electronic health records, and (iii) a software development kit (SDK). The SDK enables rapid, tailored integration of the Floodlight MS assessments into a third-party solution, for example into the DreaMS digital research tool advanced by the Research Center for Clinical Neuroimmunology and Neuroscience, Basel (RC2NB) [62][47]. The SDK approach also allows for integration into solutions developed in-house. Tailoring the deployment model for a digital solution may enable greater interoperability and the capability to address diverse and local needs on a greater scale.
References
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M.A. Multiple sclerosis. Nat. Rev. Dis. Primers 2018, 4, 43.
- Lorscheider, J.; Buzzard, K.; Jokubaitis, V.; Spelman, T.; Havrdova, E.; Horakova, D.; Trojano, M.; Izquierdo, G.; Girard, M.; Duquette, P. Defining secondary progressive multiple sclerosis. Brain 2016, 139, 2395–2405.
- Allen-Philbey, K.; Middleton, R.; Tuite-Dalton, K.; Baker, E.; Stennett, A.; Albor, C.; Schmierer, K. Can we improve the monitoring of people with multiple sclerosis using simple tools, data sharing, and patient engagement? Front. Neurol. 2020, 11, 464.
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014, 83, 278–286.
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444.
- Amato, M.P.; Portaccio, E.; Goretti, B.; Zipoli, V.; Hakiki, B.; Giannini, M.; Pastò, L.; Razzolini, L. Cognitive impairment in early stages of multiple sclerosis. Neurol. Sci. 2010, 31, 211–214.
- Inojosa, H.; Proschmann, U.; Akgün, K.; Ziemssen, T. Should we use clinical tools to identify disease progression? Front. Neurol. 2021, 11, 1890.
- Manouchehrinia, A.; Zhu, F.; Piani-Meier, D.; Lange, M.; Silva, D.G.; Carruthers, R.; Glaser, A.; Kingwell, E.; Tremlett, H.; Hillert, J. Predicting risk of secondary progression in multiple sclerosis: A nomogram. Mult. Scler. 2019, 25, 1102–1112.
- Inan, O.; Tenaerts, P.; Prindiville, S.; Reynolds, H.; Dizon, D.; Cooper-Arnold, K.; Turakhia, M.; Pletcher, M.; Preston, K.; Krumholz, H. Digitizing clinical trials. Jpn. Digit. Med. 2020, 3, 1–7.
- Haase, R.; Schultheiss, T.; Kempcke, R.; Thomas, K.; Ziemssen, T. Modern communication technology skills of patients with multiple sclerosis. Mult. Scler. 2013, 19, 1240.
- Center, P.R. Cell Phone and Smartphone Ownership Demographics. Available online: www.pewinternet.org/data-trend/mobile/cell-phone-and-smartphone-ownership-demographics/ (accessed on 15 June 2021).
- Prasad, S.; Ramachandran, R.; Jennings, C. Development of Smartphone Technology to Monitor Disease Progression in Multiple Sclerosis (P01. 144). Neurology 2012, 78.
- Bove, R.; White, C.C.; Giovannoni, G.; Glanz, B.; Golubchikov, V.; Hujol, J.; Jennings, C.; Langdon, D.; Lee, M.; Legedza, A. Evaluating more naturalistic outcome measures: A 1-year smartphone study in multiple sclerosis. Neurol. Neuroimmun. Neuroinflamm. 2015, 2, e162.
- Boukhvalova, A.K.; Kowalczyk, E.; Harris, T.; Kosa, P.; Wichman, A.; Sandford, M.A.; Memon, A.; Bielekova, B. Identifying and quantifying neurological disability via smartphone. Front. Neurol. 2018, 9, 740.
- Feys, P.; Giovannoni, G.; Dijsselbloem, N.; Centonze, D.; Eelen, P.; Lykke Andersen, S. The importance of a multi-disciplinary perspective and patient activation programmes in MS management. Mult. Scler. 2016, 22, 34–46.
- Marziniak, M.; Brichetto, G.; Feys, P.; Meyding-Lamadé, U.; Vernon, K.; Meuth, S.G. The use of digital and remote communication technologies as a tool for multiple sclerosis management: Narrative review. JMIR Rehabil. Assist. Technol. 2018, 5, e7805.
- Cancela, J.; Charlafti, I.; Colloud, S.; Wu, C. Digital health in the era of personalized healthcare: Opportunities and challenges for bringing research and patient care to a new level. Digit. Health 2021, 7–31.
- Scholz, M.; Haase, R.; Schriefer, D.; Voigt, I.; Ziemssen, T. Electronic health interventions in the case of multiple sclerosis: From theory to practice. Brain Sci. 2021, 11, 180.
- Lavorgna, L.; Russo, A.; De Stefano, M.; Lanzillo, R.; Esposito, S.; Moshtari, F.; Rullani, F.; Piscopo, K.; Buonanno, D.; Morra, V.B. Health-related coping and social interaction in people with multiple sclerosis supported by a social network: Pilot study with a new methodological approach. Interact. J. Med. Res. 2017, 6, e7402.
- Allam, A.; Kostova, Z.; Nakamoto, K.; Schulz, P.J. The effect of social support features and gamification on a Web-based intervention for rheumatoid arthritis patients: Randomized controlled trial. J. Med. Internet Res. 2015, 17, e3510.
- Fernandez-Luque, L.; Elahi, N.; Grajales, F., 3rd. An analysis of personal medical information disclosed in YouTube videos created by patients with multiple sclerosis. Stud. Health Technol. Inform. 2009, 150, 292–296.
- Biogen. Aby App—By above MS. Available online: https://www.abovems.com/en_us/home/ms-support-events/aby-app.html (accessed on 22 July 2021).
- Settle, J.R.; Maloni, H.W.; Bedra, M.; Finkelstein, J.; Zhan, M.; Wallin, M.T. Monitoring medication adherence in multiple sclerosis using a novel web-based tool: A pilot study. J. Telemed. Telecare 2016, 22, 225–233.
- De Angelis, M.; Lavorgna, L.; Carotenuto, A.; Petruzzo, M.; Lanzillo, R.; Brescia Morra, V.; Moccia, M. Digital Technology in Clinical Trials for Multiple Sclerosis: Systematic Review. J. Clin. Med. 2021, 10, 2328.
- International Medical Device Regulators Forum. Available online: http://www.imdrf.org/docs/imdrf/final/technical/imdrf-tech-140918-samd-framework-risk-categorization-141013.pdf (accessed on 12 July 2021).
- Ulwick, T. What Is Jobs-to-be-Done? Available online: https://jobs-to-be-done.com/what-is-jobs-to-be-done-fea59c8e39eb (accessed on 12 July 2021).
- Pratap, A.; Neto, E.C.; Snyder, P.; Stepnowsky, C.; Elhadad, N.; Grant, D.; Mohebbi, M.H.; Mooney, S.; Suver, C.; Wilbanks, J. Indicators of retention in remote digital health studies: A cross-study evaluation of 100,000 participants. Jpn. Digit. Med. 2020, 3, 1–10.
- Burrus, O.; Gupta, C.; Ortiz, A.; Zulkiewicz, B.; Furberg, R.; Uhrig, J.; Harshbarger, C.; Lewis, M.A. Principles for developing innovative HIV digital health interventions: The case of Positive Health Check. Med. Care 2018, 56, 756–760.
- Pagoto, S.; Bennett, G.G. How behavioral science can advance digital health. Transl. Behav. Med. 2013, 3, 271–276.
- Klonoff, D.C. Behavioral theory: The missing ingredient for digital health tools to change behavior and increase adherence. J. Diabetes Sci. Technol. 2019, 13, 276–281.
- Duhigg, C. The Power of Habit: Why We Do What We Do and How to Change; Random House: New York, NY, USA, 2013; pp. 1–371.
- Fogg, B.J. Tiny Habits: The Small Changes That Change Everything; Eamon Dolan Books: Boston, MA, USA, 2019.
- Fogg, B.J. A behavior model for persuasive design. In Proceedings of the 4th International Conference on Persuasive Technology, Claremont, CA, USA, 26–29 April 2009; pp. 1–7.
- Cugelman, B. Gamification: What it is and why it matters to digital health behavior change developers. JMIR Serious Games 2013, 1, e3139.
- Article 2 of Regulation (EU) 2017/745-MDR. Off. J. Eur. Union 2017, L 117/1–L 117/175. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ%3AL%3A2017%3A117%3ATOC (accessed on 15 September 2021).
- Bennett, K.; Bennett, A.J.; Griffiths, K.M. Security considerations for e-mental health interventions. J. Med. Internet Res. 2010, 12, e61.
- Commission, E. Green Paper on Mobile Health (“mHealth”). 2014, pp. 1–20. Available online: https://digital-strategy.ec.europa.eu/en/library/green-paper-mobile-health-mhealth (accessed on 12 July 2021).
- Recruitment, B.C.P. Leveraging Mobile Health Technology for Patient Recruitment. 2012, pp. 1–16. Available online: https://docplayer.net/10235751-Leveraging-mobile-health-technology-for-patient-recruitment-an-emerging-opportunity.html (accessed on 12 July 2021).
- Leclerc, O.; Smith, J. How New Biomolecular Platforms and Digital Technologies Are Changing R&D. Available online: https://www.mckinsey.com/industries/pharmaceuticals-and-medical-products/our-insights/how-new-biomolecular-platforms-and-digital-technologies-are-changing-r-and-d (accessed on 12 July 2021).
- Izmailova, E.S.; Wagner, J.A.; Ammour, N.; Amondikar, N.; Bell-Vlasov, A.; Berman, S.; Bloomfield, D.; Brady, L.S.; Cai, X.; Calle, R.A. Remote digital monitoring for medical product development. Clin. Transl. Sci. 2021, 14, 94–101.
- Cheng, W.-Y.; Bourke, A.K.; Lipsmeier, F.; Bernasconi, C.; Belachew, S.; Gossens, C.; Graves, J.S.; Montalban, X.; Lindemann, M. U-turn speed is a valid and reliable smartphone-based measure of multiple sclerosis-related gait and balance impairment. Gait Posture 2021, 84, 120–126.
- Creagh, A.; Simillion, C.; Scotland, A.; Lipsmeier, F.; Bernasconi, C.; Belachew, S.; van Beek, J.; Baker, M.; Gossens, C.; Lindemann, M. Smartphone-based remote assessment of upper extremity function for multiple sclerosis using the Draw a Shape Test. Physiol. Meas. 2020, 41, 054002.
- Creagh, A.P.; Simillion, C.; Bourke, A.K.; Scotland, A.; Lipsmeier, F.; Bernasconi, C.; van Beek, J.; Baker, M.; Gossens, C.; Lindemann, M. Smartphone-and smartwatch-based remote characterisation of ambulation in multiple sclerosis during the two-minute walk test. IEEE J. Biomed. Health Inform. 2020, 25, 838–849.
- Bourke, A.K.; Scotland, A.; Lipsmeier, F.; Gossens, C.; Lindemann, M. Gait characteristics harvested during a smartphone-based self-administered 2-minute walk test in people with multiple sclerosis: Test-retest reliability and minimum detectable change. Sensors 2020, 20, 5906.
- Bernat, J.L. Ethical and quality pitfalls in electronic health records. Neurology 2013, 80, 1057–1061.
- Romero, M.R.; Staub, A. Specialty Task Force: A Strategic Component to Electronic Health Record (EHR) Optimization. Stud. Health Technol. Inform. 2016, 225, 1051–1052.
- D’Souza, M.; Papadopoulou, A.; Girardey, C.; Kappos, L. Standardization and digitization of clinical data in multiple sclerosis. Nat. Rev. Neurol. 2021, 17, 119–125.
