Chronic kidney disease is a growing health crisis in the U.S. Diabetes and hypertension are the leading causes of CKD development; as the US is experiencing an increasing prevalence of both, CKD is expected to remain a critical national health issue. At ESRD, the kidneys have lost their ability function, and as a result, a series of malfunctions occur that lead to adverse health problems and health outcomes. Once diagnosed with ESRD, the patient either will be on dialysis for the rest of their life or receive a kidney transplant.
- diabetes
- chronic kidney disease
- proteinuria
- inflammation
- diet
- nutrition
- plant-based foods
- medical nutrition therapy
1. Introduction
The kidneys control many biological mechanisms such as fluid, electrolyte, pH balance, blood pressure, excretion of toxins and waste, vitamin D metabolism, and hormone synthesis. About thirty-seven million US adults are estimated to have chronic kidney disease (CKD), which is more than one in seven [1] Even more astonishing, nine in ten adults do not know they have the disease, and half of the adults with little kidney function who are not on dialysis are unaware they have CKD [1]. Chronic kidney disease often goes undiagnosed due to a lack of apparent symptoms in early stages. An estimated 94% with mild to moderate decline in renal function and about 48% of individuals with severe renal dysfunction go undiagnosed [2].
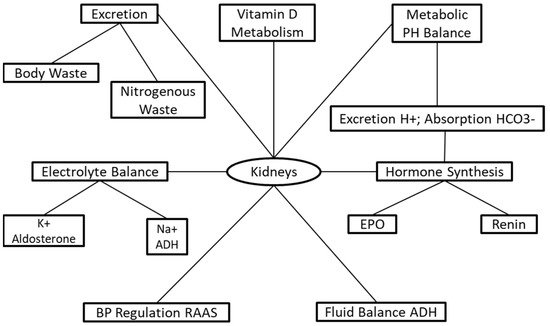
The kidneys are responsible for a series of life-sustaining mechanisms ( Figure 1 ). The primary functions of the kidneys are to sustain and maintain fluid and electrolyte and metabolic acid–base balance, which is accomplished through solute and fluid regulation, conservation of nutrients, and excretion of metabolic bodily waste [3]. The kidneys have endocrine and exocrine functions regulating and maintaining critical biological mechanisms in the body [4]. The exocrine functions involve fluid and electrolyte balance [5], acid–base regulation [6], and excretion of body waste [7] ( Figure 1 ). The endocrine functions include the activation of vitamin D for the incorporation of calcium into bones [8], and hormone synthesis for the regulation of blood pressure and synthesis of red blood cells [8][9][8,9].
The National Kidney Foundation (NKF) defines CKD as either a decline in glomerular filtration rate (GFR) to <15 mL/min/1.73 m² or the presence of kidney damage persisting for at least three months [10]. The prevalence of diabetes and hypertension is growing exponentially, predicting that CKD will continue to rise [11]. CKD patients are at increased risks for other health conditions, including acute kidney injury (AKI), T2DM, and mortality [12]. Chronic kidney disease is nationally incorporated into health promotion and disease-prevention programs to reduce its prevalence [13]. The US Department of Health and Human Services Healthy People 2020 had a target goal to minimize CKD prevalence from 14.8% in 2001 to 13.3% by 2020 [14].
Medical nutrition therapy is imperative for CKD patients because it may slow the progression of the disease through careful monitoring of protein, calcium, phosphorus, potassium, and sodium [15], relieving symptoms experienced in CKD patients while not restricting too many nutrients that would put the patient at high risk for malnutrition [16]. This review covers CKD pathophysiology, the most current diet recommendations, and the mechanisms that may delay the progression of the disease. In addition, the mechanisms of the newly explored whole food plant-based diet (WFPBD) are explained for its possible advantages in CKD prevention and progression. We performed a literature search on PubMed using “medical nutrition therapy”, “chronic kidney disease”, “clinical trials”, and “outcomes of medical nutrition therapy in chronic kidney diseases” from January 2021 to May 2021. Published articles reporting clinical trials were selected for writing this review, and the information from these papers were incorporated as tables. To be included in this narrative review, the paper had to be a clinical trial on: (a) type of protein intake and its relevance to CKD, (b) maintaining calcium, phosphate, and vitamin D (VD) levels, and (c) electrolyte balance in CDK patients. All other articles were excluded. The main contribution in this review is to provide current clinical research to dieticians and physicians in a concise manner that introduces possibilities in acquiring an appropriate CKD diet that widens dietary variation by including foods with less nutrient bioavailability than animal products and additives. In addition, we provide points for future research needed, such as RCTs, which may produce data that may support the efficacy of a whole food plant-based diet on ameliorating CKD progression.
2. Medical Nutrition Therapy
The federal Dietary Guidelines for Americans recommend an amount of 0.8 g/kg/body wt/d dietary protein intake for healthy adults [17][19]. Exceeding the recommended dietary allowance (RDA) may increase the risk of health complications even for healthy adults [17][19]. Protein intake recommendations for CKD patients are dependent on the stage of the disease, which is determined by declining GFR function [18].
An alternate protein source may be more beneficial to the patient’s health than restricting the amount of protein alone; the protein source may be of greater importance than the quantity [18][19][18,29]. Plant proteins are typically ingested along with fiber, phytonutrients, and antioxidants, although animal proteins are ingested along with saturated fat and cholesterol [2]; this may be one reason plant proteins are associated with a more vast decline in blood pressure compared to animal protein, as shown from the INTERMAP Study on micronutrients and macronutrients on blood pressure [20][33]. Additionally, animal protein is associated with decreased insulin sensitivity, increased reactive oxygen species (ROS) [21][34], and induced hyperfiltration [22][35]; ingesting an equal amount of plant protein does not promote the same effects [23][36]. Most of the food within plant-based diets come from plant sources [24][25][37,38]. These types of diets are generally lower in protein and saturated fat, contain higher levels of potassium and phosphorus, are richer in fiber, and provide the body with additional nutrients in the form of vitamins, minerals, and phytochemicals. Adopting a plant-based diet has been shown to have numerous health benefits, such as a reduction in atherosclerotic plaque buildup, decreased risk of cardiovascular disease, decreased BMI, reduced body weight, and lower blood pressure [26][27][39,40], which are parameters that are clinically relevant for management of CKD patients [26][39].
Phosphorus plays a critical role in bone formation, acid–base balance, and energy production [28][48]. The body’s ability to maintain phosphate balance is achieved by excreting excess phosphate in the urine. As CKD progresses, declining renal function prevents the kidneys from excreting enough phosphorus needed for phosphorus homeostasis [18]. The 2020 NKF guidelines recommended CKD 1–5 and HD patients receive an intake of phosphorus that keeps serum phosphorus levels within normal ranges (3.4–4.5 mg/dL) and to restrict dietary phosphate in the case of hyperphosphatemia [18][29][18,55] ( Table 12 ). Hyperphosphatemia may lead to critical pathogenic consequences, including renal osteodystrophy, cardiovascular and soft tissue calcification, secondary hyperthyroidism, cardiac disease, and mortality in ESRD patients [30][56]. Phosphorus requirements depend on the stage of renal failure combined with the consideration to not restrict phosphorus intake to the point of malnutrition, which is mainly relevant to HD patients [31][57]. Despite the KDOQI revision for phosphorus intake in CKD, nephrologists recommend a phosphorus restriction of 800–1000 mg/d [10]; however, adequate studies are lacking that demonstrate the efficacy of 800–1000 mg dietary phosphorus restriction and the outcomes in CKD patients [18].
| Electrolytes | Damage in CKD | Recommendation | Outcome | Ref |
|---|---|---|---|---|
| Total calcium CKD 3–4 w/no use of taking active vitamin D analogues |
Ca2+ deficiency ↑ risk secondary hyperparathyroidism and bone disorders. Excessive Ca2+ ↑ risk extraosseous calcification and CVD | 800–1000 mg/day | Maintain Ca2+ balance | [18][19][32] |
| CKD 5 w/use of active vitamin D analogues | Ca2+ deficiency ↑ risk secondary hyperparathyroidism and bone disorders. Excessive Ca2+ ↑ risk extraosseous calcification and CVD | Individualize Ca2+ restriction based on the use of vitamin D analogues | Maintain Ca2+ balance and prevent hypercalcemeia | [18][19][33] |
| Dietary Phosphorus * CKD 1–5 |
High dietary phosphorus intake associated w/ accelerated progression of disease and greater 5-year mortality risk | adjust dietary phosphorus intake to maintain normal serum phosphate levels between 3.4–4.5 mg/dL | Maintain Ca2+ and PTH balance. ↓ Secondary hyperparathyroidism mineral and bone disorders. Slow progression of CKD | [19][34] |
| Dietary Potassium CKD1–5 or post-transplantation | Hyper/hypokalemia associated w/muscular weakness, hypertension, ventricular arrhythmias, and death. Hypokalemia associated w/peripheral neuropathy. |
adjust dietary K+ intake to maintain serum potassium within 3.5–5.5 mEq/L | Slow progression of CKD. Prevention of peripheral neuropathy and other nerve related dysfunction. | [19][35][36] |
| Sodium (Na+) CKD 1–5 or post transplantation |
↑ BP excessive fluid retention/increased weight | <2300 mg/day | ↓ BP and normalize fluid balance/weight reduction/may ↓ proteinuria | [19][37][38][39] |
Additionally, Kalemic control is further compounded by extensive use of the renin–angiotensin–aldosterone system inhibitor (RAASI) therapy in CKD patients [40][77]. Development of hyperkalemia in CKD patients requires lowering the dose or discontinuation of the RAASI therapy to protect patients from developing cardiovascular events and end stage kidney disease.
3. The Role of a Registered Dietitian
Dietary education and patient counseling provided by a registered dietitian (RD) is essential for preventing and managing CKD. Careful and detailed dietary planning, frequent assessment of nutritional status, and dietary monitoring compliance are critical for successful dietary management.
The progressive decline in GFR is a risk factor for the development of metabolic acidosis. The main goal of therapy is to prevent or correct this metabolic acidosis, which has been shown to slow down the progression of CKD to end-stage renal disease [41][99]. The biggest contributor to this acid pool is the consumption of a diet higher in animal proteins [42][100]. The simplest treatment for this metabolic acidosis includes dietary management by reducing the protein in the diet or switching the diet to an increase in plant-based proteins [43][101]. It has been shown that dietary intervention of lowering protein intake or switching to plant-based protein reduces metabolic acidosis in stage 3–4 CKD patients [34][63].
Primary and secondary studies out of the MDRD study suggest that dietary interventions such as a low-protein diet reduce the rate of kidney function decline and lower the risk of ESKD in CKD patients [13][44][13,102]. Dietary interventions, such as low-protein diet, have been shown to retard the progression of CKD [44][102]. The dietary restriction of protein and phosphorous are shown to reduce the decline of kidney function and has been observed in type 1 diabetes patients [45][103]. The consensus among clinicians is that dietary interventions slow the rate of kidney function and potentially reduce the risk of end-stage kidney disease in patients with diabetes and CKD.
CKD patients often have or are at risk for comorbidities that entail specific diet management recommendations; this can be challenging and overwhelming. Additionally, CKD diet recommendations alter depending on the disease stage; this can create confusion for the patient. The dietitian has a more significant role than just providing dietary advice and recommendations for the patient. Counseling should be individualized and altered to the patient’s overall health, pre-existing conditions, and personal preferences. Adopting and adhering to a new diet requires the ability to motivate and inspire patients to make changes that will improve their health and prevent morbidities, although the changes may be uncomfortable for the patient. Adequate education about the rationale of the recommendations and how the patient will benefit are essential to convey. Equally as important is to assess the retention and understanding of the patient from the nutrition education. Through a thorough patient assessment and evaluation, the dietician may help prevent kidney disease by carefully monitoring their diabetic, hypertensive, and CVD patients by ordering the appropriate screening labs. It is imperative to regularly screen the patient for CVD, T2DM, malnutrition, and anemia, as they are at high risk of developing them. Providing alternative food options tailored to the patient’s likes and dislikes to replace restricted foods is more productive than focusing on the restrictions. Providing substitution education to the patient is essential to attain and maintain patient compliance and achieve successful dietary management.
4. Future Research and Clinical Practice
Secondary analysis of the MDRD study showed that patients with low protein intake during follow-up began experiencing uremic symptoms at lower GFR than patients with higher protein intake [45][103]. The reduced risk of end-stage renal failure reported may be from a delay in starting dialysis due to improved uremic symptoms rather than delayed kidney decline [45][103]. In addition, the study included 200 (24%) polycystic kidney disease (PKD) patients who may have contributed to data showing a delay in renal dysfunction due to the differences in the course of disease progression between CKD and PKD [45][103]. The INTERMAP Study lacked the use of “gold standard” diet assessments, food variation among different countries, and variation in dietary intake, which weakens the associations between nutrient intake and blood pressure [20][33].
Despite the large number of clinical trials being performed in the clinical and nutritional management of CKD, very few of these have translocated into clinical practices due to the lack of strong associations, not so clear research design, or low number of study subjects. There is a demand for future research to provide conclusive information that will assist clinicians and dietitians to make the most appropriate recommendations for their patients. Evaluating the impact of MNT on CKD progression by analysis of associated risk factors in patients with comorbidities is needed [18]. The clarity regarding which stage of CKD is most appropriate to alter protein intake is necessary. Future VD studies are required to determine the correct dosing and type of VD supplements for CKD patients. Future studies examining, comparing, and contrasting WFPBD, Mediterranean diet, and DASH diet in CKD patients to determine their effects on clinical outcomes are needed. Another challenge in CKD patients is not following the dietary recommendations. Research should be focus on boosting patient diet compliance by developing methods that will improve compliance and long-term adherence to nutrition prescriptions.
