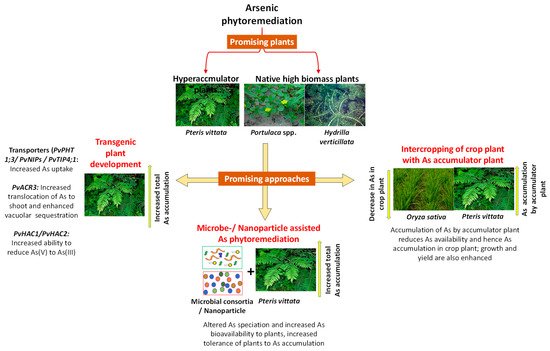
Arsenic contamination of the environment is a serious problem threatening the health of millions of people exposed to arsenic (As) via drinking water and crops grown in contaminated areas. The remediation of As-contaminated soil and water bodies needs to be sustainable, low-cost and feasible to apply in the most affected low-to-middle income countries, like India and Bangladesh. Phytoremediation is an aesthetically appreciable and successful approach that can be used for As decontamination with use of the best approach(es) and the most promising plant(s). However, phytoremediation lacks the required speed and sometimes the stress caused by As could diminish plants’ potential for remediation. To tackle these demerits, we need augment plants’ potential with appropriate technological methods including microbial and nanoparticles applications and genetic modification of plants to alleviate the As stress and enhance As accumulation in phytoremediator plants.
- arsenic
- hyperaccumulator
- nanoparticles
- microorganisms
- phytoremediation
- Pteris vittata
1. Introduction
2. Phytoremediation: A Sustainable Approach
| Plants | Arsenic Stress | Results | Ref. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arsenic Hyperaccumulator Plants | ||||||||||||
| Landolita punctata | As(V) (0.5–3.0 mg/L | ) | Plants showed As hyperaccumulation (>1000 mg/kg As) at or more than 1 mg/L As; however, higher than 1 mg/L As levels were toxic | [27] | ||||||||
| Pteris vittata | As (average 8885 mg/kg) and thallium (3.91 to 178 mg/kg) contaminated mining area | Pteris vittata | accumulated around 7215–11,110 mg/kg As, and 6.47–111 mg/kg of thallium | [28] | ||||||||
| High Biomass Producing Plants | ||||||||||||
| Calatropis prosera | Arsenic given in hydroponic and soil | C. procera | reduced As concentration by 45% and 58% in hydroponics and by 30% and 36% in soil, after 15 and 30 days, respectively. | [29] | ||||||||
| Portulaca oleracea | As (154 mg/kg and 193 mg/kg at site-I and site-II); other metals (Cd, Pb, Cu) were also present | At site I, As accumulation in stem was around 94.5 mg/kg, whereas at site II, it was 73.6 mg/kg | [30] | |||||||||
| Plants with Economic Utiliity | ||||||||||||
| Helianthus annus | Farmland soil containing As (84.85 mg/kg) | The mean As level 49.04 mg/kg in the above-ground parts. Average seed yield (45.90 kg/m | 2 | ) and oil production (34.65%) | [31] | |||||||
| Hydrilla verticillata | As(V) (15–375 μg/L) | Total As accumulation was 197.2 μg/g dry weight when As(V) was 375 μg/L | [32] | |||||||||
| Microbe-Assisted Arsenic Remediation | ||||||||||||
| Arundo donax | + consortia of two strains of | Stenotrophomonas maltophilia | and one strains of | Agrobacterium | sp. | As(III) (2–20 mg/L) | In the presence of bacterial consortium, 11.37 mg/kg As was volatilized by transpiration | [33] | ||||
| Alfalfa | + | Ensifer | sp. M14 | Soil As(III) (10 mg/kg) | As concentration in leaves of inoculated plants was 11% higher than those cultivated without microorganisms. | [34] | ||||||
| Nano-Phytoremediation Approaches | ||||||||||||
| Eucalyptus leaf extract mediated synthesis iron oxide NPs | Arsenic | Arsenic adsorption capacity was found to be 39.84 mg/g | [35] | |||||||||
| Isatis cappadocica + | glutathione modified superparamagnetic iron oxide NPs {nFe | 3 | O | 4 | @GSH} | Soil As (1000 μM) | nFe | 3 | O | 4 | @GSH treatment increased growth of plants and As tolerance by reducing As accumulation in plants | [36] |
| Genetic Engineering Approaches | ||||||||||||
| Arabidopsis thaliana | transformed with bacterial As transporter (ArsB) targeted to vacuolar membrane | As(III) (5 μM) | Transgenic plants showed higher As accumulation in shoots compared to wild type plants | [37] | ||||||||
| Nicotiana tabaccum | transformed with | PvPht1;3 | from | P. vittata | As(V) (20 μM) Soil As (9.66 mg/kg) |
Arsenic accumulation in shoot tissues of transgenic tobacco increased in both hydroponic and soil experiments | [38] | |||||
2.1. Selection of Plants for Arsenic Phytoremediation
2.1.1. Arsenic Hyperaccumulators
2.1.2. High Biomass Plants for Arsenic Cleanup
2.1.3. Plants with Bioenergy Potential and Economic Utility
References
- Srivastava, S. Arsenic in Drinking Water and Food; Springer Nature: Singapore, 2020.
- Shukla, A.; Awasthi, S.; Chauhan, R.; Srivastava, S. The Status of Arsenic Contamination in India. In Arsenic in Drinking Water and Food; Srivastava, S., Ed.; Springer Nature: Singapore, 2020; pp. 1–12.
- Medunic, G.; Fiket, Z.; Ivanic, M. Arsenic Contamination Status in Europe, Australia, and Other Parts of the World. In Arsenic in Drinking Water and Food; Srivastava, S., Ed.; Springer Nature: Singapore, 2020; pp. 183–233.
- Jankovic, M.M. Arsenic Contamination Status in North America. In Arsenic in Drinking Water and Food; Srivastava, S., Ed.; Springer Nature: Singapore, 2020; pp. 41–69.
- Srivastava, S.; Pathak, S.; Ponsin, M.; Hensawang, S.; Chanpiwat, P.; Yoeurn, C.; Phan, K. Sustainable solutions to arsenic ac-cumulation in rice grown in south and southeast Asia. Crop Pasture Sci. 2021, in press.
- Neumann, R.B.; Vincent, A.P.S.; Roberts, L.C.; Badruzzaman, A.B.M.; Ali, M.A.; Harvey, C.F. Rice Field Geochemistry and Hydrology: An Explanation for Why Groundwater Irrigated Fields in Bangladesh are Net Sinks of Arsenic from Groundwater. Environ. Sci. Technol. 2011, 45, 2072–2078.
- Upadhyay, M.K.; Majumdar, A.; Kumar, J.S.; Srivastava, S. Arsenic in Rice Agro-Ecosystem: Solutions for Safe and Sustainable Rice Production. Front. Sustain. Food Syst. 2020, 4, 53.
- Awasthi, S.; Chauhan, R.; Srivastava, S.; Tripathi, R.D. The Journey of Arsenic from Soil to Grain in Rice. Front. Plant Sci. 2017, 8, 1007.
- Himeno, S.; Sumi, D.; Fujishiro, H. Toxicometallomics of Cadmium, Manganese and Arsenic with Special Reference to the Roles of Metal Transporters. Toxicol. Res. 2019, 35, 311–317.
- Wu, C.; Huang, L.; Xue, S.G.; Shi, L.Z.; Hartley, W.; Cui, M.; Wong, M.H. Arsenic sorption by red mud-modified biochar pro-duced from rice straw. Environ. Sci. Pollut. Res. 2017, 24, 18168–18178.
- Tripathi, R.D.; Srivastava, S.; Mishra, S.; Singh, N.; Tuli, R.; Gupta, D.K.; Maathuis, F.J. Arsenic hazards: Strategies for tolerance and remediation by plants. Trends Biotechnol. 2007, 25, 158–165.
- Wan, X.; Lei, M.; Chen, T. Review on remediation technologies for arsenic-contaminated soil. Front. Environ. Sci. Eng. 2019, 14, 24.
- DalCorso, G.; Fasani, E.; Manara, A.; Visioli, G.; Furini, A. Heavy metal pollutions: State of the art and innovation in phy-toremediation. Int. J. Mol. Sci. 2019, 20, 3412.
- Kumar, P.; Kumar, A.; Kumar, R. Phytoremediation and Nanoremediation. In New Frontiers of Nanomaterials in Environmental Science; Kumar, R., Kumar, R., Kaur, G., Eds.; Springer Nature: Singapore, 2021; pp. 281–297.
- Juwarkar, A.A.; Singh, S.K.; Mudhoo, A. A comprehensive overview of elements in bioremediation. Rev. Environ. Sci. Biotechnol. 2010, 9, 215–288.
- Ernst, W.H.O. Phytoextraction of mine wastes: Opinion and impossibilities. Chem. Erde Geochem. 2005, 65, 29–42.
- Tripathi, P.; Dwivedi, S.; Mishra, A.; Kumar, A.; Dave, R.; Srivastava, S.; Shukla, M.K.; Srivastava, P.K.; Chakrabarty, D.; Trivedi, P.K.; et al. Arsenic accumulation in native plants of West Bengal, India: Prospects for phytoremediation but concerns with the use of medicinal plants. Environ. Monit. Assess. 2011, 184, 2617–2631.
- Mesa, V.; Navazas, A.; González-Gil, R.; González, A.; Weyens, N.; Lauga, B.; Gallego, J.L.R.; Sánchez, J.; Peláez, A.I. Use of Endophytic and Rhizosphere Bacteria to Improve Phytoremediation of Arsenic-Contaminated Industrial Soils by Autochthonous Betula celtiberica. Appl. Environ. Microbiol. 2017, 83, e03411-16.
- Franchi, E.; Rolli, E.; Marasco, R.; Agazzi, G.; Borin, S.; Cosmina, P.; Pedron, F.; Rosellini, I.; Barbafieri, M.; Petruzzelli, G. Phytoremediation of a multi contaminated soil: Mercury and arsenic phytoextraction assisted by mobilizing agent and plant growth promoting bacteria. J. Soils Sediments 2016, 17, 1224–1236.
- Ranjan, A.; Rajput, V.D.; Minkina, T.; Bauer, T.; Chauhan, A.; Jindal, T. Nanoparticles induced stress and toxicity in plants. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100457.
- Zuverza-Mena, N.; Martínez-Fernández, D.; Du, W.; Hernandez-Viezcas, J.A.; Bonilla-Bird, N.; López-Moreno, M.L.; Komárek, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Exposure of engineered nanomaterials to plants: Insights into the physiological and biochemical responses—A review. Plant Physiol. Biochem. 2016, 110, 236–264.
- Trujillo-Reyes, J.; Majumdar, S.; Botez, C.E.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Exposure studies of core-shell Fe/Fe(3)O(4) and Cu/CuO NPs to lettuce (Lactuca sativa) plants: Are they a potential physiological and nutritional hazard? J. Hazard. Mater. 2014, 267, 255–263.
- Liu, W.; Li, Y.; Feng, Y.; Qiao, J.; Zhao, H.; Xie, J.; Fang, Y.; Shen, S.; Liang, S. The effectiveness of nanobiochar for reducing phytotoxicity and improving soil remediation in cadmium-contaminated soil. Sci. Rep. 2020, 10, 1–10.
- Srivastav, A.; Yadav, K.K.; Yadav, S.; Gupta, N.; Singh, J.K.; Katiyar, R.; Kumar, V. Nano-phytoremediation of Pollutants from Contaminated Soil Environment: Current Scenario and Future Prospects. In Phytoremediation: Management of Environmental Contaminants; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer International Publishing: Cham, Switzerland, 2018; Volume 6, pp. 383–401.
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Alam Cheema, S.; Rehman, H.U.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778.
- Zhou, P.; Adeel, M.; Shakoor, N.; Guo, M.; Hao, Y.; Azeem, I.; Li, M.; Liu, M.; Rui, Y. Application of Nanoparticles Alleviates Heavy Metals Stress and Promotes Plant Growth: An Overview. Nanomaterials 2020, 11, 26.
- Canatto, R.A.; De Oliveira, J.A.; Da-Silva, C.J.; Albino, B.S. Tolerance of Landoltia punctata to arsenate: An evaluation of the potential use in phytoremediation programs. Int. J. Phytoremediat. 2020, 23, 102–110.
- Wei, X.; Zhou, Y.; Tsang, D.C.; Song, L.; Zhang, C.; Yin, M.; Liu, J.; Xiao, T.; Zhang, G.; Wang, J. Hyperaccumulation and transport mechanism of thallium and arsenic in brake ferns (Pteris vittata L.): A case study from mining area. J. Hazard. Mater. 2019, 388, 121756.
- Singh, S.; Fulzele, D.P. Phytoextraction of arsenic using a weed plant Calotropis procera from contaminated water and soil: Growth and biochemical response. Int. J. Phytoremediat. 2021, 1–9.
- Negi, S. Heavy metal accumulation in Portulaca oleracea Linn. J. Pharmacogn. Phytochem. 2018, 7, 2978–2982.
- Sahito, Z.A.; Zehra, A.; Tang, L.; Ali, Z.; Hashmi, M.L.R.; Ullah, M.A.; He, Z.; Yang, X. Arsenic and mercury uptake and accumulation in oilseed sunflower accessions selected to mitigate co-contaminated soil coupled with oil and bioenergy pro-duction. J. Clean. Prod. 2021, 291, 125226.
- Zhao, Y.; Zhen, Z.; Wang, Z.; Zeng, L.; Yan, C. Influence of environmental factors on arsenic accumulation and biotransformation using the aquatic plant species Hydrilla verticillata. J. Environ. Sci. 2019, 90, 244–252.
- Guarino, F.; Miranda, A.; Castiglione, S.; Cicatelli, A. Arsenic phytovolatilization and epigenetic modifications in Arundo donax L. assisted by a PGPR consortium. Chemosphere 2020, 251, 126310.
- Debiec-Andrzejewska, K.; Krucon, T.; Piatkowska, K.; Drewniak, L. Enhancing the plants growth and arsenic uptake from soil using arsenite-oxidizing bacteria. Environ. Pollut. 2020, 264, 114692.
- Kamath, V.; Chandra, P.; Jeppu, G.P. Comparative study of using five different leaf extracts in the green synthesis of iron oxide nanoparticles for removal of arsenic from water. Int. J. Phytoremediat. 2020, 22, 1278–1294.
- Souri, Z.; Karimi, N.; Norouzi, L.; Ma, X. Elucidating the physiological mechanisms underlying enhanced arsenic hyperaccu-mulation by glutathione modified superparamagnetic iron oxide nanoparticles in Isatis cappadocica. Ecotox. Environ. Saf. 2020, 53, 111336.
- Deromachi, Y.; Uraguchi, S.; Kiyono, M.; Kuga, K.; Nishimura, K.; Sato, M.H.; Hirano, T. Stable expression of bacterial trans-porter ArsB attached to SNARE molecule enhances arsenic accumulation in Arabidopsis. Plant Signal. Behav. 2020, 15, 1802553.
- Cao, Y.; Feng, H.; Sun, D.; Xu, G.; Rathinasabapathi, B.; Chen, Y.; Ma, L.Q. Heterologous Expression of Pteris vittata Phosphate Transporter PvPht1;3 Enhances Arsenic Translocation to and Accumulation in Tobacco Shoots. Environ. Sci. Technol. 2019, 53, 10636–10644.
- Baker, A.J.M.; Brooks, R.R. Terrestrial higher plants which hyperaccumulate metallic elements—A review of their distribution, ecology and phytochemistry. Biorecovery 1989, 1, 81–126.
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. A fern that hyperaccumulates arsenic. Nature 2001, 409, 579.
- Zhao, F.-J.; Dunham, S.; McGrath, S.P. Arsenic hyperaccumulation by different fern species. New Phytol. 2002, 156, 27–31.
- Srivastava, M.; Ma, L.Q.; Singh, N.; Singh, S. Antioxidant responses of hyper-accumulator and sensitive fern species to arsenic. J. Exp. Bot. 2005, 56, 1335–1342.
- Francesconi, K.; Visoottiviseth, P.; Sridokchan, W.; Goessler, W. Arsenic species in an arsenic hyperaccumulating fern, Pityrogramm acalomelanos: A potential phytoremediator of arsenic-contaminated soils. Sci. Total Environ. 2002, 284, 27–35.
- Karimi, N.; Ghaderian, S.M.; Raab, A.; Feldmann, J.; Meharg, A.A. An arsenic-accumulating, hypertolerant brassica, Isatis cap-padocica. New Phytol. 2009, 184, 41–47.
- Vetterlein, D.; Wesenberg, D.; Nathan, P.; Brautigam, A.; Schierhorn, A.; Mattusch, J.; Jahn, R. Pteris vittata—Revisited: Uptake of As and its speciation, impact of P.; role of phytochelatins and S. Environ. Pollut. 2013, 157, 3016–3024.
- Xiyuan, X.; Tongbin, C.; Zhizhuang, A.; Mei, L.; Zechun, H.; Xiaoyong, L.; Yingru, L. Potential of Pteris vittata L. for phytore-mediation of sites co-contaminated with cadmium and arsenic: The tolerance and accumulation. J. Environ. Sci. 2008, 20, 62–67.
- Liao, X.Y.; Chen, T.B.; Xie, H.; Xiao, X.Y. Effect of application of P fertilizer on efficiency of As removal in contaminated soil using phytoremediation: Field demonstration. Acta Sci. Circumst. 2004, 24, 455–462.
- Fayiga, A.O.; Ma, L.Q. Using phosphate rock to immobilize metals in soil and increase arsenic uptake by hyperaccumulator Pteris vittata. Sci. Total Environ. 2006, 359, 17–25.
- Bolan, N.; Mahimairaja, S.; Kunhikrishnan, A.; Choppala, G. Phosphorus-arsenic interactions in variable-charge soils in relation to arsenic mobility and bioavailability. Sci. Total Environ. 2013, 463–464, 1154–1162.
- Fu, J.W.; Liu, X.; Han, Y.H.; Mei, H.; Cao, Y.; de Oliveira, L.M.; Liu, Y.; Rathinasabapathi, B.; Chen, Y.; Ma, L.Q. Arse-nic-hyperaccumulator Pteris vittata efficiently solubilized phosphate rock to sustain plant growth and As uptake. J. Hazard. Mater. 2017, 330, 68–75.
- Elless, M.P.; Poynton, C.Y.; Willms, C.A.; Doyle, M.P.; Lopez, A.C.; Sokkary, D.A.; Ferguson, B.W.; Blaylock, M.J. Pilot-scale demonstration of phytofiltration for treatment of arsenic in New Mexico drinking water. Water Res. 2005, 39, 3863–3872.
- Natarajan, S.; Stamps, R.H.; Saha, U.K.; Ma, L.Q. Phytofiltration of arsenic-contaminated groundwater using Pteris vittata L.: Effect of plant density and nitrogen and phosphorus levels. Int. J. Phytoremediat. 2008, 10, 222–235.
- Huang, Y.; Miyauchi, K.; Inoue, C.; Endo, G. Development of suitable hydroponics system for phytoremediation of arse-nic-contaminated water using an arsenic hyperaccumulator plant Pteris vittata. Biosci. Biotechnol. Biochem. 2016, 80, 614–618.
- Yadav, S.K.; Juwarkar, A.A.; Phani Kumar, G.; Thawale, P.R.; Singh, S.K.; Chakrabarti, T. Bioaccumulation and phy-to-translocation of arsenic, chromium and zinc by Jatropha curcas L.: Impact of dairy sludge and biofertilizer. Bioresour. Technol. 2009, 100, 4616–4622.
- Jiang, Y.; Lei, M.; Duan, L.; Longhurst, P. Integrating phytoremediation with biomass valorisation and critical element recovery: A UK contaminated land perspective. Biomass Bioenergy 2015, 83, 328–339.
- Srivastava, S.; Srivastava, A.K.; Suprasanna, P.; D’Souza, S.F. Comparative biochemical and transcriptional profiling of two contrasting varieties of Brassica juncea L. in response to arsenic exposure reveals mechanisms of stress perception and tolerance. J. Exp. Bot. 2009, 60, 3419–3431.
- Piracha, M.A.; Ashraf, M.; Niaz, A. Arsenic fractionation and its impact on physiological behavior of sunflower (Helianthus annuus L.) in three texturally different soils under alkaline calcareous conditions. Environ. Sci. Pollut. Res. 2019, 26, 17438–17449.
- Purdy, J.J.; Smart, L.B. Hydroponic Screening of Shrub Willow (Salix spp.) for Arsenic Tolerance and Uptake. Int. J. Phytoremediat. 2008, 10, 515–528.
- Hadi, F.; Ali, N.; Ahmad, A. Enhanced phytoremediation of cadmium contaminated soil by Parthenium hysterophorus plant: Effect of gibberelic acid (GA3) and synthetic chelator, alone and in combinations. Bioremediat. J. 2014, 18, 46–55.
- Favas, P.J.; Pratas, J.; Prasad, M. Accumulation of arsenic by aquatic plants in large-scale field conditions: Opportunities for phytoremediation and bioindication. Sci. Total Environ. 2012, 433, 390–397.
- Lokhande, V.H.; Srivastava, S.; Patade, V.Y.; Dwivedi, S.; Tripathi, R.; Nikam, T.; Suprasanna, P. Investigation of arsenic accumulation and tolerance potential of Sesuvium portulacastrum (L.) L. Chemosphere 2011, 82, 529–534.
- Dwivedi, S.; Srivastava, S.; Mishra, S.; Dixit, B.; Kumar, A.; Tripathi, R. Screening of native plants and algae growing on fly-ash affected areas near National Thermal Power Corporation, Tanda, Uttar Pradesh, India for accumulation of toxic heavy metals. J. Hazard. Mater. 2008, 158, 359–365.
- Weis, J.S.; Weis, P. Metal uptake, transport and release by wetland plants: Implications for phytoremediation and restoration. Environ. Int. 2004, 30, 685–700.
- Nigam, S.; Gopal, K.; Vankar, P.S. Biosorption of arsenic in drinking water by submerged plant: Hydrilla verticilata. Environ. Sci. Pollut. Res. 2012, 20, 4000–4008.
- Mkandawire, M.; Lyubun, Y.V.; Kosterin, P.V.; Dudel, E.G. Toxicity of arsenic species to Lemna gibba L. and the influence of phosphate on arsenic bioavailability. Environ. Toxicol. 2004, 19, 26–34.
- Goswami, C.; Majumder, A.; Misra, A.K.; Bandyopadhyay, K. Arsenic uptake by Lemna minor in hydroponic system. Int. J. Phytoremediat. 2014, 16, 1221–1227.
- Zhang, X.; Lin, A.-J.; Zhao, F.-J.; Xu, G.-Z.; Duan, G.-L.; Zhu, Y.-G. Arsenic accumulation by the aquatic fern Azolla: Comparison of arsenate uptake, speciation and efflux by A. caroliniana and A. filiculoides. Environ. Pollut. 2008, 156, 1149–1155.
- Farnese, F.; Oliveira, J.; Lima, F.; Leão, G.; Gusman, G.; Silva, L. Evaluation of the potential of Pistia stratiotes L. (water lettuce) for bioindication and phytoremediation of aquatic environments contaminated with arsenic. Braz. J. Biol. 2014, 74, S108–S112.
- Rahman, M.A.; Hasegawa, H.; Ueda, K.; Maki, T. Influence of phosphate and iron ions in selective uptake of arsenic species by water fern (Salvinia natans L.). Chem. Eng. J. 2008, 145, 179–184.
- Zimmels, Y.; Kirzhner, F.; Malkovskaja, A. Application of Eichhornia crassipes and Pistia stratiotes for treatment of urban sewage in Israel. J. Environ. Manag. 2006, 81, 420–428.
- De Souza, T.D.; Borges, A.C.; de Matos, A.T.; Veloso, R.W.; Braga, A.F. Optimization of arsenic phytoremediation using Eic-chornia crassipes. Int. J. Phytoremediat. 2018, 20, 1129–1135.
- Srivastava, S.; Shrivastava, M.; Suprasanna, P.; D’Souza, S. Phytofiltration of arsenic from simulated contaminated water using Hydrilla verticillata in field conditions. Ecol. Eng. 2011, 37, 1937–1941.
- Islam, M.S.; Saito, T.; Kurasaki, M. Phytofiltration of arsenic and cadmium by using an aquatic plant, Micranthemum umbrosum: Phytotoxicity, uptake kinetics, and mechanism. Ecotoxicol. Environ. Saf. 2015, 112, 193–200.
- Aryal, R.; Nirola, R.; Beecham, S.; Kamruzzaman, M. Impact of elemental uptake in the root chemistry of wetland plants. Int. J. Phytoremediat. 2016, 18, 936–942.
- Bauddh, K.; Singh, B.; Korstad, J. Phytoremediation Potential of Bioenergy Plants; Springer: Berlin/Heidelberg, Germany, 2017.
- Chaukura, N.; Gwenzi, W.; Tavengwa, N.; Manyuchi, M.M. Biosorbents for the removal of synthetic organics and emerging pollutants: Opportunities and challenges for developing countries. Environ. Dev. 2016, 19, 84–89.
- Bote, M.A.; Naik, V.R.; Jagadeeshgouda, K.B. Review on water hyacinth weed as a potential biofuel crop to meet collective energy needs. Mater. Sci. Energy Technol. 2020, 3, 397–406.
- Nahar, K.; Sunny, S.A. Duckweed-based clean energy production dynamics (ethanol and biogas) and phyto-remediation po-tential in Bangladesh. Model. Earth Syst. Environ. 2019, 6, 1–11.
- Uchimiya, M.; Wartelle, L.H.; Klasson, K.T.; Fortier, C.A.; Lima, I.M. Influence of Pyrolysis Temperature on Biochar Property and Function as a Heavy Metal Sorbent in Soil. J. Agric. Food Chem. 2011, 59, 2501–2510.
- Zhu, N.; Zhang, J.; Tang, J.; Zhu, Y.; Wu, Y. Arsenic removal by periphytic biofilm and its application combined with biochar. Bioresour. Technol. 2018, 248, 49–55.
