The use of natural products to promote health is as old as human civilization. In recent years, the perception of natural products derived from plants as abundant sources of biologically active compounds has driven their exploitation towards the search for new chemical products that can lead to further pharmaceutical formulations. Candida fungi, being opportunistic pathogens, increase their virulence by acquiring resistance to conventional antimicrobials, triggering diseases, especially in immunosuppressed hosts. They are also pointed to as the main pathogens responsible for most fungal infections of the oral cavity. This increased resistance to conventional synthetic antimicrobials has driven the search for new molecules present in plant extracts, which have been widely explored as alternative agents in the prevention and treatment of infections.
- Candida spp.
- oral disease
- oral biofilm
- infections
- medicinal plants
- plant extracts
- natural compounds
- antibiofilm strategies
1. Introduction
2. The Bioactive Compounds of Plants
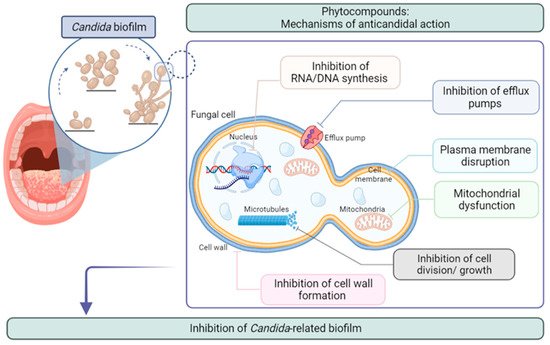
3. Plant Extracts against Oral Biofilm Formed by Candida spp.
Most of the available antifungals are either ineffective against Candida biofilms or exhibit activity at very high concentrations [55][70]. Concerning microbial resistance, pharmacotherapy has reached its limit, threatening the effective prevention and treatment of an ever-increasing range of infections. These limitations have led to the search for novel molecules with antibiofilm potential. Plants are rich sources of bioactive molecules exhibiting various biological and pharmaceutical properties. Therefore, in recent years, new clinical approaches using natural phytocompounds have been the subject of several types of research, considering the composition of natural plant products in molecules with antimicrobial and/or antibiofilm potential. Table 1 presents some of the plant species whose extracts hold compounds with antifungal/antibiofilm activity against Candida spp. Moreover, extracts able to inhibit biofilm formation and/or eradication in more than 99%, at concentrations ≤ 1 mg∙mL−1, were chosen for discussion. Allium sativum L. (Amaryllidaceae) is an aromatic herbaceous annual plant, one of the oldest authenticated and most important herbs that have been used since ancient times in traditional medicine. It is one of the most described plant species with proven antifungal, antimicrobial, anti-aging, as well as anticancer properties, which have been confirmed by epidemiological data from human clinical studies [56][71]. This specie and its active components have been also reported to reduce the risk of diabetes and cardiovascular diseases [57][58][72,73]. A. sativum antibiofilm properties against oral cavity yeast were studied by Fahim et al. [59][74] who demonstrated that, for a concentration of 8.00 µg∙mL−1, A. sativum L. essential oil presented > 99.9% of growth reduction on biofilm of C. albicans ATCC 14053. The ability of this essential oil to inhibit biofilm formation seems to be correlated with its phenolic profile, with allicin, alliin and ajoene being the major compounds found in it [60][75]. Essential oils from some plants have shown high antifungal and/or antibiofilm activity against Candida species. An example of this are the species of Cinnamomum cassia (L.) J. Presl, Cinnamomum zeylanicum Blume, Cymbopogon citratus (DC.) Stapf, Cymbopogon nardus L. Rendle, and Cymbopogon winterianus Jowitt. C. cassia (L.) J.Presl (Lauraceae), also known as “Chinese cinnamon,” is a well-known aromatic plant that has been widely cultivated and utilized to treat diabetes, ovarian cysts, stomach spasms, kidney disorders, high blood pressure, and menstrual disorders [61][76], and presents antimicrobial, antioxidant and antifungal properties [62][77]. C. zeylanicum Blume (Lauraceae) is an ever-green perennial plant that is used as a culinary herb [63][78]. This species presents several pharmacological properties such as antimicrobial, antioxidant, antifungal, and anticancer [64][79]. When it comes to oral health, a study performed by Almeida et al. [65][80] demonstrated that C. cassia essential oil, at a concentration of 1.00 mg∙mL−1, exerts more than 99.9% reduction in oral biofilm formation caused by C. albicans ATCC 90028, while C. zeylanicum, at a concentration of 1.6 µg∙mL−1, leads to more than 99.75% reduction in oral biofilm formation caused by C. albicans ATCC 10231. The high percentage of biofilm reduction shown by these two plants is attributed to the major phytocompound found in both species, the cinnamaldehyde. Cinnamaldehyde is a phenylpropanoid that may act on the cell membrane, likely binding to enzymes involved in the formation of the cytoplasmic membrane in fungal cells [66][81]. C. citratus (DC.) Stapf (Poaceae), commonly known as lemongrass, is an aromatic plant widely distributed around the world. It is used as a food flavouring, and is commonly consumed in teas and soups, but it may also be served with poultry, fish, beef, and seafood. Lemongrass essential oil exhibits a number of biological activities, including antioxidant [67][82], anti-inflammatory [68][83], antimicrobial [69][84], antifungal, and antibiofilm properties [70][85]. Almeida et al. [65][80] used the essential oil from C. citratus as an antifungal agent against C. albicans ATCC 10231 biofilms, and reported that, at the concentration of 6.4 µg∙mL−1, this essential oil was able to reduce the number of viable cells present in the biofilm by 99.79%. In this case, citral and neral were two of the main compounds found, which are known to hold antifungal properties [71][72][86,87]. C. nardus L. (Poaceae), popularly known as citronella, is a grass cultivated in subtropical and tropical regions of Asia, Africa, and America, including Brazil [73][88], The essential oil extracted from its leaves is commonly used in perfumes, the production of cosmetics, and as an insect repellent. Several studies have demonstrated the antiviral [74][89], antibacterial [75][90], and antifungal activities [76][91] of this oil. C. winterianus Jowitt (Poaceae) is an important aromatic plant cultivated in India and Brazil. In folk medicine, it is used for the treatment of anxiety, as a sedative, and for pain disorders [77][92]. Some studies demonstrated that the plant has anticonvulsant effects [78][93], anti-larvicidal effects against Aedes aegypti [79][94], and antibacterial and antifungal effects, including anti-Candida action [80][95]. The essential oils extracted from C. nardus L. and C. winterianus Jowitt species showed, in different studies, to be highly effective in combating C. albicans oral biofilms. C. nardus showed, at a concentration of 32.0 µg∙mL−1, an adherence inhibition of C. albicans ATCC 76645 higher than 99.0%, [81][68] and the application of C. winterianus essential oil, at a concentration of 1.00 mg∙mL−1, led to a reduction of C. albicans ATCC 90028 oral biofilm formation by more than 99.0%. In both species, the authors attributed the antibiofilm potential to the main compound identified in these species, namely citronellal. Citronellal is known to affect C. albicans cell growth by interfering with cell-cycle progression through the arrest of cells in S phase and affecting membrane integrity [82][96]. Solidago virgaurea L. (Asteraceae), commonly known as goldenrod, is a medicinal plant that is common throughout the world. In the literature, this plant is described as possessing a variety of medicinal properties such as antioxidant, anti-inflammatory, analgesic, spasmolytic, antihypertensive, antibacterial, antifungal and antitumor, among others [83][97]. Chevalier et al. [84][98] evaluated the effect of the extracts from two S. virgaurea subspecies, S. virgaurea subsp. alpestris and S. virgaurea subsp. virgaurea, on C. albicans oral biofilm growth. The results obtained showed that, at an extract concentration of 250 µg∙mL−1, S. virgaurea subsp. alpestris inhibition of oral biofilms from C. albicans IM003 was higher than 99.5%, and that S. virgaurea subsp. virgaurea inhibited the oral biofilm formation by C. albicans IM001 by more than 99.2%. Regarding the chemical composition of this plant, the compounds usually found in S. virgaurea are saponins, which have been attributed to the ability to inhibit the transition from yeast to hyphal growth [84][98]. This attribution seems reasonable considering the inherent surfactant properties of saponins, as well as their iron chelator qualities, iron being necessary for the growth and development of Candida spp. [85][99].| Plant Name | Plant Extract | Compound | Microorganism | Results | References | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial Activity | Antibiofilm Activity | ||||||||||||||||||||||
| Allium sativum | L. | Essential oil (bulbs) | Allicin, alliin, ajoene [60] | Allicin, alliin, ajoene [75] | C. albicans | ATCC 14053 | MIC | 8.0 μg∙mL | −1 | >99.9% reduction | 8.00 μg∙mL | −1 | [59] | [74] | |||||||||
| IZD | 19.0 mm (50.0 μg∙mL | −1 | ) | ||||||||||||||||||||
| Aloysia gratissima | (Aff & Hook) Tronc. | Essential oil (leaves) | (E) | -pinocamphone, β-pinene, guaiol |
C. albicans | CBS 562 | MIC | 0.015 mg∙mL | −1 | 12.3% inhibition | 1.00 mg∙mL | −1 | [86] | [64] | |||||||||
| MFC | 0.062 mg∙mL | −1 | |||||||||||||||||||||
| Artemisia judaica | L. | Essential oil (aerial plant parts) | Piperitone, camphor, ethyl cinnamate, chrysanthenone | C. albicans | ATCC 10231 | MIC | 1.25 μg∙mL | −1 | 50.0% reduction | 2.5 μg∙mL | −1 | [87] | [100] | ||||||||||
| Brucea javanica | (L.) Merr. | Aqeuous extract (seeds) | Quassinoids, alkaloids, |
C. albicans | ATCC 14053 | - | 94.5% CSH reduction 79.7% adherence reduction |
6.00 mg∙mL | −1 | [88] | [101] | ||||||||||||
| C. dubliniensis | ATCC MYA-2975 | 90.4% CSH reduction 27.9% adherence reduction |
|||||||||||||||||||||
| C. glabrata | ATCC 90030 | 84.8% CSH reduction 76.8% adherence reduction |
|||||||||||||||||||||
| C. krusei | ATCC 14243 | 97.0% CSH reduction 67.6% adherence reduction |
|||||||||||||||||||||
| C. lusitaniae | ATCC 64125 | 91.1% CSH reduction 89.0% adherence reduction |
|||||||||||||||||||||
| C. parapsilosis | ATCC 22019 | 98.8% CSH reduction 49.0% adherence reduction |
|||||||||||||||||||||
| C. tropicalis | ATCC 13803 | 88.4% CSH reduction 89.9% adherence reduction |
|||||||||||||||||||||
| Cassia spectabilis | DC. | Methanol extract (leaves) | (+)-spectaline; (−)-iso-6-cassine [89] | (+)-spectaline; (−)-iso-6-cassine [102] | C. albicans | 1 (CI) | MIC IZD |
6.25 mg∙mL | −1 | 20 mm (100 mg∙mL | −1 | ) | 97% inhibition | 6.25 mg∙mL | −1 | [90] | [103] | ||||||
| C. albicans | 2 (CI) | MIC IZD |
6.25 mg∙mL | −1 | 21 mm (100 mg∙mL | −1 | ) | ||||||||||||||||
| C. albicans | 3 (CI) | MIC IZD |
6.25 mg∙mL | −1 | 23 mm (100 mg∙mL | −1 | ) | ||||||||||||||||
| Chenopodium ambrosioides | L. | Aqueous extract (leaves) | Kaempferol, quercetin | C. albicans | ATCC 90028 | MIC | 0.250 mg∙mL | −1 | >99.0% reduction | 1.25 mg∙mL | −1 | [91] | [104] | ||||||||||
| MFC | 0.250 mg∙mL | −1 | |||||||||||||||||||||
| Cinnamomum cassia | L. J.Presl | Essential oil (leaves, bark, stalk) | Cinnamaldehyde, benzyl benzoate, α-pinene | C. albicans | ATCC 90028 | MIC | 65.5 µg∙mL | −1 | >99.9% reduction | 1.00 mg∙mL | −1 | [65] | [80] | ||||||||||
| MFC | |||||||||||||||||||||||
| Cinnamomum verum | J.Presl | Essential oil (leaves) | Eugenol, benzyl benzoate, | trans | -caryophyllene, acetyle eugenol, linalool | C. albicans | ATCC MYA-2876 | MIC | 1.0 mg∙mL | −1 | 50% reduction | 0.15 mg∙mL | −1 | [92] | [105] | ||||||||
| 50% inhibition | 1.0 mg∙mL | −1 | |||||||||||||||||||||
| C. tropicalis | ATCC 750 | 50% reduction | 0.35 mg∙mL | −1 | |||||||||||||||||||
| 50% inhibition | >2.0 mg∙mL | −1 | |||||||||||||||||||||
| C. dubliniensis | ATCC MYA-646 | 50% reduction | 0.2 mg∙mL | −1 | |||||||||||||||||||
| 50% inhibition | 0.2 mg∙mL | −1 | |||||||||||||||||||||
| Cinnamomum zeylanicum | Blume | Essential oil (leaves) | Cinnamaldehyde, cinnamyl acetate, cinnamyl benzoate [64] | Cinnamaldehyde, cinnamyl acetate, cinnamyl benzoate [79] | C. albicans | ATCC 10231 | MIC | 0.1 µg∙mL | −1 | 99.75% reduction | 1.6 µg∙mL | −1 | [93] | [106] | |||||||||
| MFC | 0.4 µg∙mL | −1 | |||||||||||||||||||||
| IZD | 42.5 mm (50 µg∙mL | −1 | ) | ||||||||||||||||||||
| Coriandrum sativum | L. | Essential oil (leaves) | Decanal, | trans | -2-decenal, 2-decen-1-ol, cyclodecane, cis-2-dodecenal | C. albicans | CBS 562 | MIC | 15.6 µg∙mL | −1 | 53.43% inhibition | 62.50 µg∙mL | −1 | [94] | [107] | ||||||||
| MFC | 31.2 µg∙mL | −1 | |||||||||||||||||||||
| C. tropicalis | CBS 94 | MIC | 31.2 µg∙mL | −1 | 89.76% inhibition | 125 µg∙mL | −1 | ||||||||||||||||
| MFC | 62.5 µg∙mL | −1 | |||||||||||||||||||||
| C. krusei | CBS 573 | MIC | 15.6 µg∙mL | −1 | 42.13% inhibition | 15.62 µg∙mL | −1 | ||||||||||||||||
| MFC | 31.2 µg∙mL | −1 | |||||||||||||||||||||
| C. dubliniensis | CBS 7987 | MIC | 31.2 µg∙mL | −1 | 61.51% inhibition | 62.50 µg∙mL | −1 | ||||||||||||||||
| MFC | 62.5 µg∙mL | −1 | |||||||||||||||||||||
| C. rugosa | CBS 12 | MIC | 15.6 µg∙mL | −1 | 68.03% inhibition | 62.50 µg∙mL | −1 | ||||||||||||||||
| MFC | 31.2 µg∙mL | −1 | |||||||||||||||||||||
| Croton urucurana | Baill. | Methanol extract (stems) | (epi)-catechin dimer I [95] | (epi)-catechin dimer I [108] | C. albicans | ATCC 10231 | - | 46.0% inhibition | 0.500 mg∙mL | −1 | [96] | [109] | |||||||||||
| Cymbopogon citratus | (DC.) Stapf |
Essential oil (leaves) | Citral, neral, β-myrcene, geraniol [97] | Citral, neral, β-myrcene, geraniol [110] | C. albicans | ATCC 10231 | MIC | 0.1 µL∙mL | −1 | 99.79% reduction | 6.4 µL∙mL | −1 | [93] | [106] | |||||||||
| MFC | 0.4 µL∙mL | −1 | |||||||||||||||||||||
| IZD | 18.2 mm (5% v.v | −1 | ) | ||||||||||||||||||||
| Ethanol extract (leaves) | Citral, geraniol, neral, camphene, limonene [98] | Citral, geraniol, neral, camphene, limonene [111] | C. albicans | ATCC 18804 | MIC | 0.625 mg∙mL | −1 | >99.9% inhibition | 3.13 mg∙mL | −1 | [99] | [112] | |||||||||||
| MFC | 2.50 mg∙mL | −1 | 94.0% reduction | 6.25 mg∙mL | −1 | ||||||||||||||||||
| Cymbopogon nardus | L. Rendle | Essential oil (leaves) | Citronellal, citronellol, geraniol | C. albicans | ATCC 76645 | MIC | 32.0 µg∙mL | −1 | >99.0% inhibition | 32.0 µg∙mL | −1 | [100] | [113] | ||||||||||
| MFC | |||||||||||||||||||||||
| Cymbopogon winterianus | Jowitt | Essential oil (leaves) | Citronellal, citronellol, geraniol | C. albicans | ATCC 90028 | MIC | 250 µg∙mL | −1 | >99.0% reduction | 1.00 mg∙mL | −1 | [65] | [80] | ||||||||||
| MFC | |||||||||||||||||||||||
| Cyperus articulatus | L. | Essential oil (bulbs) | α-pinene, mustakone, α-bulnesene | C. albicans | CBS 562 | MIC | 0.125 mg∙mL | −1 | 28.1% inhibition | 1.00 mg∙mL | −1 | [99] | [112] | ||||||||||
| MFC | 0.500 mg∙mL | −1 | |||||||||||||||||||||
| Eucalyptus globulus | Labill. | Essential oil (leaves) | Hyperoside, quercitrin, myricetin [101] | Hyperoside, quercitrin, myricetin [114] | C. albicans | ATCC 14053 | MFC | 0.219 mg∙mL | −1 | 86% reduction | 22.5 mg∙mL | −1 | [102] | [115] | |||||||||
| C. tropicalis | ATCC 66029 | 0.885 mg∙mL | −1 | 85% reduction | |||||||||||||||||||
| C. glabrata | ATCC 66032 | 0.219 mg∙mL | −1 | 85.2% reduction | |||||||||||||||||||
| Houttuynia cordata | Thunb | Ethanol extract (leaves) | Aldehydes | C. albicans | CAD1 | MFC | >2.17 mg∙mL | −1 | 70.0% reduction | 1.00% ( | v/v | ) | [103] | [116] | |||||||||
| Lippia sidoides | Cham. | Essential oil (leaves) | Thymol, | p | -cymene, α-caryophyllene | C. albicans | CBS 562 | MIC | 0.250 mg∙mL | −1 | 16.5% inhibition | 1.00 mg∙mL | −1 | [104] | [117] | ||||||||
| MFC | 0.500 mg∙mL | −1 | |||||||||||||||||||||
| Melaleuca alternifolia | (Maiden & Betche) Cheel | Essential oil (leaves) | Terpinen-4-ol, | γ | -terpinene, | p | -cymene, | α | -terpinene,1,8-cineole, | α | -terpineol, | α | -pinene | C. albicans | ATCC 18804 | MIC | 1.95 mg∙mL | −1 | MBEC | 125 mg∙mL | −1 | [105] | [118] |
| Essential oil (leaves) | Terpinen-4-ol, | γ | -terpinene, | α | -terpinene, terpinolene, 1,8-cineole | C. albicans | ATCC 10231 | MIC | 3.40 mg∙mL | −1 | 131% adherence reduction | 0.75% ( | v/v | ) | [106] | [119] | |||||||
| C. albicans | SC5314 | MIC | 0.84 mg∙mL | −1 | 76.0% adherence reduction | ||||||||||||||||||
| Mikania glomerata | Spreng | Essential oil (leaves) | Germacrene D, α-caryophyllene, bicyclogermacrene | C. albicans | CBS 562 | MIC | 0.250 mg∙mL | −1 | 22.7% inhibition | 1.00 mg∙mL | −1 | [104] | [117] | ||||||||||
| MFC | 0.250 mg∙mL | −1 | |||||||||||||||||||||
| Piper betle | L. | Aqueous extract (leaves) | Hydroxychavicol, cinnamoyl derivatives, luteolin, apigenin [107] | Hydroxychavicol, cinnamoyl derivatives, luteolin, apigenin [120] | C. albicans | ATCC 14053 | - | 38.6% CSH reduction 61.4% adherence reduction |
6.00 mg∙mL | −1 | [88] | [101] | |||||||||||
| C. dubliniensis | ATCC MYA-2975 | 78.3% CSH reduction 21.4% adherence reduction |
|||||||||||||||||||||
| C. glabrata | ATCC 90030 | 71.4% CSH reduction 12.4% adherence reduction |
|||||||||||||||||||||
| C. krusei | ATCC 14243 | 31.6% CSH reduction 56.4% adherence reduction |
|||||||||||||||||||||
| C. lusitaniae | ATCC 64125 | 67.5% CSH reduction 47.6% adherence reduction |
|||||||||||||||||||||
| C. parapsilosis | ATCC 22019 | 48.1% CSH reduction 46.5% adherence reduction |
|||||||||||||||||||||
| C. tropicalis | ATCC 13803 | 29.7% CSH reduction 86.9% adherence reduction |
|||||||||||||||||||||
| Rosmarinus officinalis | L. | Liposoluble extract (leaves) | Carnosic acid, carnosol [108] | Carnosic acid, carnosol [121] | C. albicans | ATCC 18804 | MIC | 0.78 mg∙mL | −1 | 99.9% reduction | 200 mg∙mL | −1 | [109] | [122] | |||||||||
| MMC | 3.13 mg∙mL | −1 | |||||||||||||||||||||
| Satureja hortensis | L. | Essential oil (leaves and flowers) | Thymol, λ-terpinene, carvacrol, | p | -cymene | C. albicans | F81 (CI) | MIC MFC |
300 µg∙mL | −1 | 400 µg∙mL | −1 | 91.0% inhibition 91.0% reduction |
4.80 mg∙mL | −1 | [110] | [123] | ||||||
| C. albicans | F94 (CI) | 200 µg∙mL | −1 | 300 µg∙mL | −1 | 90.0% inhibition 80.0% reduction |
|||||||||||||||||
| C. albicans | F87 (CI) | 300 µg∙mL | −1 | 400 µg∙mL | −1 | 86.0% inhibition 76.0% reduction |
|||||||||||||||||
| C. albicans | F49 (CI) | 400 µg∙mL | −1 | 600 µg∙mL | −1 | 92.0% inhibition 92.0% reduction |
|||||||||||||||||
| C. albicans | F82 (CI) | 400 µg∙mL | −1 | 600 µg∙mL | −1 | 89.0% inhibition 89.0% reduction |
|||||||||||||||||
| C. albicans | F95 (CI) | 400 µg∙mL | −1 | 81.0% inhibition 81.0% reduction |
|||||||||||||||||||
| C. albicans | F92 (CI) | 300 µg∙mL | −1 | 600 µg∙mL | −1 | 90.0% inhibition 90.0% reduction |
|||||||||||||||||
| C. albicans | F60 (CI) | 400 µg∙mL | −1 | 600 µg∙mL | −1 | 80.0% inhibition 80.0% reduction |
|||||||||||||||||
| C. albicans | F86 (CI) | 200 µg∙mL | −1 | 300 µg∙mL | −1 | 87.0% inhibition 87.0% reduction |
|||||||||||||||||
| C. albicans | F91 (CI) | 300 µg∙mL | −1 | 400 µg∙mL | −1 | 83.0% inhibition 83.0% reduction |
|||||||||||||||||
| C. albicans | F69 (CI) | 200 µg∙mL | −1 | 300 µg∙mL | −1 | 91.0% inhibition 80.0% reduction |
|||||||||||||||||
| C. albicans | F1 (CI) | 87.0% inhibition 79.0% reduction |
|||||||||||||||||||||
| C. albicans | F34 (CI) | 86.0% inhibition 91.0% reduction |
|||||||||||||||||||||
| C. albicans | F19 (CI) | 90.0% inhibition 85.0% reduction |
|||||||||||||||||||||
| C. albicans | F78 (CI) | 400 µg∙mL | −1 | 600 µg∙mL | −1 | 84.0% inhibition 84.0% reduction |
|||||||||||||||||
| Schinus terebinthifolia | Raddi. |
Methanol extract (leaves) | Phenolic compounds, anthraquinones, terpenoids, alkaloids | C. albicans | ATCC 10231 | - | 47.0% inhibition | 0.007 mg∙mL | −1 | [96] | [109] | ||||||||||||
| Solidago virgaurea | subsp. | alpestris | Waldst. & Kit. ex Willd. | Aqueous extract (aerial plant parts) | Saponins | C. albicans | ATCC 10231 | NA (IZD) | 95.9% inhibition 92.4% reduction |
0.250 mg∙mL | −1 | 0.750 mg∙mL | −1 | [84] | [98] | ||||||||
| C. albicans | IM001 (CI) | 96.0% inhibition 82.2% reduction |
0.250 mg∙mL | −1 | 0.750 mg∙mL | −1 | |||||||||||||||||
| C. albicans | IM003 (CI) | 99.5% inhibition 76.3% reduction |
0.250 mg∙mL | −1 | 0.750 mg∙mL | −1 | |||||||||||||||||
| C. albicans | IM007 (CI) | 95.1% inhibition 91.9% reduction |
0.250 mg∙mL | −1 | 0.750 mg∙mL | −1 | |||||||||||||||||
| Solidago virgaurea | L. subsp. | virgaurea | . | Aqueous extract (aerial plant parts) | Saponins | C. albicans | ATCC 10231 | NA (IZD) | 98.4% inhibition 77.9% reduction |
0.250 mg∙mL | −1 | 0.750 mg∙mL | −1 | ||||||||||
| C. albicans | IM001 (CI) | 99.2% inhibition 91.1% reduction |
0.250 mg∙mL | −1 | 0.750 mg∙mL | −1 | |||||||||||||||||
| C. albicans | IM003 (CI) | 97.3% inhibition 79.2% reduction |
0.250 mg∙mL | −1 | 0.750 mg∙mL | −1 | |||||||||||||||||
| C. albicans | IM007 (CI) | 96.5% inhibition 90.9% reduction |
0.250 mg∙mL | −1 | 0.750 mg∙mL | −1 | |||||||||||||||||
| Terminalia catappa | L. |
Ethanol extract (leaves) | Caffeic acid, quercitrin, kaempferol, gallic acid, chlorogenic acid, isoquercitrin [111] | Caffeic acid, quercitrin, kaempferol, gallic acid, chlorogenic acid, isoquercitrin [124] | C. albicans | ATCC 90028 | MIC MFC |
6.25 mg∙mL | −1 | 12.5 mg∙mL | −1 | >98.0% reduction | 62.5 mg∙mL | −1 | [112] | [125] | |||||||
| n | -butanol fraction from ethanol extract (leaves) | C. albicans | ATCC 90028 | MIC MFC |
250 μg∙mL | −1 | >99.5% reduction | 2.50 mg∙mL | −1 | [113] | [126] | ||||||||||||
| C. glabrata | ATCC 2001 | MIC MFC |
250 μg∙mL | −1 | >99.0% reduction | 2.50 mg∙mL | −1 | ||||||||||||||||
| Trachyspermum ammi | (L.) Sprague | Aromatic water (aerial plant parts) | Thymol, carvacrol, carvotanacetone | C. albicans | CBS1905 | - | - | 95.2% inhibition | 0.5% ( | v | / | v | ) | [114] | [127] | ||||||||
| Zataria multiflora | Boiss. | Aqueous extract (whole plant) | Thymol, hydroxyl benzoic acid, and cymene [115] | Thymol, hydroxyl benzoic acid, and cymene [128] | C. albicans | PTCC-5027 | MIC | 1.50 mg∙mL | −1 | 87% reduction | 25 mg∙mL | −1 | [116] | [129] | |||||||||
| Ethanolic extract (whole plant) | MIC | 0.84 mg∙mL | −1 | 97% reduction | |||||||||||||||||||
