Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Janusz Blasiak.
Migraine, the leading cause of disability in the population aged below 50, is frequently associated with functional gastrointestinal (GI) disorders (FGIDs) such as functional nausea, cyclic vomiting syndrome, and irritable bowel syndrome (IBS). Conversely, changes in intestinal GI transit may cause diarrhea or constipation and are a component of the autonomic symptoms associated with pre- and post-dorsal phases of migraine attack.
- tryptophan metabolism
- migraine
- kynurenines
- functional gastrointestinal diseases
- irritable bowel syndrome
1. Introduction
Functional disorders of the gastrointestinal (GI) tract are frequently associated with neurological diseases, including migraine, and are a serious diagnostic problem due to nonspecific syndromes (reviewed in [1,2][1][2]). Migraine is bidirectionally comorbid with several other disorders, including neurological, psychiatric, cardio- and cerebrovascular, GI, metaboloendocrine, and immunological diseases [3]. Each of these comorbid diseases shares some mechanism of pathogenesis with migraine and can contribute to the activation of the trigeminovascular system along with the neuroendocrine hypothalamic system, the causative mechanisms in migraine [4]. This reflects the gut–brain axis, a network of complex interactions between the nervous and GI systems with the involvement of the intestinal microbiota. Therefore, the treatment of migraine should be multipathway to identify and eliminate risk and comorbidity factors. Functional disorders of the GI tract may be especially important in this context as the enteric nervous system, cyclic vomiting syndrome, and abdominal migraine directly point to the association between functional GI disorders and migraine headaches. Changes in intestinal GI transit may cause diarrhea or constipation and are a component of the autonomic symptoms associated with pre- and post-dorsal phases of migraine attack [5].
Tryptophan (l-Trp) is an essential exogenous amino acid metabolized in the human body in three main pathways: serotonin (5-hydroxyptamine, 5-HT), kynurenine (l-kyn), and microbiota-related indole pathway. Although the role of 5-HT in the central nervous system (CNS) is well established, the kynurenine pathway of l-Trp metabolism is the main route of l-Trp transformation as it accounts for about 95% of l-Trp metabolism in normal conditions and is implicated in behavioral and cognitive symptoms of neurological diseases [6,7,8][6][7][8].
Recently, we showed that l-Trp metabolism might be implicated in the pathogenesis of lymphocytic colitis, a functional GI disorder [9]. We also reported that the serotonin pathway of l-Trp metabolism is impaired in patients with small bacterial overgrowth (SIBO) [10], a condition which may contribute to the pathogenesis of functional GI diseases [11]. We also showed that older adults with moderate depression had different l-Trp intake and metabolism as compared with controls [12]. Several other works confirm an important role of l-Trp metabolism in functional GI disorders associated with neurological syndromes (reviewed in [13]). These studies suggest that the intake of dietary and supplementary l-Trp may be beneficial in the prevention and therapy of GI disorders associated with neurological symptoms. The GI microbiota may be an important intermediate between l-Trp, GI disorders, and neurological symptoms (reviewed in [14,15][14][15]).
Some reports indicate that increased intake of dietary l-Trp may prevent migraine occurrence or attenuate its GI-related symptoms, such as nausea and vomiting, and photophobia ([14,16][14][16]). We observed a decrease in depressive mood disorders after an increase in dietary intake of l-Trp [12].
Migraine therapy is still challenging, but significant progress has occurred lately in this field. Triptans, serotonin 5-HT1B/1D and 5-HT1F receptor agonists, gepants, calcitonin gene-related peptide (CGRP) and its receptor antagonists; ditans, antagonists of metabotropic glutamate receptor 5, and others are used in migraine treatment (reviewed in [17]). The drugs targeting CGRP and its receptors and ligands are a hope for a breakthrough in migraine therapy (reviewed in [18]). Several other antimigraine drugs, apart from anti-CGRP monoclonal antibodies, are considered, including G-protein coupled receptors, glutamate, ion channels, and neuromodulatory devices (reviewed in [19]). A kynurenic acid analogue was reported to abolish nitroglycerin-induced hyperalgesia resulted in an increased CGRP expression in a rat migraine-like headaches model [20,21][20][21]. Therefore, expected breakthrough in migraine prevention and treatment may be related to the kynurenine pathway of l-Trp metabolism. Although new antimigraine drugs are still under research, 5-hydroxytryptamine 1 (5-HT1) receptor agonists remain a main group of drugs in migraine prevention and treatment [22]. However, the exact mechanism of the action of 5-HT1 receptor agonists in migraine is not known, and it has been suggested recently that the metabolites of the kynurenine pathway of l-Trp metabolism may be important for this mechanism [23].
The involvement of the gastrointestinal microbiota in the kynurenine metabolic pathway and the activation of this pathway in neurological and psychiatric diseases have recently been reviewed [24,25,26][24][25][26].
Many papers report an association between migraine and functional GI disorders, and most of them underline the need for further research to identify common areas of physiology, but few works show a specific direction of such studies (reviewed in, e.g., [1,2,5,27,28][1][2][5][27][28]).
2. The Gut–Brain Axis
The movement and functions of the GI tract are normally regulated by the brain, although it shows a substantial degree of autonomy due to intrinsic neural plexuses within the enteric nervous system (ENS) [30][29]. The ENS is a part of the peripheral nervous system controlling GI reaction independently of the CNS [31][30]. In particular, stomach and esophagus strongly depend on extrinsic neural inputs, particularly from the parasympathetic and sympathetic pathways, contrary to intestines, which can function without extrinsic inputs [32][31]. Removal of the innervation of the GI tract results in its disfunctions, manifested as nausea and vomiting, abdominal pain, and diarrhea [33][32]. The gut microbiota may influence the CNS functions and its impairment may cause neurological diseases, including Alzheimer’s disease, mood and anxiety disorders, multiple sclerosis, Parkinson’s disease, and migraine (reviewed in [34][33]). Therefore, the mutual interaction between the CNS, ENS, and the GI tract justifies the use of the term “gut–brain axis”, which is not limited to the brain, but is extended to the entire CNS.
The composition of the intestinal microbiota plays a major role in the gut–brain axis as the microbiota-derived neurotransmitters, hormones, and inflammatory mediators may influence CNS functions [35][34]. The intestinal microbiota may directly modulate the gut–brain axis through the stimulation of the end terminals of the vagus nerve [36][35]. CNS can modulate the gut microbiota through the sympathetic and parasympathetic systems and by the secretion of neuroendocrine peptides [37][36]. Physical and psychological stresses may induce alterations in the intestinal microbiota as they promote secretion of the corticotrophin-releasing hormone in hypothalamus, which induces cortisol release from the adrenal glands, resulting in changes in intestine permeability through alterations in the microbiota profile (dysbiosis) (Figure 1) [2]. Therefore, the microbiota can be involved not only in the control of the GI functions, but also in regulating behavior and brain functions (reviewed in [38][37]). Consequently, the gut–brain axis may be extended to the microbiota–gut–brain axis, but the gut microbiota can be considered as a component of the gut, so the term the gut–brain axis is still justified [39][38].
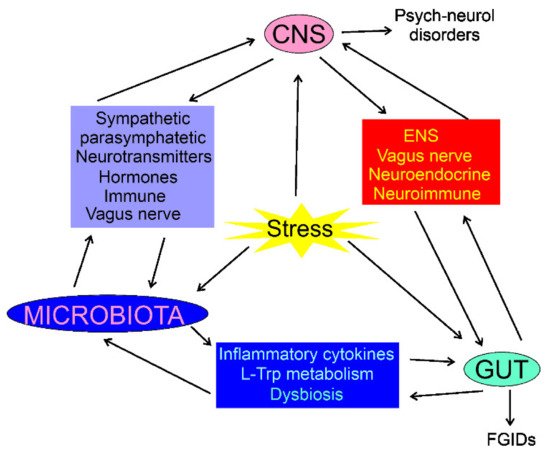

Figure 1. Interaction of the central nervous system (CNS), enteric nervous system (ENS), the gastrointestinal (GI) tract, and the gut microbiota in normal and stress conditions. In each case, the interaction is bidirectional and can be mediated by many factors; some of them are shown. Stress conditions may influence, directly or indirectly, CNS, gut, and microbiota, resulting in psychoneurological disorders and functional GI diseases (FGIDs). The stress may also result in organic GI or CNS diseases, which are not subjected to this review.
The blood–brain barrier (BBB) is a physical and functional separator between the brain and gut/microbiota. Therefore, it is rational to ask how the BBB influences the communication within the gut–brain–microbiota axis. The concept of the BBB has evolved from a static physical barrier between the brain and blood to a part of neovascular unit containing brain microvascular endothelial cells, neurons, microglia, astrocytes, pericytes, and extracellular matrix (reviewed in [40][39]). Therefore, the BBB can act as an important modulator of the communication within the gut–brain–microbiota axis (reviewed in [41][40]). Intestinal neuroendocrine cells detect luminal metabolites and send corresponding signals to the brain through the activation of the vagus nerve, release of hormones and neurotransmitters, and through neural routes, including neuroepithelial connections (reviewed in [41][40]). The mechanisms of the modulation of the gut–brain–microbiota are complex and cannot be thoroughly discussed here due to space limitation. The role of the BBB in the transport of l-kyn and its metabolites is presented further in this review.
As both migraine and functional GI disorders are characterized by pain symptoms, some general information about pain pathogenesis may clarify the interconnection between these two classes of diseases. According to the International Association for the Study of Pain, pain is “an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage” [42][41]. The biogenesis of pain can be underlined by nociceptic, neuropathic, and neuroplastic mechanisms. The kynurenine pathway of l-Trp metabolism may be involved in pain pathogenesis, and the most likely effect associated with this involvement is neuroinflammation (reviewed in [43][42]). The most common form of pain is nociceptive pain, which is caused by a structural dysfunction, including tissue damage occurring, e.g., in bone fractures (reviewed in [44][43]). The activation of nociceptors might take place in migraines, and trigeminal perivascular sensory nerve terminals are a prime candidate responsible for this effect (reviewed in [45,46][44][45]). Gastrointestinal pain is a form of visceral pain, common in some disorders, including functional GI diseases (reviewed in [47][46]). In general, GI pain can be nociceptive, neuropathic, and associated with cancer. Mechanisms of GI pain are complex and may include peripheral and central sensitization. Moreover, GI pain may occur with the involvement of the autonomic nervous system, which may be responsible for symptoms associated with GI pain. The exact origin of pain in migraine and functional GI disorders is not completely clear, and is discussed in other recent reviews [48,49][47][48].
Nutritional status and diet are reported as critical modifiable factors regulating microbiota both in physiological and pathological conditions (reviewed in [50][49]). The microbiota is a modifiable target of pharmaceutical intervention, and the gut microbiome, directly and indirectly, may affect drug metabolism [51][50]. Therefore, the microbiota may play an important intermediatory role between nutritional or pharmaceutical intervention and brain health [39][38]. In particular, migraine can be considered in therapeutic interventions through targeting the microbiota.
References
- Altamura, C.; Corbelli, I.; de Tommaso, M.; Di Lorenzo, C.; Di Lorenzo, G.; Di Renzo, A.; Filippi, M.; Jannini, T.B.; Messina, R.; Parisi, P.; et al. Pathophysiological Bases of Comorbidity in Migraine. Front. Hum. Neurosci. 2021, 15, 640574.
- Arzani, M.; Jahromi, S.R.; Ghorbani, Z.; Vahabizad, F.; Martelletti, P.; Ghaemi, A.; Sacco, S.; Togha, M. Gut-brain Axis and migraine headache: A comprehensive review. J. Headache Pain 2020, 21, 15.
- Aurora, S.K.; Papapetropoulos, S.; Kori, S.H.; Kedar, A.; Abell, T.L. Gastric stasis in migraineurs: Etiology, characteristics, and clinical and therapeutic implications. Cephalalgia 2013, 33, 408–415.
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. 2017, 97, 553–622.
- Aurora, S.K.; Shrewsbury, S.B.; Ray, S.; Hindiyeh, N.; Nguyen, L. A link between gastrointestinal disorders and migraine: Insights into the gut-brain connection. Headache 2021, 61, 576–589.
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794.
- Israelyan, N.; Del Colle, A.; Li, Z.; Park, Y.; Xing, A.; Jacobsen, J.P.R.; Luna, R.A.; Jensen, D.D.; Madra, M.; Saurman, V.; et al. Effects of Serotonin and Slow-Release 5-Hydroxytryptophan on Gastrointestinal Motility in a Mouse Model of Depression. Gastroenterology 2019, 157, 507–521.e504.
- Israelyan, N.; Margolis, K.G. Serotonin as a link between the gut-brain-microbiome axis in autism spectrum disorders. Pharm. Res. 2018, 132, 1–6.
- Chojnacki, C.; Popławski, T.; Gasiorowska, A.; Chojnacki, J.; Blasiak, J. Serotonin in the Pathogenesis of Lymphocytic Colitis. J. Clin. Med. 2021, 10, 285.
- Chojnacki, C.; Popławski, T.; Konrad, P.; Fila, M.; Chojnacki, J.; Błasiak, J. Serotonin Pathway of Tryptophan Metabolism in Small Intestinal Bacterial Overgrowth—A Pilot Study with Patients Diagnosed with Lactulose Hydrogen Breath Test and Treated with Rifaximin. J. Clin. Med. 2021, 10, 2065.
- Shimura, S.; Ishimura, N.; Mikami, H.; Okimoto, E.; Uno, G.; Tamagawa, Y.; Aimi, M.; Oshima, N.; Sato, S.; Ishihara, S.; et al. Small Intestinal Bacterial Overgrowth in Patients with Refractory Functional Gastrointestinal Disorders. J. Neurogastroenterol. Motil. 2016, 22, 60–68.
- Chojnacki, C.; Popławski, T.; Chojnacki, J.; Fila, M.; Konrad, P.; Blasiak, J. Tryptophan Intake and Metabolism in Older Adults with Mood Disorders. Nutrients 2020, 12, 3183.
- Gao, K.; Mu, C.L.; Farzi, A.; Zhu, W.Y. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723.
- Drummond, P.D. Tryptophan depletion increases nausea, headache and photophobia in migraine sufferers. Cephalalgia 2006, 26, 1225–1233.
- Bosi, A.; Banfi, D.; Bistoletti, M.; Giaroni, C.; Baj, A. Tryptophan Metabolites Along the Microbiota-Gut-Brain Axis: An Interkingdom Communication System Influencing the Gut in Health and Disease. Int. J. Tryptophan Res. 2020, 13, 1178646920928984.
- Razeghi Jahromi, S.; Togha, M.; Ghorbani, Z.; Hekmatdoost, A.; Khorsha, F.; Rafiee, P.; Shirani, P.; Nourmohammadi, M.; Ansari, H. The association between dietary tryptophan intake and migraine. Neurol. Sci. 2019, 40, 2349–2355.
- Ducros, A.; de Gaalon, S.; Roos, C.; Donnet, A.; Giraud, P.; Guégan-Massardier, E.; Lantéri-Minet, M.; Lucas, C.; Mawet, J.; Moisset, X.; et al. Revised guidelines of the French headache society for the diagnosis and management of migraine in adults. Part 2: Pharmacological treatment. Rev. Neurol. 2021, 177, 734–752.
- Assas, B.M. Anti-migraine agents from an immunological point of view. J. Transl. Med. 2021, 19, 23.
- Do, T.P.; Al-Saoudi, A.; Ashina, M. Future prophylactic treatments in migraine: Beyond anti-CGRP monoclonal antibodies and gepants. Rev. Neurol. 2021, 177, 827–833.
- Greco, R.; Demartini, C.; Zanaboni, A.M.; Redavide, E.; Pampalone, S.; Toldi, J.; Fülöp, F.; Blandini, F.; Nappi, G.; Sandrini, G.; et al. Effects of kynurenic acid analogue 1 (KYNA-A1) in nitroglycerin-induced hyperalgesia: Targets and anti-migraine mechanisms. Cephalalgia 2017, 37, 1272–1284.
- Vamos, E.; Pardutz, A.; Klivenyi, P.; Toldi, J.; Vecsei, L. The role of kynurenines in disorders of the central nervous system: Possibilities for neuroprotection. J. Neurol. Sci. 2009, 283, 21–27.
- Monteith, T.S.; Goadsby, P.J. Acute migraine therapy: New drugs and new approaches. Curr. Treat. Options Neurol. 2011, 13, 1–14.
- Tanaka, M.; Török, N.; Vécsei, L. Are 5-HT(1) receptor agonists effective anti-migraine drugs? Expert Opin. Pharm. 2021, 22, 1221–1225.
- Tanaka, M.; Toldi, J.; Vécsei, L. Exploring the Etiological Links behind Neurodegenerative Diseases: Inflammatory Cytokines and Bioactive Kynurenines. Int. J. Mol. Sci. 2020, 21, 2431.
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734.
- Török, N.; Tanaka, M.; Vécsei, L. Searching for Peripheral Biomarkers in Neurodegenerative Diseases: The Tryptophan-Kynurenine Metabolic Pathway. Int. J. Mol. Sci. 2020, 21, 9338.
- Anderson, G. Integrating Pathophysiology in Migraine: Role of the Gut Microbiome and Melatonin. Curr. Pharm. Des. 2019, 25, 3550–3562.
- Cámara-Lemarroy, C.R.; Rodriguez-Gutierrez, R.; Monreal-Robles, R.; Marfil-Rivera, A. Gastrointestinal disorders associated with migraine: A comprehensive review. World J. Gastroenterol. 2016, 22, 8149–8160.
- Kulkarni, S.; Ganz, J.; Bayrer, J.; Becker, L.; Bogunovic, M.; Rao, M. Advances in Enteric Neurobiology: The “Brain” in the Gut in Health and Disease. J. Neurosci. 2018, 38, 9346–9354.
- Rao, M.; Gershon, M.D. The bowel and beyond: The enteric nervous system in neurological disorders. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 517–528.
- Browning, K.N.; Travagli, R.A. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr. Physiol. 2014, 4, 1339–1368.
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.J. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Adv. Exp. Med. Biol. 2014, 817, 39–71.
- Gwak, M.G.; Chang, S.Y. Gut-Brain Connection: Microbiome, Gut Barrier, and Environmental Sensors. Immune Netw. 2021, 21, e20.
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209.
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49.
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The Brain-Gut-Microbiome Axis. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 133–148.
- Suganya, K.; Koo, B.S. Gut-Brain Axis: Role of Gut Microbiota on Neurological Disorders and How Probiotics/Prebiotics Beneficially Modulate Microbial and Immune Pathways to Improve Brain Functions. Int. J. Mol. Sci. 2020, 21, 7551.
- Sandhu, K.V.; Sherwin, E.; Schellekens, H.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Feeding the microbiota-gut-brain axis: Diet, microbiome, and neuropsychiatry. Transl. Res. J. Lab. Clin. Med. 2017, 179, 223–244.
- Hawkins, B.T.; Davis, T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharm. Rev. 2005, 57, 173–185.
- Parker, A.; Fonseca, S.; Carding, S.R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 2020, 11, 135–157.
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982.
- Tanaka, M.; Török, N.; Tóth, F.; Szabó, Á.; Vécsei, L. Co-Players in Chronic Pain: Neuroinflammation and the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 897.
- Warwick, C.A.; Keyes, A.L.; Woodruff, T.M.; Usachev, Y.M. The complement cascade in the regulation of neuroinflammation, nociceptive sensitization, and pain. J. Biol. Chem. 2021, 297, 101085.
- Dux, M.; Rosta, J.; Messlinger, K. TRP Channels in the Focus of Trigeminal Nociceptor Sensitization Contributing to Primary Headaches. Int. J. Mol. Sci. 2020, 21, 342.
- Olesen, J.; Burstein, R.; Ashina, M.; Tfelt-Hansen, P. Origin of pain in migraine: Evidence for peripheral sensitisation. Lancet Neurol. 2009, 8, 679–690.
- Drewes, A.M.; Olesen, A.E.; Farmer, A.D.; Szigethy, E.; Rebours, V.; Olesen, S.S. Gastrointestinal pain. Nat. Rev. Dis. Primers 2020, 6, 1.
- Grinberg, K.; Sela, Y.; Nissanholtz-Gannot, R. New Insights about Chronic Pelvic Pain Syndrome (CPPS). Int. J. Environ. Res. Public Health 2020, 17, 3005.
- Robblee, J.; Secora, K.A. Debunking Myths: Sinus Headache. Curr. Neurol. Neurosci. Rep. 2021, 21, 42.
- Mentella, M.C.; Scaldaferri, F.; Pizzoferrato, M.; Gasbarrini, A.; Miggiano, G.A.D. Nutrition, IBD and Gut Microbiota: A Review. Nutrients 2020, 12, 944.
- Walsh, J.; Griffin, B.T.; Clarke, G.; Hyland, N.P. Drug-gut microbiota interactions: Implications for neuropharmacology. Br. J. Pharmacol. 2018, 175, 4415–4429.
More
