The R2R3-MYB transcription factors (TFs) play several key roles in numerous plant biological processes. Hedychium coronarium is an important ornamental plant well-known for its elegant flower shape and abundant aroma type. The floral aroma of H. coronarium is due to the presence of a large amount of terpenes and benzenoids. However, less is known about the role of R2R3-MYB TFs in the regulatory mechanism of floral aroma production in this breed. Herein, we isolate and functionally characterize the R2R3-MYB TF HcMYB132, which is potentially involved in regulating floral aroma synthesis. Sequence alignment analysis revealed that it includes a nuclear localization signal NLS(s) and a 2R, 3R motif signature in the sequences. A subcellular localization assay revealed that HcMYB132 protein localizes to the nucleus. Real-time qPCR assays showed that HcMYB132 is specifically expressed in flowers and its expression pattern correlates with the emission of floral volatile compounds. In HcMYB132-silenced flowers, the levels of floral volatile compounds were significantly reduced, and the expression of key structural volatile synthesis genes was downregulated compared to control. Collectively, these results suggest that HcMYB132 might play a significant role in the regulation of terpenoid biosynthesis in H. coronarium.
- floral scent
- Hedychium coronarium
- R2R3-MYB
- structural genes
- terpenes
1. Introduction
2. Characterization of HcMYB132
3. Subcellular Localization of HcMYB132
2.3. Expression Pattern of HcMYB132


4. Discussion
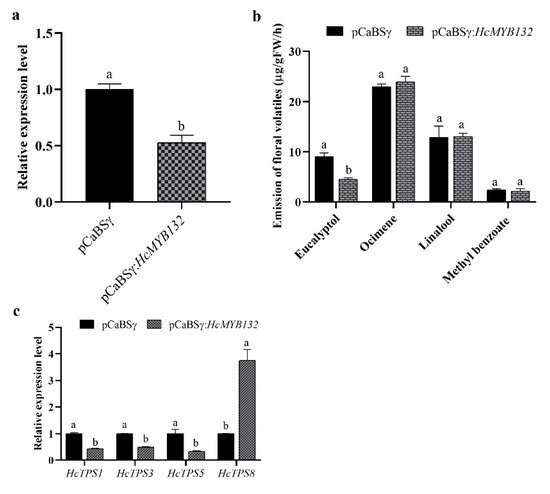
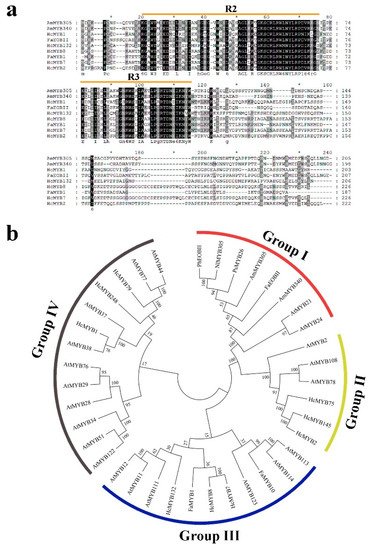
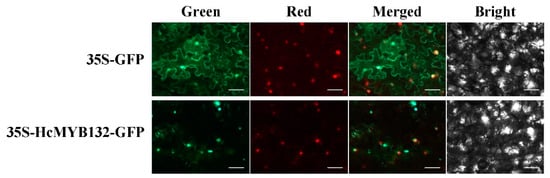
H. coronarium is popular in tropical and subtropical parts of the world due to its appealing strong aroma type and medicinal properties [3][33][3,33]. R2R3-MYB TFs are the main regulators of terpenes and phenylpropanoids [34][35][34,35]. However, less is known about the transcriptional regulatory mechanism of floral aroma production. Until now, a few MYB TFs have been reported that control the regulatory network of floral scent production [29][30][36][37][29,30,36,37]. Herein, we identified and functionally characterized a R2R3-MYB TF (HcMYB132) that is potentially involved in floral aroma synthesis in H. coronarium. Multiple sequence analyses of HcMYB132 revealed the existence of 2R and 3R repeats in the sequences (Figure 1a). Several previous findings suggest that the R2 and R3 signature motifs are highly conserved and regulate various aspects of plant secondary metabolites [13][38][39][40][13,38,39,40]. We generated a phylogenic tree using the previously characterized R2R3-MYB TFs involved in the regulatory network of secondary metabolism, together with HcMYB132 (Figure 1b). HcMYB132 was classified into Group III with FaMYB1, FaMYB10, and AtMYB11/12/111/113/114/123. The functional characterization of aforementioned genes revealed their role in the regulation of the flavonoid/phenylpropanoid metabolism [14][41][42][43][14,41,42,43], indicating that HcMYB132 might play a significant role in secondary metabolism. It has been reported that MYB TFs in same subclade have identical functions [13][35][13,35]. The structure analysis revealed that the HcMYB132 contains two exons, which are in line with the previous reports [44]. A subcellular localization assay revealed that HcMYB132 protein is localized to the nucleus, which is consistent with the previous findings [1][7][13][45][1,7,13,45]. The process of floral scent production is interrelated with flower development [46][47][48][46,47,48]. Our previous studies revealed that production and emission of floral volatile compounds and the expression of key structural volatile biosynthesis genes were low during the bud stage and peaked during the full bloom stage [7][8][9][10][7,8,9,10]. Previous studies also showed that volatile emission content was significantly larger from the flower than from the rhizome and leaf, which is consistent with the expression pattern of HcMYB132 [7]. In the current findings, it was revealed that HcMYB132 was mainly expressed in the flowers and its expression pattern increased with flower development, peaked during the fully bloomed stage, and dropped down thereafter (Figure 4a,b), implying that it might potentially be involved in the floral aroma production and emission mechanism. A similar expression pattern was observed in Fragaria ananassa EOBII, EOBI, and ODO1, and was involved in the regulatory network of eugenol [15][29][15,29]. Likewise, Prunus persica MYBF1 and MYB15 showed the highest expression in the flower and were involved in flavanol biosynthesis regulation [31]. In Lilium hybrid, ODO1 TF had highest expression in the flower and plaedy a crucial role in the regulation of phenylpropanoid/ benzenoid volatile production [49]. These results suggest that HcMYB132 potentially regulates the process of floral scent production. To reveal the role of HcMYB132 in floral aroma production in H. coronarium, the activity of HcMYB132 was repressed via gene silencing. The data showed that the volatile contents of eucalyptol were substantially decreased in HcMYB132-silenced flowers compared to control flowers. Furthermore, in HcMYB132-silenced flowers, the transcript levels of key eucalyptol volatile biosynthesis genes (HcTPS1 and HcTPS3) were significantly decreased (Figure 5). Likewise, strawberry MYB10 regulates the expression of numerous key genes involved in the flavonoid and phenylpropanoid biosynthesis process [14]. In petunia ODO1-suppressed plants, the mRNA levels of several scent-related genes were downregulated [29]. Similarly, litchi MYB5 activates the transcript levels of key genes involved in the synthesis of anthocyanin [23]. In HcMYB1/2/7/8/75/79/145/238/248-silenced flowers, the emission of floral volatiles and the expression of structural genes were significantly decreased [1][7][1,7]. Moreover, the emission of eucalyptol and the expression of HcMYB132 were influenced by auxin treatments, which are consistent with previous findings [7][50][7,50]. These data endorse the previous findings that R2R3-MYB TFs are involved in the regulation of volatile formation in H. coronarium.