Lactococcosis, particularly that caused by Lactococcus garvieae, is a major re-emerging bacterial disease seriously affecting the sustainability of aquaculture industry. Medicinal herbs and plants do not have very much in vitro antagonism and in vivo disease resistance towards lactococcosis agents in aquaculture. Most in vitro studies with herbal extractives were performed against L. garvieae with no strong antibacterial activity, but essential oils, especially those that contain thymol or carvacrol, are more effective.
- lactococcosis
- Lactococcus garvieae
- phytotherapy
- aquaculture
1. Introduction
Among the species of Lactococcus genus, L. L. garvieaegarvieae has been highlighted as one of the most serious global bacterial pathogens in the aquaculture sector, both in freshwater and marine fish, especially at water temperatures of >15 °C, but L. lactis and L. L. pisciumpiscium seem to be limited to some highly valuable aquaculture species, such as salmonids and sturgeons, at various water temperatures [11,22,23][1][2][3]. Due to the widespread sources of the bacterial agents and disease spreading, as well as the heterogenicity of the bacterial stains implicated in the disease outbreaks, both vaccination and chemotherapy require more attention in future. The application of co-friendly environmental substances, such as medicinal herbs and probiotics, are nowadays a potential best alternative to antibiotic therapy and an immune enhancer against such bacterial diseases.
2. Diseases Caused by Lactococcus Members in Aquaculture
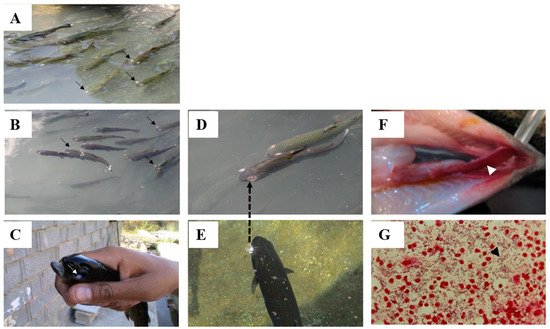
2.1. Disease Caused by L. garvieae
2.1. Disease Caused by L. garvieae
2.2. Diseases Caused by Other Species of Lactococcus Genus
L. lactis
2.2. Diseases Caused by Other Species of Lactococcus Genus
L. lactis strains are genetically classified into four subspecies of lactis, cremoris, tractae, and hordniae [13][20]. It is not a common veterinary pathogen, although it can cause cattle mastitis and be involved in septic arthritis of the neonatal calf. For example, several variants of L. L. lactislactis have been associated with bovine mastitis [79][21]. In humans, it has been reported as a cause of endocarditis, arthritis, and septicemia in patients, although this requires more clarification [80,81,82,83,84][22][23][24][25][26].
Up to date, there are only four reports of lactococcosis by L. L. lactislactis in an aquatic organisms. The first report was an outbreak of white tail disease in cultured giant freshwater prawn (Macrobrachium rosenbergii) in Taiwan [85][27]. The affected prawns were cloudy and whitish in the muscles, showing remarkable edema and necrosis and inflammation in the muscles and hepatopancreas. In subsequent report by Chen et al. [3] [23]L. L. lactislactis subsp. Lactis was isolated from affected hybrid sturgeon, Bester (Huso huso x Acipenser ruthenus) with signs of anorexia, pale body color, reddish spots on the abdomen, enteritis, enlarged abdomen, rapid respiration rate ascites, and 70%–100% mortality. Microscopically, the affected sturgeons demonstrated extensive haemorrhagic multifocal necrotic foci of spleen and liver with degeneration of hepatic cells, lipid droplets and glycogen granules, necrosis and renal tubule epithelial swelling and hydropic degeneration in kidney, skin ulcers deep in underling muscles, appearance of present of immunocompetent cells in the stomach, and small focus on tips of gills and on the myocardium [23][3]. No histopathological changes were, however seen in the eyeball, cerebrum and meninges of affected fish. The third report was from silver carp (Hypophthalmichthys molitrix) with extensive skin lesions near the caudal peduncle and musculoskeletal lesion in the USA [86][28]. The fourth outbreak of infection by L. L. lactislactis has been reported as the cause of endocarditis valvularis, parientalis thromboticans in mature allis shad (Alosa alosa) in Europe in 2018 that could be associated with the stressors, such as capturing, transport, breeding, and low oxygen level [87][29]. Although, in some cases the disease was reproduced experimentally, the mechanisms of pathogenesis by L. lactis in aquatic animals warranted future research works.
3. Phytotherapy of Lactococcosis in Aquaculture
3.1. In Vitro Studies
Almost all in vitro studies with vegetable and lichens extractives were performed against L. garvieae. For convenience, details of in vitro and in vivo studies have been included in3.2. In Vivo Studies
| Origin/ Source of L. garvieae |
Plant | Extractive | Major Compounds | MIC |
|---|
| Host | Plant | Extractive | Dosage/DurationMBC | Survival Increase Compared to Control (%) Reference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | Reference | ||||||||||||
| Rainbow trout | Camellia sinensis (green tea-leaves) | Methanolic extract | Unknown | 800 | 1100 | Akbary, 2014 | |||||||
| Giant freshwater prawn (Macrobrachium rosenbergii) |
Eichhornia crassipes (water hyacinth-leaves) | Hot-water extract | 1 g kg diet−1, 12 days 2 g kg diet−1, 12 days 3 g kg diet−1, 12 days |
↑57.3 ↑128.6 ↑171.4 |
Chang et al., 2013 | Rainbow trout | Thymus vulgaris (thyme), Origanum vulgare (oregano) and Eucalyptus sp. | Mix-oil® | |||||
| Giant freshwater prawn (M. rosenbergii) | (essential oils from leaves) | Citraconic anhydride, 1, 8-cineole, thymol | 6.25 | 12.5 | Eichhornia crassipes (leaves) | Powder | 20 g kg diet−1, 120 days | ↑44.3Amiri et al., 2020 | |||||
| Chang et al., 2016 | Unknown | Glycyrrhiza glabra L. (black sugar-root) | n-hexane extract | Unknown | Unknown | 5630 | Asan-Ozusaglam et al., 2014 | ||||||
| Hot-water extract | 20 g kg diet−1, 120 days | ↑89.0 | Unknown | Glycyrrhiza glabra L. (root) | Dichloromethane extract | Unknown | Unknown | 1410 | Asan-Ozusaglam et al., 2014 | ||||
| Aqueous extract 1 | 2 g kg diet−1, 120 days | ↑89.0 | Type culture collection | Lavandula angustifolia (lavender) | Essential oil | Unknown | 500 | Unknown | Baba, 2020 | ||||
| Type culture collection | Eugenia caryophyllus | Essential oil | |||||||||||
| Dreg of aqueous extract 1 | 18 g kg diet−1, 120 days | ↑77.7 | Unknown | 250 | |||||||||
| Giant freshwater prawn (M. rosenbergii) |
Musa acuminate (banana-peel) | Unknown | Aqueous extract | 1 g kg diet−1, 120 days 3 g kg dietBaba, 2020 |
|||||||||
| −1 | , 120 days | 6 g kg diet−1, 120 days | ↑200 ↑300 ↑467 |
Rattanavichai et al., 2015 | Rainbow trout | Mentha piperitae (pepper mint) | Essential oil | Unknown | 500 | Unknown | Baba, 2020 | ||
| Giant freshwater prawn (M. rosenbergii) |
Morinda cutrifolia (noni) | Aqueous extract | 0.6 g kg diet−1, 21 days 3 g kg diet−1, 21 days 6 g kg diet−1, 21 days |
↑250 ↑50.4 NS |
Halim et al., 2017 | Rainbow trout | Rosmarinus officinalis (rosemary) | Essential oil | Unknown | 500 | Unknown | ||
| Nile tilapia (Oreochromis niloticus) |
Argania spinosa (argan-seeds) | Baba, 2020 | |||||||||||
| Oil | 5 mL kg diet−1, 45 days 10 mL kg diet−1, 45 days 20 mL kg diet−1, 45 days |
↑66.7 ↑91.7 ↑86.1 |
Baba et al., 2017 | Rainbow trout | Cinnamomum zeylanicum (cinnamon) | Essential oil | |||||||
| Rainbow trout | Unknown | (Oncorhynchus mykiss) | Lentinula edodes (Shiitake mushroom) | Aqueous extract | 10 g kg diet−1250 | Unknown | Baba, 2020 | ||||||
| , 45 days | 20 g kg diet | −1, 45 days | ↑79.0 ↑109.7 |
Baba et al., 2015 | Rainbow trout | Nigella sativa (black cumin) | Essential oil | Unknown | 250 | Unknown | Baba, 2020 | ||
| Rainbow trout (O. mykiss) |
Pleurotus ostreatus (oyster mushroom) | Aqueous extract | 10 g kg diet−1, 42 days 20 g kg diet−1, 42 days |
↑40.0 ↑60.1 |
Uluköy et al., 2016 | Strain O41 | |||||||
| Rainbow troutLavandula officinalis (true lavender-flowers) | Ethanolic extract |
(O. mykiss) | Usnea barbata (beard lichen) | Methanolic extractEssential oil (linalyl acetate), tannins, coumarins, flavonoids, and phytosterols |
4200 | 8400 | Bulfon et al., 2014 | ||||||
| 230 mg kg fish | −1 (a) | 460 mg kg fish−1 (a) 690 mg kg fish−1 (a) |
↑62.4 ↑45.3 NS |
Bilen et al., 2019 | Strain O41 | Melissa officinalis (lemon balm-leaves) | Ethanolic extract | Rosmarinic acid, essential oil (citral, citronellal, β-caryophyllen), caffeic acid, and chlorogenic acidderivatives |
8400 | 33,600 | Bulfon et al., 2014 | ||
| Strain O41 | Ocimum basilicum (sweet basil-flowering plant) | Ethanolic extract | Essential oil (linalool, estragol, camphor, eugenol, ocimene, cineol, sesquiterpenes), tannins, favonoids, caffeic acid, and esculoside | ||||||||||
| Three spotted tilapia (Oreochromis andersonii) | Capsaicin | Isolated compound | 1.97 mg kg fish−1 (b) | 80% survival vs. 0% survival in control | Ndashe et al., 2020 | 16,800 | Unknown | Bulfon et al., 2014 | |||||
| Rainbow trout (O. mykiss) |
Origanum onites (oregano) | Essential oil | 0.125 mL kg diet−1, 56 days 1.5 mL kg diet−1, 56 days 2.5 mL kg diet−1, 56 days 3.0 mL kg diet−1, 56 days |
↑54 ↑92 ↑84 No mortality |
Diler et al. 2017 | Strain O41 | Origanum vulgare (oregano-inflorescence) | Ethanolic extract | Carvacrol, thymol, γ-terpinene, p-cymene, limonene, linolool, and borneol | 4200 | 33,600 | Bulfon et al., 2014 | |
| Nile tilapia (Oreochromis niloticus) |
Syzygium aromaticum (clove-buds) | Essential oil | 5 mL kg diet−1, 5 days 10 mL kg diet−1, 5 days 20 mL kg diet−1, 5 days 30 mL kg diet−1, 5 days |
↑40 ↑70 ↑80 No mortality |
Rattanachaikunsopon et al., 2009 | Strain O41 | Orthosiphon stamineus (Java tea-leaves) | Ethanolic extract | Essential oil (sesquiterpenes), flavones, triterpenoid, saponins, vitamins, and organic salts | ||||
| Mullet (Mugil cephalus) | * TCM | Aqueous extract of the powder | 5 g kg fish−1, 28 days 33,600 |
Unknown | Bulfon et al., 2014 | ||||||||
| 10 g kg fish | −1 | , 28 days | 20 g kg fish−1, 28 days |
NS ↑230.8 ↑184.6 |
Choi et al., 2014 | Strain O41 | Rosmarinus officinalis (leaves) | Ethanolic extract | Essential oil (eucalyptol, α-pinene, camphor, borneol), flavonoids, rosmarinic acid, and terpenes | 8400 | Unknown | Bulfon et al., 2014 | |
| Rainbow trout (O. mykiss) |
Citrus paradisi (grapefruit), Citrus reticulata (tangerine), Citrus aurantium ssp. bergamia (bergamot), Citrus sinensis (sweet orange) | Biocitro ® (blend of these extracts) | 0.75 g kg diet−1 | Strain O41 | Salvia officinalis (sage-leaves) | Ethanolic extract | Essential oil (thujone, monoterpenes, and sesquiterpenes), tannins, bitter substances, and flavonoids | 4200 | 33,600 | Bulfon et al., 2014 | |||
| Strain O41 | Thymus vulgaris (leaves) | Ethanolic extract | Essential oil (thymol, carvacrol, p-cimol, and terpinene), tannins, flavonoids and triterpenes | Unknown | Unknown | Bulfon et al., 2014 | |||||||
| Strain O41 | Vaccinium vitis-idaea (lingonberry-leaves) | Ethanolic extract | Phenolic glycosides (arbutin and hydroquinone), tannins, flavonoids (iperoside, avicularin, isoquercitrin), terpenic acids (ursolic and oleanolic acids), organic acids, and mineral salts | 4200 | Unknown | Bulfon et al., 2014 | |||||||
| Rainbow trout | Glycyrrhiza glabra L. (root) | Ethanolic extract | Unknown | 920 | Unknown | Fereidouni et al., 2013 | |||||||
| Rainbow trout | Peganum harmala (wild rue-seed) | Methanolic extrac | Unknown | 105 | Unknown | Fereidouni et al., 2013 | |||||||
| , 28 days | Rainbow trout | Trachyspermum copticum (carum ajowan-seed) | Ethanolic extract | Unknown | 453 | Unknown | Fereidouni et al., 2013 | ||||||
| Rainbow trout | Myrtus communis (myrtle-leaves) | Essential oil | Unknown | 672 | Unknown | Fereidouni et al., 2013 | |||||||
| Rainbow trout | Juglans regia (English walnut-leaves) | Ethanolic extract | Unknown | 510 | Unknown | Fereidouni et al., 2013 | |||||||
| Rainbow trout | Quercus branti Lindley (Brant’s oak-seed) | Ethanolic extract | Unknown | 978 | Unknown | Fereidouni et al., 2013 | |||||||
| Rainbow trout | Tanacetum parthenium (feverfew-leaves) | Essential oil | Unknown | 824 | Unknown | Fereidouni et al., 2013 | |||||||
| Rainbow trout | Satureja bachtiarica Bung. (savory-leaves) | Essential oil | Unknown | 126 | Unknown | Fereidouni et al., 2013 | |||||||
| Rainbow trout | Glycyrrhiza glabra L.(root) | Ethanolic extract | Glycyrrhizinic acid | >1000 | >1000 | Goudarzi et al., 2011 | |||||||
| Rainbow trout | Satureja bachtiarica Bung. (aerial parts) | Essential oil | Phenols: Carvacrol, thymol | 8 | 16 | Goudarzi et al., 2011 | |||||||
| Rainbow trout | Satureja bachtiarica Bung. (aerial parts) | Ethanolic extract | Phenols: Carvacrol, thymol | >1000 | >1000 | Goudarzi et al., 2011 | |||||||
| Rainbow trout | Punica granatum (pomegranate-flowers) | Ethanolic extract | Polyphenols: pomegranatate | >1000 | >1000 | Goudarzi et al., 2011 | |||||||
| Rainbow trout | Quercus branti Lindley (seed/flour) | Ethanolic extract | Tannins | >1000 | >1000 | Goudarzi et al., 2011 | |||||||
| Rainbow trout | Echinophora platyloba DC. (prickly parsnip-aerial parts) | Essential oil | Monoterpenes: trans-β-ocimene | >1000 | >1000 | Goudarzi et al., 2011 | |||||||
| Rainbow trout | Echinophora platyloba DC. (aerial parts) | Ethanolic extract | Monoterpenes: trans-β-ocimene | >1000 | >1000 | Goudarzi et al., 2011 | |||||||
| Rainbow trout | Heracleum lasiopetalum Boiss. (fruits) | Ethanolic extract | Sesquiterpene hydrocarbons: Germacrene-D | >1000 | >1000 | Goudarzi et al., 2011 | |||||||
| Rainbow trout |
Kelussia odoratissima Mozaff. (wild celery-leaves) | Ethanolic extract | Z-ligustilide | >1000 | >1000 | Goudarzi et al., 2011 | |||||||
| Rainbow trout | Stachys lavandulifolia Vahl (wood betony-flowers) | Ethanolic extract | Sabinene, α-pinene, β-myrcene | >1000 | >1000 | Goudarzi et al., 2011 | |||||||
| Rainbow trout | Thymus daenensis Celak. (common thyme-aerial part/inflorescence) | Ethanolic extract | Phenols: thymol, carvacrol | >1000 | >1000 | Goudarzi et al., 2011 | |||||||
| Rainbow trout | Thymus daenensis Celak. (Aerial part/inflorescence) | Essential oil | Phenols: thymol, carvacrol | 8 | 16 | Goudarzi et al., 2011 | |||||||
| Rainbow trout | Myrtus communis (leaves) | Ethanolic extract | α-pinene, 1,8-cineole, myrtenyl acetate | >250 | >500 | Goudarzi et al., 2011 | |||||||
| Rainbow trout | Myrtus communis (leaves) | Essential oil | α-pinene, 1,8-cineole, myrtenyl acetate | >1000 | >1000 | Goudarzi et al., 2011 | |||||||
| Rainbow trout | Thymbra spicata (spiked thyme-aerial part/inflorescence) | Essential oil | Phenols: thymol, carvacrol | 8 | 16 | Goudarzi et al., 2011 | |||||||
| Rainbow trout | Bunium persicum (Boiss.) K.- Pol. (black caraway-fruits) |
Essential oil | γ-terpinen-7-al, cuminaldehyde, γ terpinene | 8 | 16 | Goudarzi et al., 2011 | |||||||
| Rainbow trout | Teucrium polium (felty germander-aerial parts) | Essential oil | α-pinene, linalool | >1000 | >1000 | Goudarzi et al., 2011 | |||||||
| Rainbow trout | Alhagi maurorum (camelthorn-aerial parts) | Essential oil | Alhagidin, alhagitin, quercetin, catechin | >1000 | >1000 | Goudarzi et al., 2011 | |||||||
| Rainbow trout | Zataria multifora Boiss. (Shirazi thyme) | Essential oil | Phenols: thymol, carvacrol | 4 | 8 | Goudarzi et al., 2011 | |||||||
| Olive flounder (P. olivaceus) | Zingiber officinale (ginger) | Essential oil | 2000 | 2000 | Hossain et al., 2019 | ||||||||
| L. garvieae GQ850376 | Eucalyptus globulus (southern blue gum-aerial parts) | Essential oil | 1,8-eucalypol, pinene, terpineol acetae, globulol | 250 | 250 | Mahmoodi et al., 2012 | |||||||
| L. garvieae GQ850376 | Eucalyptus globulus (aerial parts) | Methanolic extract | 1,8-eucalypol, pinene, terpineol acetae, globulol | 500 | 500 | Mahmoodi et al., 2012 | |||||||
| L. garvieae GQ850376 | Zataria multiflora (aerial parts) | Essential oil | phenolic monoterpene, Carvacrol, alpha-pinene | 7.8 | 15.6 | Mahmoodi et al., 2012 | |||||||
| L. garvieae GQ850376 | Zataria multiflora (aerial parts) | Methanolic extract | phenolic monoterpene Carvacrol, alpha-pinene | 15.6 | 15.6 | Mahmoodi et al., 2012 | |||||||
| L. garvieae GQ850376 | Anethum graveolens (dill-seed) | Essential oil | D-carvacrol, limonene, dill apiole, E-dihydrocarvone, Z-dihydrocarvone | 62.4 | 125 | Mahmoodi et al., 2012 | L. garvieae GQ850376 | Anethum graveolens (seed) | Methanolic extract | D-carvacrol, limonene, dill apiole, E-dihydrocarvone, Z-dihydrocarvone | 125 | 125 | Mahmoodi et al., 2012 |
| ↑120 | L. garvieae GQ850376 | Rosmarinus officinalis | Essential oil | 1,8-cineole, alpha-pinene, toluene | 15.6 | 31.2 | Mahmoodi et al., 2012 | ||||||
| L. garvieae GQ850376 | Rosmarinus officinalis | Methanolic extract | 1,8-cineole, alpha-pinene, toluene | 31.2 | 31.2 | Mahmoodi et al., 2012 | |||||||
| Rainbow trout | Citrus paradisi (grapefruit), Citrus reticulata (tangerine), Citrus aurantium ssp. bergamia (bergamot), Citrus sinensis (sweet orange) | Biocitro ® (blend of citrus extracts) | Ascorbic acid, citrus bioflavonoids (hesperidin, naringin, quercetin, rutin) and organic acids | 2.0 | Unknown | Mora-Sánchez et al., 2020 | |||||||
| Mora-Sánchez et al., 2020 | Tilapia (O. andersonii) | Capsicum annum (Chili pepper) | Methanolic extract (capsaicin) | Unknown | Unknown | 196.7 | Ndashe et al., 2020 | ||||||
| Olive flounder | Citrus aurantifolia (key lime-peel) | Essential oil | Limonene, γ-terpinene, β-pinene | 0.125% (v/v) | 1% (v/v) | Pathirana et al., 2018 | |||||||
| Olive flounder | Limonene | Commercial trans-limonene (>99%) | Limonene | 0.031% (v/v) | 0.025% (v/v) | Pathirana et al., 2018 | |||||||
| Olive flounder | Syzygium aromaticum (clove-buds) | Essential oil | Eugenol, β-caryophyllene, α-humulen, eugenyl-acetate | 0.5% (v/v) | 1% (v/v) | Pathirana et al., 2019a | |||||||
| Olive flounder | Commercial eugenol (>99%) | Isolated compound eugenol |
Eugenol | 1% (v/v) | 1% (v/v) | Pathirana et al., 2019a | |||||||
| Olive flounder | Cinnamomum zeylanicum | Essential oil | Cinnamaldehyde, eugenol, β-Caryophyllene |
0.015% (v/v) | 0.031% (v/v) | Pathirana et al., 2019b | |||||||
| Olive flounder | Commercial trans-cinnamaldehyde (>99%) (Sigma-Aldrich) | cinnamaldehyde | Cinnamaldehyde | 0.003% (v/v) | 0.015% (v/v) | Pathirana et al., 2019b | |||||||
| Rainbow trout | Argania spinose L. (argan-oil) | Essential oil | Oleic acid, linoleic acid, palmitic acid, stearic acid | 250 | Unknown | Öntas et al., 2016 | |||||||
| Rainbow trout | Citrus limon L. (lemon-peel) | Essential oil | Limonene, γ-terpinene, β-pinene, α-terpineol, myrecene and terpinolene | 500 | Unknown | Öntas et al., 2016 | |||||||
| Strain ATCC43921 | Cinnamomum verum (cinnamon-bark) | Essential oil | Unknown | 120 | Unknown | Rattanachaikunsopon et al., 2009 | |||||||
| Strain ATCC43921 | Ocimum sanctum (holy basil-leaves) | Essential oil | Unknown | 240 | Unknown | Rattanachaikunsopon et al., 2009 | |||||||
| Strain ATCC43921 | Zingiber officinale (roots) | Essential oil | Unknown | 120 | Unknown | Rattanachaikunsopon et al. 2009 | |||||||
| Strain ATCC43921 | Syzygium aromaticum (flower buds) | Essential oil | Unknown | 30 | Unknown | Rattanachaikunsopon et al., 2009 | |||||||
| Rainbow trout | Zataria multiflora (aerial parts) | Essential oil | Carvacrol, benzene and phenol | 0.12 | 0.12 | Soltani et al., 2014 | |||||||
| Rainbow trout | Allium sativum (garlic-edible parts) | Essential oil | trisulfide, di-2-propenyl, disulfide, di-2-propenyl and trisulfide, methyl 2-propenyl | 0.5 | 1 | Soltani et al., 2014 | |||||||
| Rainbow trout | Cinnamomum zeylanicum (bark) | Essential oil | cinnamic aldehyde, linalool, ortho methoxy cinnamic aldehyde and 1,8-cineole | 0.5 | 0.5 | Soltani et al., 2014 | |||||||
| S. quinqueradiata | Chloramphenicol | 0.8 a | 1.6 b | Maki et al., 2008 | |||||||||
| S. quinqueradiata | Ciprofloxacin | 1.6 a | 3.13 b | Maki et al., 2008 | |||||||||
| S. quinqueradiata | Erythromycin | 0.1 a | 800 b | Maki et al., 2008 | |||||||||
| S. quinqueradiata | Enoxacin | 6.25 a | 12.5 b | Maki et al., 2008 | |||||||||
| S. quinqueradiata | Florfenicol | 1.6 a | 1.6 b | Maki et al., 2008 | |||||||||
| S. quinqueradiata | Floroxacin | 12.5 a | 12.5 b | Maki et al., 2008 | |||||||||
| S. quinqueradiata | Kanamycin | 25 a | 50 b | Maki et al., 2008 | |||||||||
| S. quinqueradiata | Lincomycin | 25 a | 800 b | Maki et al., 2008 | |||||||||
| S. quinqueradiata | Norfloxacin | 6.25 a | 12.5 b | Maki et al., 2008 | |||||||||
| S. quinqueradiata | Oxolinic acid | 400 a | 800 b | Maki et al., 2008 | |||||||||
| S. quinqueradiata | Orbifloxacin | 1.6 a | 1.6 b | Maki et al., 2008 | |||||||||
| S. quinqueradiata | Ofloxacin | 3.13 a | 6.25 b | Maki et al., 2008 | |||||||||
| S. quinqueradiata | Penzylpenicillin | 0.8 a | 1.6 b | Maki et al., 2008 | S. quinqueradiata | Streptomycin | 25 a | 50 b | Maki et al., 2008 | ||||
| S. quinqueradiata | Tetracycline | 12.5 a | 400 b | Maki et al., 2008 |
3.2. In Vivo Studies
All in vivo studies were related to survival against L. garvieae infection, and, in most cases, the extractives of medicinal herbs and plants were added to the diets for various periods before the treated fish being challenged with L. garvieae infection. Overall, the essential oils that showed the best in vitro antibacterial activity against L. garvieae (Table 1) were not tested for the in vivo bioassays yet. The extractives tested under in vivo conditions presented moderate in vitro antibacterial activity against this bacterium or even were not tested in vitro. However, the dietary supplementation with all tested extractives reduced mortality of infected animals (Table 2), probably because they improved immune parameters before challenging the treated fish with L. L. garvieaegarvieae. A 12-day feeding giant freshwater prawn (Macrobrachium rosenbergii) with hot-water extract of water hyacinth (Eichhornia crassipes) leaves at 1, 2, and 3 g kg−1 diet induced significantly higher survival rate after challenge with L. garvieae infection, but higher disease resistance was seen in the prawn treated with higher concentration of the extract [108][30]. In addition, the treated animals exhibited an enhancement in the immune responses including respiratory burst, phenoloxidase activity, superoxide dismutase activity, glutathione peroxidase, total hemocyte value, differential hemocyte count, transglutaminase activity, and phagocytic activity towards L. garvtieae. In the subsequent research work by Chang and Cheng [109][31], dietary addition of three tested water hyacinth extracts (Table 2) for 120 days increased survival and immune parameters, i.e., total hemocyte count, semi-granular and granular cells counts of giant freshwater prawn while phenoloxidase activity, respiratory bursts of hemocytes were not observed only with dietary addition of powder of this plant to the diet.
4. Conclusions
References
- Soltani, M.; Nikbakht, G.; Ebrahimzadeh Moussavi, H.; Ahmadzadeh, N. Epizootic outbreak of lactococcosis caused by Lactococcus garvieae in farmed rainbow trout (Oncorhynchus mykiss) in Iran. Bull. Eur. Assoc. Fish Pathol. 2008, 28, 95–106.
- Eldar, A.A.; Ghittino, C. Lactococcus garvieae and Streptococcus iniae infections in rainbow trout Oncorhynchus mykiss: Similar, but different diseases. Dis. Aquat. Org. 1999, 36, 227–231.
- Chen, M.-H.; Hung, S.-W.; Shyu, C.-L.; Lin, C.-C.; Liu, P.-C.; Chang, C.-H.; Shia, W.-Y.; Cheng, C.-F.; Lin, S.-L.; Tu, C.-Y. Lactococcus lactis subsp. lactis infection in Bester sturgeon, a cultured hybrid of Huso huso × Acipenser ruthenus, in Taiwan. Res. Vet. Sci. 2012, 93, 581–588.
- Austin, B.; Austin, D.A.; Austin, B.; Austin, D.A. Bacterial Fish Pathogens; Springer International Publishing: Cham, Switzerland, 2016; Volume 481.
- Ortega, C.; Irgang, R.; Valladares-Carranza, B.; Collarte, C.; Avendaño-Herrera, R. First identification and characterization of Lactococcus garvieae isolated from rainbow trout (Oncorhynchus mykiss) cultured in mexico. Animals 2020, 10, 1609.
- Hirono, I.; Yamashita, H.; Park, C.I.; Yoshida, T.; Aoki, T. Identification of genes in a KG—Phenotype of Lactococcus garvieae, a fish pathogenic bacterium, whose proteins react with antiKG—Rabbit serum. Microb. Pathog. 1999, 27, 407–417.
- Bragg, R.; Broere, J. Streptococcosis in rainbow trout in South Africa. Bull. Eur. Assoc. Fish Pathol. 1986, 6, 89–91.
- Bekker, A.; Hugo, C.; Albertyn, J.; Boucher, C.; Bragg, R. Pathogenic Gram-positive cocci in South African rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2011, 34, 483–487.
- Vendrell, D.; Balcázar, J.L.; Ruiz-Zarzuela, I.; De Blas, I.; Gironés, O.; Múzquiz, J.L. Lactococcus garvieae in fish: A review. Comp. Immunol. Microbiol. Infect. Dis. 2006, 29, 177–198.
- Chang, P.; Lin, C.; Lee, Y. Lactococcus garvieae infection of cultured rainbow trout, Oncorhynchus mykiss in Taiwan and associated biophysical characteristics and histopathology. Eur. Assoc. Fish Pathol. 2002, 22, 319–327.
- Avci, H.; Birincioglu, S.; Tanrikul, T.; Epikmen, E.; Metin, N.; Avsever, M. Experimental Lactococcus garvieae infection in rainbow trout, Oncorhynchus mykiss, Walbaum 1792: A comparative histopathological and immunohistochemical study. J. Fish Dis. 2014, 37, 481–495.
- Chen, S.-C.; Lin, Y.-D.; Liaw, L.-L.; Wang, P.-C. Lactococcus garvieae infection in the giant freshwater prawn Macrobranchium rosenbergii confirmed by polymerase chain reaction and 16S rDNA sequencing. Dis. Aquat. Org. 2001, 45, 45–52.
- Algöet, M.; Bayley, A.; Roberts, E.; Feist, S.; Wheeler, R.; Verner-Jeffreys, D. Susceptibility of selected freshwater fish species to a UK Lactococcus garvieae isolate. J. Fish Dis. 2009, 32, 825–834.
- Türe, M.; Haliloğlu, H.İ.; Altuntaş, C.; Boran, H.; Kutlu, İ. Comparison of experimental susceptibility of rainbow trout (Oncorhynchus mykiss), turbot (Psetta maxima), black sea trout (Salmo trutta labrax) and sea bass (Dicentrarchus labrax) to Lactococcus garvieae. Turk. J. Fish. Aquat. Sci. 2014, 14, 507–513.
- Kusuda, R.; Hamaguchi, M. Extracellular and intracellular toxins of Streptococcus sp. isolated from yellowtail. Bull. Eur. Assoc. Fish Pathol. 1988, 8, 9–10.
- Aguado-Urda, M.; López-Campos, G.H.; Gibello, A.; Cutuli, M.T.; López-Alonso, V.; Fernández-Garayzábal, J.F.; Blanco, M.M. Genome sequence of Lactococcus garvieae 8831, isolated from rainbow trout lactococcosis outbreaks in Spain. J. Bacteriol. 2011, 193, 4263–4264.
- Holbourn, K.P.; Shone, C.C.; Acharya, K. A family of killer toxins. FEBS J. 2006, 273, 4579–4593.
- Ture, M.; Altinok, I. Detection of putative virulence genes of Lactococcus garvieae. Dis. Aquat. Org. 2016, 119, 59–66.
- Meyburgh, C.; Bragg, R.; Boucher, C. Lactococcus garvieae: An emerging bacterial pathogen of fish. Dis. Aquat. Org. 2017, 123, 67–79.
- Saraoui, T.; Leroi, F.; Björkroth, J.; Pilet, M.-F. Lactococcus piscium: A psychrotrophic lactic acid bacterium with bioprotective or spoilage activity in food—A review. J. Appl. Microbiol. 2016, 121, 907–918.
- Rodrigues, M.; Lima, S.; Higgins, C.; Canniatti-Brazaca, S.; Bicalho, R. The Lactococcus genus as a potential emerging mastitis pathogen group: A report on an outbreak investigation. J. Dairy Sci. 2016, 99, 9864–9874.
- Mannion, P.; Rothburn, M. Diagnosis of bacterial endocarditis caused by Streptococcus lactis and assisted by immunoblotting of serum antibodies. J. Infect. 1990, 21, 317–318.
- Clark, I.; Burnie, J. Immunoblotting and culture positive endocarditis. J. Clin. Pathol. 1991, 44, 152–156.
- Campbell, P.; Dealler, S.; Lawton, J. Septic arthritis and unpasteurised milk. J. Clin. Pathol. 1993, 46, 1057–1058.
- Durand, J.M.; Rousseau, M.C.; Gandois, J.M.; Kaplanski, G.; Mallet, M.N.; Soubeyrand, J. Streptococcus lactis septicemia in a patient with chronic lymphocytic leukemia. Am. J. Hematol. 1995, 50, 64–65.
- Zechini, B.; Cipriani, P.; Papadopoulou, S.; Di Nucci, G.; Petrucca, A.; Teggi, A. Endocarditis caused by Lactococcus lactis subsp. lactis in a patient with atrial myxoma: A case report. Diagn. Microbiol. Infect. Dis. 2006, 56, 325–328.
- Wang, P.-C.; Lin, Y.-D.; Liaw, L.-L.; Chern, R.-S.; Chen, S.-C. Lactococcus lactis subspecies lactis also causes white muscle disease in farmed giant freshwater prawns Macrobrachium rosenbergii. Dis. Aquat. Org. 2008, 79, 9–17.
- Khoo, L.H.; Austin, F.W.; Quiniou, S.M.; Gaunt, P.S.; Riecke, D.K.; Jacobs, A.M.; Meals, K.O.; Dunn, A.W.; Griffin, M.J. Lactococcosis in silver carp. J. Aquat. Anim. Health 2014, 26, 1–8.
- Wünnemann, H.; Eskens, U.; Prenger-Berninghoff, E.; Ewers, C.; Lierz, M. Lactococcus lactis, causative agent of an endocarditis valvularis and parietalis thromboticans in the allis shad, Alosa alosa (L.). J. Fish Dis. 2018, 41, 1207–1215.
- Chang, C.-C.; Tan, H.-C.; Cheng, W. Effects of dietary administration of water hyacinth (Eichhornia crassipes) extracts on the immune responses and disease resistance of giant freshwater prawn, Macrobrachium rosenbergii. Fish Shellfish Immunol. 2013, 35, 92–100.
- Chang, C.C.; Cheng, W. Multiple dietary administrating strategies of water hyacinth (Eichhornia crassipes) on enhancing the immune responses and disease resistance of giant freshwater prawn, Macrobrachium rosenbergii. Aquac. Res. 2016, 47, 140–152.
