The establishment of proper conceptus–endometrial communication is essential for conceptus implantation and subsequent successful placentation in mammals including ruminants. Extracellular vesicles (EVs) present in uterine lumen are now considered to play a role in conceptus–endometrial interactions during the preimplantation period. In fact, EV transport a variety of bioactive molecules, including soluble and membrane-bound proteins, lipids, DNA, and RNAs, into target cells. EVs thus regulate gene expression and elicit biological effects including increased cell proliferation, migration, and adhesion in recipient cells. EVs of conceptus as well as endometrial origins are interactive in the uterine microenvironment for improved pregnancy success.
- extracellular vesicles
- conceptus
- endometrium
- implantation
- pregnancy
In ruminants, the establishment of proper conceptus–endometrial communication is essential for conceptus implantation and subsequent successful placentation. Accumulated evidence supports the idea that extracellular vesicles (EVs) present in uterine lumen are involved in conceptus–endometrial interactions during the preimplantation period. EVs make up a new field of intercellular communicators, which transport a variety of bioactive molecules, including soluble and membrane-bound proteins, lipids, DNA, and RNAs. EVs thus regulate gene expression and elicit biological effects including increased cell proliferation, migration, and adhesion in recipient cells. Uterine EVs are interactive and coordinate with ovarian progesterone (P4), trophectoderm-derived interferon tau (IFNT) and/or prostaglandins (PGs) in the physiological or pathological microenvironment.
1. Introduction
In domestic ruminants, conceptus implantation to the uterine endometrium is a unique physiological process, consisting of blastocyst hatching, elongation, migration, apposition/attachment, and subsequent placentation [1]. The morula-stage embryo enters the uterus on days 4–6 postmating and then forms a blastocyst that contains an inner cell mass (ICM) and a blastocoel or central cavity surrounded by a monolayer of trophectoderm (TE). The blastocyst then hatches from the zona pellucida on day 8, and slowly grows into a tubular or ovoid form, which is termed a conceptus (embryo-fetus and associated extraembryonic membranes) [2][3]. The ovoid conceptus then begins to elongate into a filamentous form on days 12–13 in sheep or days 14–15 in cattle, respectively. During this period, elongating conceptus begins to produce a major cytokine, interferon tau (IFNT) [4][5][6]. IFNT prevents secretion of luteolytic pulses of prostaglandin F2-alpha (PGF2a) by uterine epithelium for the prolongation of the corpus luteum (CL) life span, which is a process described as the maternal recognition of pregnancy (MRP) [7][8][9][10]. After several days to a week of elongation, the ruminant conceptus occupies the entire length of the uterine horn ipsilateral to the CL, with extraembryonic membranes extending into the contralateral uterine horn, and begins its attachment to the uterine epithelium on day 16 in sheep and day 19 in cattle, followed by adhesion and placentation [11]. During this period, conceptus IFNT, together with maternal progesterone (P4) from functional CL, regulates endometrial gene expression, which sets up the uterine environment necessary for the establishment of conceptus migration, apposition, and initial attachment to the uterine epithelial cells in the ruminant species [12][13]. Thus, the establishment of proper conceptus–endometrial communication is required to allow conceptus implantation to the endometrium and subsequent maintenance of pregnancy.
The communication between different cell types is generally maintained through secretory soluble factors such as hormones and cytokines. In recent years, however, emerging evidence indicates that extracellular vesicles (EVs) produced by cells are also involved in cellular communication. In fact, EVs have been observed to transfer information to other cells, regulating cellular activities of the recipient cells [14]. Novel approaches and insights have made possible the extensive characterization of EVs, which contain surface receptors/ligands and cargo of proteins, lipids, metabolites, DNAs, and RNAs from the originating cells. Thus, EVs could be indicative of the cellular physiological state and function. In addition, the lipid bilayer of EVs made up of relatively high concentrations of cholesterol, sphingomyelin, ceramide, and detergent-resistant membrane domains, making these vesicles stable in extracellular spaces [15]. Following contact or uptake by recipient cells, EVs can regulate gene expression and elicit biological effects, including increased cell proliferation, migration, and adhesion. Evidence gathered indicates that EVs are produced by reproductive tissues/cells including follicular [16][17], oviductal [18], and endometrial cells [19], as well as in-vitro- and in-vivo-produced embryos [20][21][22]. Evidence is mounting that EVs of maternal or embryonic origin participate in the conceptus/fetal–endometrial interactions that are critical to pregnancy’s early stages, possibly continuing throughout the entire processes [23][24][25][26].
2. General Concepts of EVs: Biogenesis, Secretion, and Cargo
In order to organize the data generated in different laboratories throughout the world, the International Society for Extracellular Vesicles (ISEV), which is the leading global professional society, constantly updates newly discovered EVs along with the available information. The documents entitled Minimal Information for Studies of Extracellular Vesicles (“MISEV”) Guidelines have been published and updated by the ISEV to provide information on better isolation and characterization of EV preparations, as well as suggestions to specific activities associated with EVs [27]. In this section, general information including subtypes and cargo of EVs based on the MISEV guidelines and the recent findings are provided to better understand the roles of EVs for conceptus–endometrial communication.
2.1. Subtypes of EVs
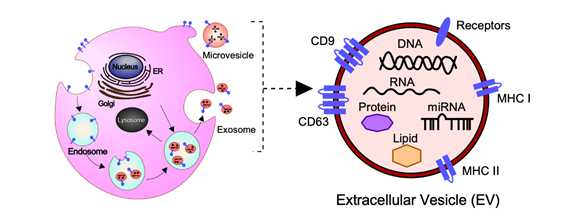
In general, EVs are defined as nano-sized, membrane-enclosed vesicles naturally released from the cells [27][28] and commonly classified as exosomes, microvesicles (MVs), and apoptotic bodies according to their sizes, biogenesis, and secretion (Figure 1) [29].
Exosomes are small membrane-bound vesicles with a diameter of 50–150 nm, which are derived from endosomal multivesicular bodies (MVBs). The formation is derived from the invagination of the plasma membrane (early endosome) and the subsequent fusion of endocytic vesicles mediated by the endosomal sorting complex responsible for transport (ESCRTs) and/or other components such as ceramides and tetraspanins [30][31]. Exosomes are the intraluminal vesicles secreted into the extracellular space by the fusion of MVBs with the plasma membrane.
MVs have a diameter of 100–1000 nm and are released directly from the plasma membrane into the extracellular space by budding and fission [30][31]. Their biogenesis mechanism is only a partially known; however, it has been found that MVs arise from the result of dynamic interplay between phospholipid redistribution and cytoskeletal protein contraction [32].
Apoptotic bodies are the largest vesicles with a diameter of 100–5000 nm, which originate from the fragmentation of the plasma membrane of cells undergoing the apoptotic processes [33]. Apoptotic bodies contain cell organelles, proteins, DNA fragments, and histones deriving directly from the intracellular environment. Apoptotic bodies are known to contribute to cellular waste management, unlike exosomes or MVs.
Figure 1. Biogenesis, secretion, and cargo of exosomes and microvesicles (MVs). Exosomes are small membrane-bound vesicles with a diameter of 50–150 nm, which are derived from endosomal multivesicular bodies. MVs are released directly from the plasma membrane with a diameter of 100–1000 nm into the extracellular space by budding and fission. Exosomes and MVs contain a variety of bioactive molecules including soluble and membrane-bound protein, lipids, metabolites, DNA, and RNA (mRNA, miRNAs, and other small regulatory RNAs), which regulate cellular activities of the recipient cells.
2.2. The Molecular Cargo of EVs
At present, the MISEV2018 guidelines suggest that three categories of protein markers should be demonstrated in all EV preparations for the presence of EVs (Categories 1 and 2) and their purity from common contaminants (Category 3) [34]. EVs transport a variety of bioactive molecules including soluble and membrane-bound protein, lipids, metabolites, DNA, and RNA (mRNA, miRNAs, and other small regulatory RNAs) [35]. Lipidomic analysis has shown that EVs, independent of their biogenesis, contain a multitude of lipids such cholesterol, sphingomyelin, ceramide, glycerophospholipids, phosphatidylcholine, and phosphatidylserine [34][36][37]. In addition, several proteomic analyses have shown that EVs contain different types of proteins, such as heat shock proteins (HSP70 and HSP90), major histocompatibility complex class I and II (MHC class I and II), tetraspanins (CD9, CD63 and others), endosomal sorting complex proteins required for transport (Alix and Tsg101) and chaperones, which are often used as protein markers [34][38][39]. Moreover, receptors including epidermal growth factor receptor (EGFR), membrane trafficking proteins (GTPases, Flotillin and Annexins), cytoskeletal proteins (tubulin and actin), and cytosolic proteins are also enriched in EVs. It should be noted that the cargo of EVs varies depending not only on their cellular origin, but also on their physiological and/or pathological condition.
3. Potential Role of EVs for Clinical Application in Farm Animals
The embryo transfer (ET) industry has been growing rapidly. Indeed, ET data provided that a total of 1,129,041 bovine embryos and 17,868 ovine embryos were transferred commercially worldwide in 2018 [40]. It is generally accepted that the majority of bovine embryonic losses occur during the second and third weeks of pregnancy [41][42][43][44], suggesting that reducing embryonic losses at these stages result in higher productivity and economic efficiency in ruminants.
First, EVs may be of clinical significance for in-vitro-fertilized (IVF)-ET settings because selection of high-quality IVF blastocysts is required to increase successful IVF-ET rates. It was reported that EVs are secreted by in-vitro-cultured bovine embryos into culture media and their characteristics associated with embryo quality [45]. Human embryos secrete miRNAs, which could be packed in exosomes into culture media [46]. Notably, some of these miRNAs, including miRNA-191, were differentially expressed according to the fertilization method, chromosomal status, and pregnancy outcome. These findings suggest that cargo of EVs in the spent embryo medium could be biomarkers predictive of high-quality blastocysts, which would be a powerful noninvasive approach and improve IVF-ET successes.
Second, the application of EVs as biomarkers in early embryonic mortality or early pregnancy diagnosis could improve the rates of pregnancy successes as well. It is noteworthy that the cargo of EVs changes based on the extracellular environment. Comprehensive miRNA sequencing on serum EVs in day 17 pregnant and embryonic-mortality cattle identified that 27 miRNAs were significantly increased in day 17 embryonic mortality compared to those of the pregnant group [47]. Furthermore, miRNA profiles from EVs in the maternal blood of cattle on pregnant day 21 found that the somatic cell nuclear transfer-derived embryonic loss group exhibited lower abundance of 27 miRNAs than were found in successful pregnancy groups [48]. These observations indicate that pregnant and embryonic-mortality animals could be diagnosed through the detection of individual miRNA from the circulating EVs.
Given the observations that EVs secreted by the conceptus and/or endometrium are likely to promote conceptus implantation to the endometrium, third, potential clinical application would be to deliver specific cargo via EVs into the uterine cavity during the early pregnant stages. Recent studies have reported that the addition of bovine embryo- and uterus- derived exosomes increased the cleavage rate and blastocyst formation in cloned embryos [49], whereas the uterine exosomes derived from cows with endometritis significantly decreased the blastocyst formation rate of in-vitro-fertilized embryos compared to those derived from healthy cows [50]. These results provide support for the idea that the delivery of specific cargo via EVs into the uterine cavity conditions the uterine environment to result in better embryo development or endometrium receptivity for improved pregnancy success.
4. Conclusions
It is well documented that proper biochemical and cellular communication between the conceptus and the uterine endometrium are required for conceptus implantation and subsequent placentation. Recent progress suggests that uterine EVs may gain recognition as critical to conceptus–endometrial communication during the peri-implantation period in concert with the well-characterized molecules. It should be noted that uterine EVs could have autocrine and/or paracrine biological effects at different stages in the early pregnancy. As discussed earlier, uterine EVs are interactive and coordinated with ovarian P4, trophectoderm-derived IFNT, and/or lipids, including PGs, for conceptus elongation and conceptus implantation and subsequent placentation in the physiological or pathological microenvironment. Although significant details including the biogenesis, cargo, and definitive roles of uterine EVs have not yet been established, the rapid technological advances in the field of EV research will provide a strong impetus to clarify their effects on the peri-implantation processes.
In addition, EVs could become advanced tools for diagnosing and/or therapeutic agents useful to the reproductive field. For example, EVs in the spent embryo medium would be a predictive biomarker for selection of high-quality IVF blastocysts. In addition, the serum EVs following artificial insemination or embryo transfer would be a noninvasive biomarker for detecting pregnancy status. The last potential application would be to deliver specific cargo via EVs into the uterine cavity to improve embryo development or endometrium receptivity for more pregnancy success. Further research is required to characterize differences in the cargo of EVs between pregnant and nonpregnant or embryonic-mortality animals, which ultimately improves fertility rates in agriculturally important animals.
References
- Bazer, F.W.; Spencer, T.E.; Johnson, G.A.; Burghardt, R.C.; Wu, G. Comparative aspects of implantation. Reproduction 2009, 138, 195–209.
- Guillomot, M. Cellular interactions during implantation in domestic ruminants. J. Reprod. Fertil. Suppl. 1995, 49, 39–51.
- Hue, I.; Degrelle, S.A.; Turenne, N. Conceptus elongation in cattle: Genes, models and questions. Anim. Reprod. Sci. 2012, 134, 19–28.
- Klemann, S.W.; Li, J.Z.; Imakawa, K.; Cross, J.C.; Francis, H.; Roberts, R.M. The production, purification, and bioactivity of recombinant bovine trophoblast protein-1 (bovine trophoblast interferon). Mol. Endocrinol. 1990, 10, 1506–1514.
- Imakawa, K.; Anthony, R.V.; Kazemi, M.; Marotti, K.R.; Polites, H.G.; Roberts, R.M. Interferon-like sequence of ovine trophoblast protein secreted by embryonic trophectoderm. Nature 1987, 330, 377–379.
- Gnatek, G.G.; Smith, L.D.; Duby, R.T.; Godkin, J.D. Maternal recognition of pregnancy in the goat: Effects of conceptus removal on interestrus intervals and characterization of conceptus protein production during early pregnancy. Biol. Reprod. 1989, 41, 655–663.
- Godkin, J.D.; Bazer, F.W.; Moffatt, J.; Sessions, F.; Roberts, R.M. Purification and properties of a major, low molecular weight protein released by the trophoblast of sheep blastocysts at day 13–21. J. Reprod. Fertil. 1982, 65, 141–150.
- Stewart, H.J.; McCann, S.H.; Barker, P.L.; Lee, K.E.; Lamming, G.E.; Flint, A.P. Interferon sequence homology and receptor binding activity of ovine trophoblast antiluteolytic protein. J. Endocrinol. 1987, 115, R13–R15.
- Charpigny, G.; Reinaud, P.; Huet, J.C.; Guillomot, M.; Charlier, M.; Pernollet, J.C.; Martal, J. High homology between a trophoblastic protein (trophoblastin) isolated from ovine embryo and alpha-interferons. FEBS Lett. 1988, 228, 12–16.
- Roberts, R.M.; Cross, J.C.; Leaman, D.W. Interferons as hormones of pregnancy. Endocr. Rev. 1992, 13, 432–452.
- Guillomot, M.; Fléchon, J.E.; Wintenberger-Torres, S. Conceptus attachment in the ewe: An ultrastructural study. Placenta 1981, 2, 169–182.
- Nagaoka, K.; Nojima, H.; Watanabe, F.; Chang, K.T.; Christenson, R.K.; Sakai, S.; Imakawa, K. Regulation of blastocyst migration, apposition, and initial adhesion by a chemokine, interferon gamma-inducible protein 10 kDa (IP-10), during early gestation. J. Biol. Chem. 2003, 278, 29048–29056.
- Imakawa, K.; Nagaoka, K.; Nojima, H.; Hara, Y.; Christenson, R.K. Changes in immune cell distribution and IL-10 production are regulated through endometrial IP-10 expression in the goat uterus. Am. J. Reprod. Immunol. 2005, 53, 54–64.
- Yáñez-Mó, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066.
- Pollet, H.; Conrard, L.; Cloos, A.S.; Tyteca, D. Plasma Membrane Lipid Domains as Platforms for Vesicle Biogenesis and Shedding? Biomolecules 2018, 8, 94.
- Da Silveira, J.C.; Veeramachaneni, D.N.; Winger, Q.A.; Carnevale, E.M.; Bouma, G.J. Cell-secreted vesicles in equine ovarian follicular fluid contain miRNAs and proteins: A possible new form of cell communication within the ovarian follicle. Biol. Reprod. 2012, 86, doi:10.1095/biolreprod.111.093252.
- Andrade, G.; Meirelles, F.; Perecin, F.; Da Silveira, J. Cellular and extracellular vesicular origins of miRNAs within the bovine ovarian follicle. Reprod. Domest. Anim. 2017, 52, 1036–1045.
- Gatien, J.; Mermillod, P.; Tsikis, G.; Bernardi, O.; Janati Idrissi, S.; Uzbekov, R.; Le Bourhis, D.; Salvetti, P.; Almiñana, C.; Saint-Dizier, M. Metabolomic Profile of Oviductal Extracellular Vesicles across the Estrous Cycle in Cattle. Int. J. Mol. Sci. 2019, 20, 6339.
- Evans, J.; Rai, A.; Nguyen, H.P.T.; Poh, Q.H.; Elglass, K.; Simpson, R.J.; Salamonsen, L.A.; Greening, D.W. Human Endometrial Extracellular Vesicles Functionally Prepare Human Trophectoderm Model for Implantation: Understanding Bidirectional Maternal-Embryo Communication. Proteomics 2019, 19, e1800423.
- Mellisho, E.A.; Velásquez, A.E.; Nuñez, M.J.; Cabezas, J.G.; Cueto, J.A.; Fader, C.; Castro, F.O.; Rodríguez-Álvarez, L. Identification and characteristics of extracellular vesicles from bovine blastocysts produced in vitro. PLoS ONE 2017, 12, e0178306.
- Kropp, J.; Salih, S.M.; Khatib, H. Expression of microRNAs in bovine and human pre-implantation embryo culture media. Front. Genet. 2014, 5, 91.
- Nakamura, K.; Kusama, K.; Bai, R.; Sakurai, T.; Isuzugawa, K.; Godkin, J.D.; Suda, Y.; Imakawa, K. Induction of IFNT-Stimulated Genes by Conceptus-Derived Exosomes during the Attachment Period. PLoS ONE 2016, 11, e0158278.
- Ruiz-González, I.; Xu, J.; Wang, X.; Burghardt, R.C.; Dunlap, K.A.; Bazer, F.W. Exosomes, endogenous retroviruses and toll-like receptors: Pregnancy recognition in ewes. Reproduction 2015, 149, 281–291.
- Bidarimath, M.; Khalaj, K.; Kridli, R.T.; Kan, F.W.K.; Koti, M.; Tayade, C. Extracellular vesicle mediated intercellular communication at the porcine maternal-fetal interface: A new paradigm for conceptus-endometrial cross-talk. Sci. Rep. 2017, 7, 1–14.
- O’Neil, E.V.; Burns, G.W.; Ferreira, C.R.; Spencer, T.E. Characterization and regulation of extracellular vesicles in the lumen of the ovine uterus. Biol. Reprod. 2020, Epub ahead of Print.
- Nakamura, K.; Kusama, K.; Ideta, A.; Imakawa, K.; Hori, M. IFNT-independent effects of intrauterine extracellular vesicles (EVs) in cattle. Reproduction 2020, 159, 503–511.
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750.
- Théry, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593.
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383.
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247.
- Devhare, P.B.; Ray, R.B. Extracellular vesicles: Novel mediator for cell to cell communications in liver pathogenesis. Mol. Asp. Med. 2018, 60, 115–122.
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neurooncol. 2013, 113, 1–11.
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, E.; Pap, E.; Kittel, A.; et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell. Mol. Life Sci. 2011, 68, 2667–2688.
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750.
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017, 27, 172–188.
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228.
- Royo, F.; Gil-Carton, D.; Gonzalez, E.; Mleczko, J.; Palomo, L.; Perez-Cormenzana, M.; Mayo, R.; Alonso, C.; Falcon-Perez, J.M. Differences in the metabolite composition and mechanical properties of extracellular vesicles secreted by hepatic cellular models. J. Extracell. Vesicles 2019, 8, 1575678.
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977.
- Haraszti, R.A.; Didiot, M.C.; Sapp, E.; Leszyk, J.; Shaffer, S.A.; Rockwell, H.E.; Gao, F.; Narain, N.R.; DiFiglia, M.; Kiebish, M.A.; et al. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J. Extracell. Vesicles 2016, 5, 32570.
- Burns, G.W.; Brooks, K.E.; Spencer, T.E. Extracellular Vesicles Originate from the Conceptus and Uterus During Early Pregnancy in Sheep. Biol. Reprod. 2016, 94, 1–11.
- Ng, Y.H.; Rome, S.; Jalabert, A.; Forterre, A.; Singh, H.; Hincks, C.L.; Salamonsen, L.A. Endometrial exosomes/microvesicles in the uterine microenvironment: A new paradigm for embryo-endometrial cross talk at implantation. PLoS ONE 2013, 8, e58502.
- Giacomini, E.; Vago, R.; Sanchez, A.M.; Podini, P.; Zarovni, N.; Murdica, V.; Rizzo, R.; Bortolotti, D.; Candiani, M.; Viganò, P. Secretome of in vitro cultured human embryos contains extracellular vesicles that are uptaken by the maternal side. Sci. Rep. 2017, 7, 1–13.
- Schultz, G.A.; Heyner, S. Growth factors in preimplantation mammalian embryos. Oxf. Rev. Reprod. Biol. 1993, 15, 43–81.
- Qu, P.; Qing, S.; Liu, R.; Qin, H.; Wang, W.; Qiao, F.; Ge, H.; Liu, J.; Zhang, Y.; Cui, W.; et al. Effects of embryo-derived exosomes on the development of bovine cloned embryos. PLoS ONE 2017, 12, e0174535.
- Saadeldin, I.M.; Kim, S.J.; Choi, Y.B.; Lee, B.C. Improvement of cloned embryos development by co-culturing with parthenotes: A possible role of exosomes/microvesicles for embryos paracrine communication. Cell. Reprogram. 2014, 16, 223–234.
- Desrochers, L.M.; Bordeleau, F.; Reinhart-King, C.A.; Cerione, R.A.; Antonyak, M.A. Microvesicles provide a mechanism for intercellular communication by embryonic stem cells during embryo implantation. Nat. Commun. 2016, 7, 1–11.
- Mellisho, E.A.; Briones, M.A.; Velásquez, A.E.; Cabezas, J.; Castro, F.O.; Rodríguez-Álvarez, L. Extracellular vesicles secreted during blastulation show viability of bovine embryos. Reproduction 2019, 158, 477–492.
- Spencer, T.E.; Palmarini, M. Endogenous retroviruses of sheep: A model system for understanding physiological adaptation to an evolving ruminant genome. J. Reprod. Dev. 2012, 58, 33–37.
- Dunlap, K.A.; Palmarini, M.; Adelson, D.L.; Spencer, T.E. Sheep endogenous betaretroviruses (enJSRVs) and the hyaluronidase 2 (HYAL2) receptor in the ovine uterus and conceptus. Biol. Reprod. 2005, 73, 271–279.
- Dunlap, K.A.; Palmarini, M.; Varela, M.; Burghardt, R.C.; Hayashi, K.; Farmer, J.L.; Spencer, T.E. Endogenous retroviruses regulate periimplantation placental growth and differentiation. Proc. Natl. Acad. Sci. USA 2006, 103, 14390–14395.