Many phase III trials failed to demonstrate a survival benefit from the addition of molecular therapy to conventional chemotherapy for advanced and metastatic gastric cancer, and only three agents were approved by the FDA. Despite recent advances in surgical techniques and in anticancer drugs, and the adoption of perioperative treatments mostly based on conventional chemotherapy, the prognosis of advanced and metastatic gastric cancer remains poor. In the last decade, the addition of molecular therapy did not show any significant survival advantage, and the first reports available documented an increase of the rate of severe adverse effects and related mortality. The survival benefits of molecular therapies available to date for advanced and metastatic gastric cancer are rather unclear, mostly due to inaccurate patient selection, particularly concerning oncogene amplification and copy number.
- gastric cancer
- molecular target therapy
- chemotherapy
- EGFR inhibitors
- angiogenesis inhibitors
- MET inhibitors
1. Introduction
2. Molecular Targets and Target Agents
2.1. Epidermal Growth Factor Receptor
2.1.1. Anti-HER1
2.1.2. Anti HER2
2.2. Vascular Endothelial Growth Factor
VEGFs are proteins promoting blood vessel formation. Four types of VEGF (VEGF-A, VEGF-B, VEGF-C, and VEGF-D) have been identified, with three types of corresponding receptors (VEGFR-1, VEGFR-2, and VEGFR-3). Several studies have reported the fundamental role of these signaling proteins in new blood vessel formation and cancer cell proliferation [34][29]. Furthermore, VEGF expression has been found in approximately 40% of GC [35][30]. Bevacizumab is an anti-VEGF-A monoclonal antibody that inhibits circulating VEGF-A activity [36][31]. The efficacy of this monoclonal antibody has been widely documented in several solid tumor treatments [37,38,39][32][33][34] but bevacizumab is still under investigation for its benefit in GC. Some phase II/III trials proved its efficacy in association with conventional chemotherapy in AGC, while others did not report any clear benefits [40,41][35][36].2.3. Mammalian Target of Rapamycin
mTOR is a serine/threonine protein kinase identified in mammalian cells with a leading role in controlling mechanisms of cell growth and proliferation. Human cancers can be characterized by hyperactivity or inactivity of the mTOR pathway, which plays a crucial role in maintaining tumor-modified phenotypes [48][37]..4. Hepatocyte Growth Factor Receptor
2.4. Hepatocyte Growth Factor Receptor
HGFR, also known as c-MET, is a proto-oncogenic receptor tyrosine kinase that, after binding to hepatocyte growth factor, induces cell migration and proliferation, promotes mitosis, and inhibits apoptosis. C-MET overexpression and gene amplification are related to a poor prognosis [50,51][38][39]. Crizotinib (PF-02341066) is a tyrosine kinase inhibitor of the c-MET receptor and of the TKR anaplastic lymphoma kinase; it has been approved by the FDA for treatment of ALK-positive NSCLC patients. Another promising agent targeting the HGF-cMET complex is rilotumumab. This human monoclonal antibody impairs the c-MET signaling pathway by binding to and inactivating its ligand HGF [53][40]. Clinical trials of this drug in GC (including two phase III trials) were halted due to a significant increase in mortality in the experimental arm (rilotumumab in combination with conventional chemotherapy) in one of these trials, but new investigations have begun. In Figure 1, targeted therapies and oncogenic pathways in gastric cancer are detailed.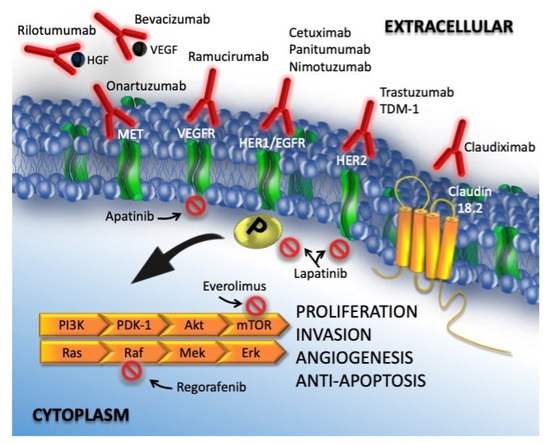
2.5. Preclinical Trials
3. Molecularly Targeted Therapies for Gastric Cancer
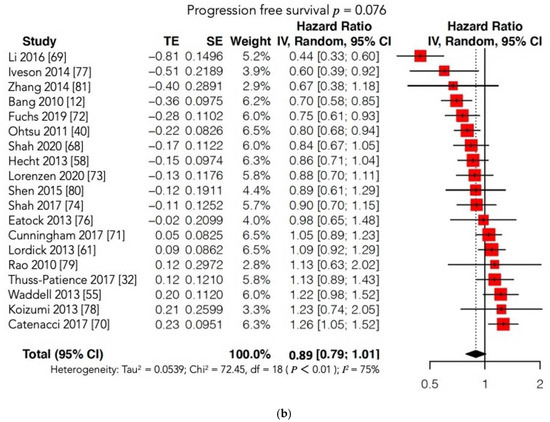
GC is still characterized by a poor prognosis, particularly in cases of metastatic or recurrent disease and in locally advanced stages. The identification and introduction of effective and safe molecular therapies in clinical practice lag behind other malignancies, such as lung and breast cancers. Unfortunately, findings showed that molecular therapies do not provide a clear survival benefit compared to conventional CT in the case of advanced or metastatic GC. In 2016, the Cochrane group published the largest systematic review and meta-analysis investigating the survival benefit of MTs for GC patients, with or without conventional treatment. The Cochrane authors identified 11 RCTs (phase II and III studies), and the conclusion was “Adding molecular-targeted treatment to chemotherapy may have a small effect on survival and on stopping further development of the disease, compared with chemotherapy alone, but the evidence is of low quality”. In the past five years, only eight new phase III RCTs have been conducted. Most of these studies failed to demonstrate the superiority of MT with or without conventional CT compared with conventional treatment alone or with placebo in terms of survival outcomes. Moreover, two of these eight trials were terminated prematurely. The METGastric Phase III trial was stopped early because of negative results reported in a concomitant Phase II study that concluded: “The addition of onartuzumab to mFOLFOX6 in gastric cancer did not improve efficacy in an unselected population or in a MET immunohistochemistry-positive population” [74,75][43][44]. The RILOMET-1 was interrupted prematurely because a safety control committee found more deaths in the experimental arm than in the control arm during a planned interim analysis of safety and survival outcomes [70][45]. The RCT published by Li was the only positive study; it reported a clear survival benefit in patients with GC treated with apatinib (a VEGFR2 inhibitor) compared with those receiving a placebo in terms of both OS (7.6 vs. 5.0 months, p = 0.0027) and PFS (2.8 vs. 1.9 months, p < 0.001), with an acceptable SAE rate [69][46]. Accordingly, in 2014, the China Food and Drug Administration approved the use of apatinib as a third-line treatment for metastatic GC. Despite this positive report, the overall meta-analysis did not show any significant differences in OS and PFS between the experimental (MT) and control arms. Furthermore, the subgroup analysis according to the type of MT administered (VEGFR or c-MET inhibitors) failed to show a significant prolongation of OS and/or PFS in the experimental arm. Notably, our results may have been unable to identify significant differences between the two arms due to the high heterogeneity found among the included studies. On account of this statistical bias, we conducted two further meta-analyses matching our OS and PFS findings with those reported in the Cochrane review [76,77,78,79,80,81][47][48][49][50][51][52] (Figure 72a,b; Table S1). Regrettably, these new cumulative analyses maintained high heterogeneity and could not document any survival advantage when MT was added to conventional treatment or administered alone compared to conventional CT or to a placebo.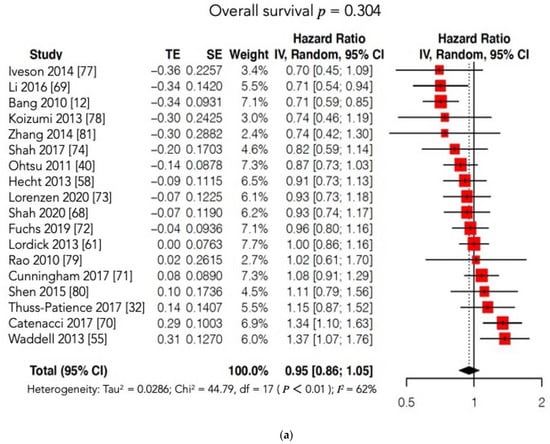
4. Conclusions
The results showed that despite their newly documented safety, the molecular therapies available to date for advanced and metastatic gastric cancer do not present clear survival benefits. These unfavorable results are mostly related to inadequate patient selection. Targeted therapies are promising treatments for patients with locally advanced, metastatic, or recurrent gastric cancer as they are for other types of tumors. However, their clinical validation requires accurate patient selection, particularly related to driver oncogene amplification and copy number, and it should take into account preclinical models investigating cancer heterogeneity and escape mechanisms.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- The Italian Gastric Cancer Study Group. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br. J. Surg. 2014, 101, 23–31.
- Songun, I.; Putter, H.; Kranenbarg, E.M.-K.; Sasako, M.; van de Velde, C.J. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010, 11, 439–449.
- Degiuli, M.; Sasako, M.; Ponti, A.; Calvo, F. Survival results of a multicentre phase II study to evaluate D2 gastrectomy for gastric cancer. Br. J. Cancer 2004, 90, 1727–1732.
- Hartgrink, H.; Van De Velde, C.; Putter, H.; Bonenkamp, J.; Kranenbarg, E.M.-K.; Songun, V.; Welvaart, K.; Van Krieken, J.; Meijer, S.; Plukker, J.; et al. Extended Lymph Node Dissection for Gastric Cancer: Who May Benefit? Final Results of the Randomized Dutch Gastric Cancer Group Trial. J. Clin. Oncol. 2004, 22, 2069–2077.
- Degiuli, M.; Reddavid, R.; Tomatis, M.; Ponti, A.; Morino, M.; Sasako, M.; Rebecchi, F.; Garino, M.; Vigano, L.; Scaglione, D.; et al. D2 dissection improves disease-specific survival in advanced gastric cancer patients: 15-year follow-up results of the Italian Gastric Cancer Study Group D1 versus D2 randomised controlled trial. Eur. J. Cancer 2021, 150, 10–22.
- Reddavid, R.; Sofia, S.; Chiaro, P.; Colli, F.; Trapani, R.; Esposito, L.; Solej, M.; Degiuli, M. Neoadjuvant chemotherapy for gastric cancer. Is it a must or a fake? World J. Gastroenterol. 2018, 24, 274–289.
- National Comprehensive Cancer Network Gastric Cancer (Version 3.2020). Available online: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf (accessed on 5 December 2020).
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v38–v49.
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2020, 24, 1–21.
- Atlass, A.J.; Thorsson, V.; Shmulevich, I.; Reynolds, S.M.; Miller, M.; Bernard, B.; HiNoue, T.; Laird, P.W.; Curtis, C.; Shen, H.; et al. The Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nat. Cell Biol. 2014, 513, 202–209.
- Bang, Y.-J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697.
- Jüttner, S.; Wiβmann, C.; Jöns, T.; Vieth, M.; Hertel, J.; Gretschel, S.; Schlag, P.M.; Kemmner, W.; Höcker, M. Vascular Endothelial Growth Factor-D and Its Receptor VEGFR-3: Two Novel Independent Prognostic Markers in Gastric Adenocarcinoma. J. Clin. Oncol. 2006, 24, 228–240.
- Corso, S.; Isella, C.; Bellomo, S.E.; Apicella, M.; Durando, S.; Migliore, C.; Ughetto, S.; D’Errico, L.; Menegon, S.; Rull, D.M.; et al. A Comprehensive PDX Gastric Cancer Collection Captures Cancer Cell–Intrinsic Transcriptional MSI Traits. Cancer Res. 2019, 79, 5884–5896.
- Reddavid, R.; Corso, S.; Moya-Rull, D.; Giordano, S.; Degiuli, M. Patient-Derived Orthotopic Xenograft models in gastric cancer: A systematic review. Updates Surg. 2020, 72, 951–966.
- Song, H.; Zhu, J.; Lu, D. Molecular-targeted first-line therapy for advanced gastric cancer. Cochrane Database Syst. Rev. 2016, 7, CD011461.
- Zhang, Z.; Tang, H.; Lin, J.; Hu, Y.; Luo, G.; Luo, Z.; Cheng, C.; Wang, P. Clinicopathologic and prognostic significance of human epidermal growth factor receptor in patients with gastric cancer: An updated meta-analysis. Oncotarget 2017, 8, 17202–17215.
- Navarini, D.; Gurski, R.R.; Madalosso, C.; Aita, L.; Meurer, L.; Fornari, F. Epidermal Growth Factor Receptor Expression in Esophageal Adenocarcinoma: Relationship with Tumor Stage and Survival after Esophagectomy. Gastroenterol. Res. Pract. 2012, 2012, 1–5.
- Hara, M.; Nakanishi, H.; Tsujimura, K.; Matsui, M.; Yatabe, Y.; Manabe, T.; Tatematsu, M. Interleukin-2 potentiation of cetuximab antitumor activity for epidermal growth factor receptor-overexpressing gastric cancer xenografts through antibody-dependent cellular cytotoxicity. Cancer Sci. 2008, 99, 1471–1478.
- Lordick, F.; Allum, W.; Carneiro, F.; Mitry, E.; Tabernero, J.; Tan, P.; Van Cutsem, E.; van de Velde, C.; Cervantes, A. Unmet needs and challenges in gastric cancer: The way forward. Cancer Treat. Rev. 2014, 40, 692–700.
- Rojo, F.; Tabernero, J.; Albanell, J.; Van Cutsem, E.; Ohtsu, A.; Doi, T.; Koizumi, W.; Shirao, K.; Takiuchi, H.; Cajal, S.R.; et al. Pharmacodynamic Studies of Gefitinib in Tumor Biopsy Specimens From Patients With Advanced Gastric Carcinoma. J. Clin. Oncol. 2006, 24, 4309–4316.
- Rodriguez, C.P.; Adelstein, D.J.; Rice, T.W.; Rybicki, L.A.; Videtic, G.M.M.; Saxton, J.P.; Murthy, S.C.; Mason, D.P.; Ives, D.I. A Phase II Study of Perioperative Concurrent Chemotherapy, Gefitinib, and Hyperfractionated Radiation Followed by Maintenance Gefitinib in Locoregionally Advanced Esophagus and Gastroesophageal Junction Cancer. J. Thorac. Oncol. 2010, 5, 229–235.
- Dragovich, T.; McCoy, S.; Fenoglio-Preiser, C.M.; Wang, J.; Benedetti, J.K.; Baker, A.F.; Hackett, C.B.; Urba, S.G.; Zaner, K.S.; Blanke, C.D.; et al. Phase II Trial of Erlotinib in Gastroesophageal Junction and Gastric Adenocarcinomas: SWOG 0127. J. Clin. Oncol. 2006, 24, 4922–4927.
- Maron, S.B.; Alpert, L.; Kwak, H.A.; Lomnicki, S.; Chase, L.; Xu, D.; O’Day, E.; Nagy, R.J.; Lanman, R.B.; Cecchi, F.; et al. Targeted Therapies for Targeted Populations: Anti-EGFR Treatment for EGFR-Amplified Gastroesophageal Adenocarcinoma. Cancer Discov. 2018, 8, 696–713.
- Corso, S.; Pietrantonio, F.; Apicella, M.; Migliore, C.; Conticelli, D.; Petrelli, A.; D’Errico, L.; Durando, S.; Moya-Rull, D.; Bellomo, S.E.; et al. Optimized EGFR Blockade Strategies in EGFR Addicted Gastroesophageal Adenocarcinomas. Clin. Cancer Res. 2021, 27, 3126–3140.
- Hynes, N.E.; Stern, D.F. The biology of erbB-2/nue/HER-2 and its role in cancer. BBA Rev. Cancer 1994, 1198, 165–184.
- Satoh, T.; Xu, R.-H.; Chung, H.; Sun, G.-P.; Doi, T.; Xu, J.-M.; Tsuji, A.; Omuro, Y.; Li, J.; Wang, J.-W.; et al. Lapatinib Plus Paclitaxel Versus Paclitaxel Alone in the Second-Line Treatment ofHER2-Amplified Advanced Gastric Cancer in Asian Populations: TyTAN—A Randomized, Phase III Study. J. Clin. Oncol. 2014, 32, 2039–2049.
- Hughes, J.B.; Berger, C.; Rødland, M.S.; Hasmann, M.; Stang, E.; Madshus, I.H. Pertuzumab increases epidermal growth factor receptor down-regulation by counteracting epidermal growth factor receptor-ErbB2 heterodimerization. Mol. Cancer Ther. 2009, 8, 1885–1892.
- Jung, Y.; Mansfield, P.; Akagi, M.; Takeda, A.; Liu, W.; Bucana, C.; Hicklin, D.; Ellis, L. Effects of combination anti-vascular endothelial growth factor receptor and anti-epidermal growth factor receptor therapies on the growth of gastric cancer in a nude mouse model. Eur. J. Cancer 2002, 38, 1133–1140.
- Maeda, K.; Chung, Y.-S.; Ogawa, Y.; Kang, S.-M.; Ogawa, M.; Sawada, T.; Sowa, M. Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer 1996, 77, 858–863.
- Presta, L.G.; Chen, H.; O’Connor, S.J.; Chisholm, V.; Meng, Y.G.; Krummen, L.; Winkler, M.; Ferrara, N. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997, 57, 4593–4599.
- Miller, K.; Wang, M.; Gralow, J.; Dickler, M.; Cobleigh, M.; Perez, E.A.; Shenkier, T.; Cella, D.; Davidson, N.E. Paclitaxel plus Bevacizumab versus Paclitaxel Alone for Metastatic Breast Cancer. N. Engl. J. Med. 2007, 357, 2666–2676.
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N. Engl. J. Med. 2004, 350, 2335–2342.
- Yang, J.C.; Haworth, L.; Sherry, R.M.; Hwu, P.; Schwartzentruber, D.J.; Topalian, S.L.; Steinberg, S.M.; Chen, H.X.; Rosenberg, S.A. A Randomized Trial of Bevacizumab, an Anti–Vascular Endothelial Growth Factor Antibody, for Metastatic Renal Cancer. N. Engl. J. Med. 2003, 349, 427–434.
- Ohtsu, A.; Shah, M.A.; Van Cutsem, E.; Rha, S.Y.; Sawaki, A.; Park, S.R.; Lim, H.Y.; Yamada, Y.; Wu, J.; Langer, B.; et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: A randomized, double-blind, placebo-controlled phase iii study. J. Clin. Oncol. 2011, 29, 3968–3976.
- Shah, M.A.; Jhawer, M.; Ilson, D.H.; Lefkowitz, R.A.; Robinson, E.; Capanu, M.; Kelsen, D.P. Phase II Study of modified docetaxel, cisplatin, and fluorouracil with bevacizumab in patients with metastatic gastroesophageal adenocarcinoma. J. Clin. Oncol. 2011, 29, 868–874.
- Bjornsti, M.-A.; Houghton, P.J. The tor pathway: A target for cancer therapy. Nat. Rev. Cancer 2004, 4, 335–348.
- Scagliotti, G.V.; Novello, S.; von Pawel, J. The emerging role of MET/HGF inhibitors in oncology. Cancer Treat. Rev. 2013, 39, 793–801.
- Graziano, F.; Galluccio, N.; Lorenzini, P.; Ruzzo, A.; Canestrari, E.; D’Emidio, S.; Catalano, V.; Sisti, V.; Ligorio, C.; Andreoni, F.; et al. Genetic Activation of the MET Pathway and Prognosis of Patients with High-Risk, Radically Resected Gastric Cancer. J. Clin. Oncol. 2011, 29, 4789–4795.
- Waddell, T.; Moorcraft, S.Y.; Cunningham, D. Potential role of rilotumumab in the treatment of gastric cancer. Immunotherapy 2014, 6, 1243–1253.
- Ughetto, S.; Migliore, C.; Pietrantonio, F.; Apicella, M.; Petrelli, A.; D’Errico, L.; Durando, S.; Moya-Rull, D.; Bellomo, S.E.; Rizzolio, S.; et al. Personalized therapeutic strategies in HER2-driven gastric cancer. Gastric Cancer 2021, 24, 897–912.
- Gomez-Martin, C.; Plaza, J.C.; Pazo-Cid, R.A.; Salud, A.; Pons, F.; Fonseca, P.; Leon, A.; Alsina, M.; Visa, L.; Rivera, F.; et al. Level of HER2 Gene Amplification Predicts Response and Overall Survival in HER2-Positive Advanced Gastric Cancer Treated With Trastuzumab. J. Clin. Oncol. 2013, 31, 4445–4452.
- Shah, M.A.; Bang, Y.-J.; Lordick, F.; Alsina, M.; Chen, M.; Hack, S.P.; Bruey, J.M.; Smith, D.; McCaffery, I.; Shames, D.S.; et al. Effect of Fluorouracil, Leucovorin, and Oxaliplatin with or Without Onartuzumab in HER2-Negative, MET-Positive Gastroesophageal Adenocarcinoma. JAMA Oncol. 2017, 3, 620–627.
- Shah, M.A.; Cho, J.-Y.; Tan, I.B.; Tebbutt, N.C.; Yen, C.-J.; Kang, A.; Shames, D.S.; Bu, L.; Kang, Y.-K. A Randomized Phase II Study of FOLFOX with or Without the MET Inhibitor Onartuzumab in Advanced Adenocarcinoma of the Stomach and Gastroesophageal Junction. Oncologist 2016, 21, 1085–1090.
- Catenacci, D.V.T.; Tebbutt, N.C.; Davidenko, I.; Murad, A.M.; Al-Batran, S.-E.; Ilson, D.H.; Tjulandin, S.; Gotovkin, E.; Karaszewska, B.; Bondarenko, I.; et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1467–1482.
- Li, J.; Qin, S.; Xu, J.; Xiong, J.; Wu, C.; Bai, Y.; Liu, W.; Tong, J.; Liu, Y.; Xu, R.; et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J. Clin. Oncol. 2016, 34, 1448–1454.
- Eatock, M.M.; Tebbutt, N.C.; Bampton, C.L.; Strickland, A.H.; Valladares-Ayerbes, M.; Swieboda-Sadlej, A.; Van Cutsem, E.; Nanayakkara, N.; Sun, Y.N.; Zhong, Z.D.; et al. Phase II randomized, double-blind, placebo-controlled study of AMG 386 (trebananib) in combination with cisplatin and capecitabine in patients with metastatic gastro-oesophageal cancer. Ann. Oncol. 2013, 24, 710–718.
- Iveson, T.; Donehower, R.C.; Davidenko, I.; Tjulandin, S.; Deptala, A.; Harrison, M.; Nirni, S.; Lakshmaiah, K.; Thomas, A.; Jiang, Y.; et al. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: An open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study. Lancet Oncol. 2014, 15, 1007–1018.
- Koizumi, W.; Yamaguchi, K.; Hosaka, H.; Takinishi, Y.; Nakayama, N.; Hara, T.; Muro, K.; Baba, H.; Sasaki, Y.; Nishina, T.; et al. Randomised phase II study of S-1/cisplatin plus TSU-68 vs S-1/cisplatin in patients with advanced gastric cancer. Br. J. Cancer 2013, 109, 2079–2086.
- Rao, S.; Starling, N.; Cunningham, D.; Sumpter, K.; Gilligan, D.; Ruhstaller, T.; Valladares-Ayerbes, M.; Wilke, H.; Archer, C.; Kurek, R.; et al. Matuzumab plus epirubicin, cisplatin and capecitabine (ECX) compared with epirubicin, cisplatin and capecitabine alone as first-line treatment in patients with advanced oesophago-gastric cancer: A randomised, multicentre open-label phase II study. Ann. Oncol. 2010, 21, 2213–2219.
- Shen, L.; Li, J.; Xu, J.; Pan, H.; Dai, G.; Qin, S.; Wang, L.; Wang, J.; Yang, Z.; Shu, Y.; et al. Bevacizumab plus capecitabine and cisplatin in Chinese patients with inoperable locally advanced or metastatic gastric or gastroesophageal junction cancer: Randomized, double-blind, phase III study (AVATAR study). Gastric Cancer 2014, 18, 168–176.
- Zhang, Z.D.; Kong, Y.; Yang, W.; Zhang, B.; Zhang, Y.L.; Ma, E.M.; Liu, H.X.; Chen, X.B.; Hua, Y.W. Clinical evaluation of cetuximab combined with an S-1 and oxaliplatin regimen for Chinese patients with advanced gastric cancer. World J. Surg. Oncol. 2014, 12, 115.
- Fuchs, C.S.; Shitara, K.; Di Bartolomeo, M.; Lonardi, S.; Al-Batran, S.-E.; Van Cutsem, E.; Ilson, D.H.; Alsina, M.; Chau, I.; Lacy, J.; et al. Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 420–435.
- Chen, H.-D.; Zhou, J.; Wen, F.; Zhang, P.-F.; Zhou, K.-X.; Zheng, H.-R.; Yang, Y.; Li, Q. Cost-effectiveness analysis of apatinib treatment for chemotherapy-refractory advanced gastric cancer. J. Cancer Res. Clin. Oncol. 2017, 143, 361–368.
- Bai, Y.; Xu, Y.; Wu, B. Cost-effectiveness and budget impact analysis of apatinib for advanced metastatic gastric cancer from the perspective of health insurance system. Gastroenterol. Res. Pract. 2017, 2017.
- Wilke, H.; Muro, K.; Van Cutsem, E.; Oh, S.-C.; Bodoky, G.; Shimada, Y.; Hironaka, S.; Sugimoto, N.; Lipatov, O.; Kim, T.-Y.; et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1224–1235.
- Li, S.; Peng, L.; Tan, C.; Zeng, X.; Wan, X.; Luo, X.; Yi, L.; Li, J. Cost-Effectiveness of ramucirumab plus paclitaxel as a second-line therapy for advanced gastric or gastro-oesophageal cancer in China. PLoS ONE 2020, 15, e0232240.
- Saito, S.; Muneoka, Y.; Ishikawa, T.; Akazawa, K. Cost-effectiveness of Paclitaxel + Ramucirumab Combination Therapy for Advanced Gastric Cancer Progressing After First-line Chemotherapy in Japan. Clin. Ther. 2017, 39, 2380–2388.
- Apicella, M.; Corso, S.; Giordano, S. Targeted therapies for gastric cancer: Failures and hopes from clinical trials. Oncotarget 2017, 8, 57654–57669.
- Liu, X.; Meltzer, S.J. Gastric Cancer in the Era of Precision Medicine. CMGH 2017, 3, 348–358.
- Kwak, E.L.; LoRusso, P.; Hamid, O.; Janku, F.; Kittaneh, M.; Catenacci, D.V.T.; Chan, E.; Bekaii-Saab, T.S.; Amore, B.; Hwang, Y.C.; et al. Clinical activity of AMG 337, an oral MET kinase inhibitor, in adult patients (pts) with MET-amplified gastroesophageal junction (GEJ), gastric (G), or esophageal (E) cancer. J. Clin. Oncol. 2015, 33, 1.
- Asioli, S.; Maletta, F.; Verdun Di Cantogno, L.; Satolli, M.A.; Schena, M.; Pecchioni, C.; Botta, C.; Chiusa, L.; Molinaro, L.; Conti, L.; et al. Approaching heterogeneity of human epidermal growth factor receptor 2 in surgical specimens of gastric cancer. Hum. Pathol. 2012, 43, 2070–2079.
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; Tarpey, P.; et al. Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. N. Engl. J. Med. 2012, 366, 883–892.
- Apicella, M.; Migliore, C.; Capelôa, T.; Menegon, S.; Cargnelutti, M.; Degiuli, M.; Sapino, A.; Sottile, A.; Sarotto, I.; Casorzo, L.; et al. Dual MET/EGFR therapy leads to complete response and resistance prevention in a MET-amplified gastroesophageal xenopatient cohort. Oncogene 2017, 36, 1200–1210.
- Smolen, G.A.; Sordella, R.; Muir, B.; Mohapatra, G.; Barmettler, A.; Archibald, H.; Kim, W.J.; Okimoto, R.A.; Bell, D.W.; Sgroi, D.C.; et al. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc. Natl. Acad. Sci. USA 2006, 103, 2316–2321.
- Corso, S.; Ghiso, E.; Cepero, V.; Sierra, J.R.; Migliore, C.; Bertotti, A.; Trusolino, L.; Comoglio, P.M.; Giordano, S. Activation of HER family members in gastric carcinoma cells mediates resistance to MET inhibition. Mol. Cancer 2010, 9, 121.
- Corso, S.; Comoglio, P.M.; Giordano, S. Cancer therapy: Can the challenge be MET? Trends Mol. Med. 2005, 11, 284–292.
- Hidalgo, M.; Amant, F.; Biankin, A.V.; Budinská, E.; Byrne, A.T.; Caldas, C.; Clarke, R.B.; de Jong, S.; Jonkers, J.; Mælandsmo, G.M.; et al. Patient-derived Xenograft models: An emerging platform for translational cancer research. Cancer Discov. 2014, 4, 998–1013.
