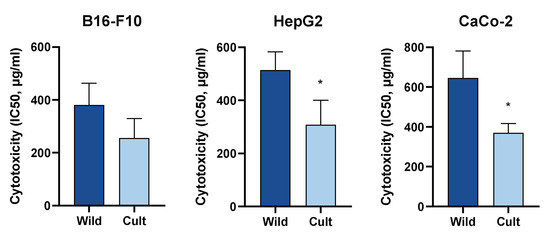
The effect of cultivation practises on both the phytochemical profile and biological activity of aqueous ethanol extracts of Chelidonium majus L. was studied. Extracts were prepared from aerial parts of the same plant population collected in the wild and grown under organic farming conditions. Both qualitative and quantitative analyses of alkaloids and flavonoid derivatives were performed by LC/MS methods, and the cytotoxicity of lyophilised extracts was studied in B16-F10, HepG2, and CaCo-2 cells. Coptisine was the dominant alkaloid of extracts prepared from wild-grown plants, whereas after cultivation, chelidonine was the most abundant alkaloid. The total alkaloid content was significantly increased by cultivation. Ten flavonol glycoconjugates were identified in C. majus extracts, and quantitative analysis did not reveal significant differences between extracts prepared from wild-grown and cultivated specimens. Treatment with C. majus extracts resulted in a dose-dependent increase in cytotoxicity in all three cell lines. The extracts prepared from cultivated specimens showed higher cytotoxicity than the extracts prepared from wild-grown plants. The strongest cytotoxic effect of cultivated C. majus was observed in B16-F10 cells (IC50 = 174.98 ± 1.12 µg/mL). Cultivation-induced differences in the phytochemical composition of C. majus extracts resulted in significant increases in the cytotoxic activities of the preparations.
1. Introduction
Greater celandine,
Chelidonium majus L. (
Papaveraceae Juss), is a valuable medicinal plant that is widely distributed throughout Europe, Asia, Northwest Africa, and North America
[1]. In Latvia, it is considered a native species occurring throughout the country from solitarily specimens to dense growths
[2]. In traditional medicine,
C. majus has been used to treat bile and liver disorders
[3]. Fresh latex from plants has been used externally for the treatment of warts, corns, fungal infections, eczema, and tumours of the skin
[4][5]. In Latvian folklore materials, fresh latex and tea made from
C. majus were reported to be used for treating diarrhoea, eye problems, and skin diseases such as lichen and warts
[6]. The treatment of ophthalmological problems and gastrointestinal and skin disorders are mentioned among many other ethnobotanical studies across Europe
[7][8][9][10]. The European Medicines Agency (EMA) has proposed two possible therapeutic indications in the monograph on
Chelidonii herba: for symptomatic relief of digestive disorders such as dyspepsia and flatulence (oral intake), as well as for treatment of warts, calluses, and corns (cutaneous use)
[11]. However, these indications were not supported due to a lack of information on clinical safety. From a research point of view, this plant is still very interesting because it is widely used in folk medicine, but it has not yet acquired the status of an officially approved and evidence-based herbal medicine.
This species is known to produce a broad range of secondary metabolites, ensuring its therapeutic properties. The main constituents of
C. majus responsible for biological properties are isoquinoline alkaloids such as chelidonine, chelerythrine, sanguinarine, coptisine, berberine, allocryptopine, and protopine
[1]. They are reported to have anti-inflammatory, antimicrobial, antibacterial, antiviral, immunomodulatory, anticancer, choleretic, hepatoprotective, and analgesic properties
[1][3].
C. majus alkaloids are a subject of interest due to their cytotoxic effects against various types of cancer cell lines
[12][13][14]. The well-known product Ukrain
®, a preparation consisting of a mixture of
C. majus alkaloids, is marketed for its anticancer properties. However, many previous clinical studies are considered untrustworthy
[15]. Most in vitro anticancer activity studies of
C. majus refer to sanguinarine, chelidonine, chelerythrine, and berberine. Sanguinarine, which interacts strongly with DNA, has been shown to be the most potent anticancer agent obtained from
C. majus. The IC50 values of sanguinarine in leukaemia cell lines are reported to be up to 0.10 µM
[12] and 0.2 µM in human keratinocyte (HaCaT) cell lines
[14]. Chelerythrine, berberine, and chelidonine are also active, but are less potent as cytostatic agents
[12].
Comprehensive reports on the alkaloid profile of
C. majus [16][17][18][19] are available. More than 50 alkaloids have been detected in greater celandine
[17][20]. Quantitative analyses of the main alkaloids chelerythrine, sanguinarine, and coptisine in
C. majus extracts were performed by HPLC-DAD and LC–MS/MS, and tentative identification of minor alkaloids was performed with data from the literature
[16][19][21][22][23]. In contrast, data on flavonoid composition and content in
C. majus are fragmented and mainly qualitative. Grosso et al.
[16] quantified the flavonoid content with HPLC-DAD for the first time, and MRM methods were used for the determination of quercetin and phenolic acids
[23].
Greater celandine, like other wild plants, shows interesting features with potential commercial viability. The market demand for biologically active ingredients from plants is increasing, and the cultivation of medicinal plants offers several benefits over collection of wild plants, e.g., reliable supply, standardised and improved production, and certainty of botanical identity. It is well known that the content of biologically active components of celandine is significantly affected by growing conditions
[1][24]. Therefore, it is important to assess the influence of cultivation practices (growing in the wild or under organic farming conditions) on the phytochemical composition of
C. majus populations.
2. Results and Discussion on Chelidonium majus L.
2.1. Alkaloid Profile and Quantitative Analysis of Aqueous Ethanol C. majus Extracts
LC/MS-TOF analyses of aqueous ethanol
C. majus extracts revealed the presence of 12 alkaloids. The identities of chelidonine, sanguinarine, and chelerythrine were confirmed with available reference standards. Tentative identification of other alkaloids was performed by comparison of their chromatographic retention times and detected m/z values with literature data
[19][25]. A summary of the identification results is shown in
Table 1.
Table 1. List of tentatively identified alkaloids in the ethanol extracts of aerial parts of C. majus.
18][24] have focused on the total phenolics content or total flavonoids content. Mass spectrometry was sporadically applied for the identification of individual compounds
[16].
To achieve nontarget identification of flavonoid derivatives, we screened the corresponding aglycone masses of quercetin, isorhamnetin, and kaempferol on an HRMS instrument and then performed MRM analyses on a tandem mass spectrometer (
Supplementary Materials Figure S1). The identified key flavonols quercetin, kaempferol, and isorhamnetin were found to be present in various glycosylated forms. In contrast to roots showing the presence of only quercetin aglycone
[23], in aerial parts we identified 10 mono-, di-, and triglycosides of flavonols, as shown in
Table 3.
Table 3. List of tentatively identified flavonoids in the ethanol extracts of aerial parts of C. majus.
| Peak # |
RT, min | Compound |
p |
Characteristic ions MW (Monoisotopic) |
Value | 1 ESICalculated Elemental Composition |
| + | , m/z |
Characteristic ions | 1 ESI−, m/z |
Compound |
MW (Monoisotopic) |
Calculated Elemental Composition 2 |
Parent Scan of Aglycone Fragment Ion, m/z |
Wild 2019 |
Cultivated 2020 |
|---|
| 18.02 |
354.134 |
Protopine 1 |
353.126 |
C20 |
| 1 |
11.2 | H | 19 | NO5 |
| Sanguinarine |
1.9 ± 2.1 |
12.8 ± 3.6 |
0.0004 |
627.144 |
|
Quercetin Triglycoside |
772.206 |
| 18.81 |
354.133 |
Chelidonine |
353.126 |
| Chelerythrine | C | 20 |
3.5 ± 1.3 | H19NO5 |
| 17.5 ± 8.5 |
0.007 |
), carbohydrate residues with three saccharide moieties were identified in extracts. Peak
2 showed a precursor ion at m/z 757 [M + H]
+ (C
33H
40O
20), and its MS/MS spectrum presented a product ion at m/z 611 attributed to the elimination of a glycosyl residue and a product ion at m/z 449 produced after loss of a rutinoside residue. Based on aglycone formation at m/z 287, this compound was tentatively identified as kaempferol 3-O-rhamnosylglycoside-7-O-glucoside
[30]. Similarly, quercetin 3-O-triglycoside consisting of one rhamnosidylglycoside and one glucoside (peak
1) and isorhamnetin 3-O-rhamnosylglycoside-7-O-glucoside (peak
3) were identified. The identification of peak
3 was based on the MRM parent scan, as HR full scan mass spectra did not yield protonated molecular ions. These three glycosides (peaks
1,
2, and
3) were formed from the same carbohydrates attached to the same positions of the three different flavonols and have not been previously reported in
C. majus.
Two peaks with molecular masses equivalent to that of kaempferol diglycoside were detected (peaks
6 and
7). Although both molecular and aglycone ions coincided, retention times were different. The later eluting peak (
7) was identified as kaempferol-3-rutinose by comparison with the reference compound.
Quantitative analyses of flavonoids showed the predominance of rutinoside-type glycoconjugates (
Table 4,
Supplementary Materials Table S2), similar to the findings of Grosso et al.
[16] and Parvu et al.
[29]. Quercetin 3-O-rutinoside (rutin) was the predominant compound in extracts prepared from both wild-grown and cultivated
C. majus specimens. The next most abundant flavonol glycosides were isorhamnetin 3-O-rutinoside and kaempferol 3-O-rutinoside. The total flavonol glycoside content was slightly higher in extracts prepared from cultivated
C. majus specimens; however, the major contribution was the increase in rutin content, and the changes were not statistically significant.
Table 4. Content of flavonoids (µg/g of dry material) in the ethanol extracts of aerial parts of wild-grown and cultivated C. majus.
| Compound |
Average Flavonoid Content (n = 5) |
p Value |
| |
Wild 2019 |
Cultivated 2020 |
|
| C | 33 | H | 40 | O | 21 |
773, 627,465 (303) | 3 |
| Kaempferol |
13.1 ± 9.2 |
6.9 ± 4.2 |
0.2 |
| 2 |
13.0 |
|
755.201 |
Kaempferol Triglycoside |
756.211 |
C33H40O20 |
| Isorhamnetin | 757, 611, 449 (287) |
19.00 |
| 8.8 ± 6.7 |
4.0 ± 1.6 |
0.2 |
370.163 |
Allocryptopine 1 |
3369.158 |
13.6C21H |
479.11923NO5 |
| |
Isorhamnetin Triglycoside |
786.222 |
C | 34 | H | 42O21 |
787, 641, 479 (317) |
19.09 |
320.092 |
| Quercitrin |
1.4 ± 1.2 |
2.0 ± 0.9 |
0.4 |
Coptisine 1 |
320.092 |
C19H14NO4+ |
| 4 |
16.8 |
611.159 |
609.143 |
Quercetin 3-O-Rutinoside |
610.153 |
C |
| Isorhamnetin 3-O-Rutinoside |
1612.7 ± 722.9 | 27 |
1857.2 ± 326.0 | H | 30 | O16 |
611, 465 (303) |
| 0.5 |
20.2 |
370.165 |
5Allocryptopine 1 |
17.0369.158 |
465.101 |
|
Quercetin 3-O-Galactoside |
464.100 |
C21H20O12 |
465 (303) |
| Kaempferol 3-O-Rutinoside |
653.8 ± 377.4 |
600.7 ± 216.4 |
0.8 |
20.56 |
340.153 |
Canadine 1 |
339.147 |
C20H21NO4 |
| 21.05 |
340.118 |
351.56 ± 1.38Norchelidonine 1 |
339.111 |
279.25 ± 1.08C19 |
361.41 ± 1.84H17NO5 |
| 21.28 |
332.091 |
Sanguinarine |
332.092 |
C20H |
| Chelidonine |
63.6 ± 35.4 |
252.2 ± 133.2 |
0.02 |
| Coptisine 1 |
138.5 ± 35.6 |
143.5 ± 32.2 |
0.8 |
| Berberine 1 | C | 21 | H | 23NO5 |
| 9.4 ± 6.6 |
12.8 ± 8.4 |
0.6 |
20.33 |
340.118 |
Norchelidonine 1 |
339.111 |
| Allocryptopine 1 |
6 |
| Quercetin 3-O-Rutinoside | 17.4 |
287.056 |
3007.2 ± 1270.1 |
4385.1 ± 1150.8C |
0.119H |
5.2 ± 3.017NO5 |
11.9 ± 7.4 |
0.1 |
| Total Content |
222.0 |
450.6 |
| Quercetin 3-O-Galactoside | 0.02 |
220.2 ± 269.9 |
195.9 ± 114.0 |
0.9 |
| Kaempferol Glucoside 1 |
135.9 ± 130.8 |
53.5 ± 12.9 |
0.2 |
| Total |
5653.1 |
7105.3 |
0.3 |