Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Vivi Li and Version 1 by Małgorzata Starowicz.
The evaluation of volatiles in food is an important aspect of food production. It gives knowledge about the quality of foods and their relationship to consumers’ choices. Alcohols, aldehydes, acids, esters, terpenes, pyrazines, and furans are the main chemical groups that are involved in aroma formation. They are products of food processing: thermal treatment, fermentation, storage, etc. Food aroma is a mixture of varied molecules. Because of this, the analysis of aroma composition can be challenging.
- aroma profile
- electronic nose
- food chemistry
- food aroma
- gas chromatography
- GCxGC
- mass spectrometry
- microextraction
- olfactometry
- volatolomics
1. Introduction
Aroma (odour) is an important aspect in designing/formulating novel food products [1]. Thereby, the characterization of aroma is the main goal in many studies. Aroma characterization can give data in the area of food products’ quality and their relationship to consumers’ choices [2]. The aroma evaluation is often used to identify key molecules accounting for the aroma characteristics of specific food products. Most aroma molecules are volatile organic compounds. These compounds come from, e.g., the ripening of plants, the development of oils or during natural processes such as fermentation [3][4][5][6], but also food contamination [7]. Moreover, the volatile compounds are likely to form during long exposure to high temperatures in the reaction of sugars with amino groups of either amino acids or peptides/proteins, which is called the Maillard reaction [8]. The Maillard-derived volatile components are responsible for the characteristic odour of bakery or roasted products, e.g., meat, nuts, and coffee. The listing of naturally occurring aroma chemicals is very broad, ranging from acids, alcohols, aldehydes, amines, esters and ketones, heterocyclic compounds. About 10k of diverse volatile chemicals have been recognized in foodstuffs plus beverages; e.g., coffee aroma is a mix of over 1k volatiles. Lots of these compounds are frequent in several varied food products. For example, methional is determined in almost every food sample in an olfactometry study. Aldehydes were detected as quality markers of edible oils during their storage at ambient temperature [9]. Additionally, Zhou et al. [10] determined aldehydes and alcohols as significant volatiles in oil quality changes during storage, and the formation is negatively correlated with poly-unsaturated fatty acids amount. As was described by Ahn et al. [11] in their study, turkey meat is also exposed to the oxidation process and as a result, off-flavour aldehydes could be formed. According to the authors’ recommendations, negative volatiles formation could be limited by the addition of vitamin E with antioxidant activity. The example of chemical components in charge of the odour of different foodstuffs is presented in Table 1. However, the contribution of the individual compound to the food aroma depends on several factors, like aroma description, adsorption to the food matrices, content in the food samples, the threshold of odour, vapour pressure, chemicals interactions, and co-reaction with other volatiles [12]. Certain volatile compounds do not contribute to food aroma, whereas others define the flavour of food products; for instance, benzaldehyde is a characteristic compound in the aroma of almonds, and citral is a defining aroma of lemons [13]. However, in most cases, the aroma depends on the contribution of a mix of volatile components from various chemical classes.
Table 1. The most frequent volatile compounds from different family groups, their characteristic aroma and their occurrence in food products.
| Family Group | Compounds Example | Aroma Description | Examples of Food Sample | References |
|---|---|---|---|---|
| alcohols | hexanol | bitter, floral | watermelon | [14][15] |
| terpenes | myrcene | earthy, fruity, and clove-like | lemongrass, clove, bay leaves, basil and thyme | [15] |
| α- and β-pinene | woody, green, pine-like 1 | black pepper | [16][17] | |
| limonene | citrus-like | pistachio, nutmeg, gin | [18][19][20][21] | |
| linalool | floral | saffron, oregano, basil | [22][23][24] | |
| aldehydes | hexanal | freshly cut grass | almond, chestnut peanut, walnut, lamb meat |
[10][25][26][27] |
| octanal | fruity | orange juice | [28] | |
| esters | ethyl octanoate | sweet, fruity, brandy-, apple-, apricot- and banana-like | fermented and dried fish; wine; Xiaoqu Liquor | [29][30] |
| ethyl hexanoate | floral, fruity, pineapple-like | alcoholic beverages | [30][31] | |
| furans | furan | sweet, woody, almond-like, baked bread | coffee | [32] |
| furfural | almond-like, sweet | cookies, bread | [33][34][35] | |
| 2,5-dimethyl-4-hydroxy-3(2H)-furanone (furaneol) | fruity, strawberry-like | kiwifruit, strawberry | [36][37] | |
| pyrazines | 2,5-dimethylpyrazine | roast, coffee-line, peanut-like 2 | peanut, cookies | [3][26][33] |
| ethylpyrazine | nutty, buttery, peanut | cookies, roasted beef | ||
| methylpyrazine | nutty, cocoa, roasted meat | cocoa, cookies | [33][38] | |
| acids | acetic acid | acidic 1 | hazelnut; bread sourdough | [35][39] |
The complexity of food aroma makes the preparation of the sample an essential stage prior to the final investigation. In a matter of technology, the certain detection and categorization of aroma components is a demanding part. Moreover, volatile molecules can naturally interact with the food matrix and also with other food components. The interactions can be classified into three groups. The first is synergism, the second compensation and the third one is masking. Following the definitions created by Mottram and Elmore [12]: “Synergism is defined as the interaction when two or more distinct substances produce a mutual scent which is stronger than those of individual components. Compensation is the case when one component counteracts another constituent. Masking is the combination of one pleasant odour with an unpleasant.” Therefore, as was mentioned before the challenging part, the proper preparation of food samples. Generally, the preparation of food samples prior to analysis could be divided into three main phases. These phases are named as follows: sample pretreatment, preparation and analysis by adequate apparatus [42][43].
2. Isolation and Concentration as a First Step to the Analysis of Volatile Components
2.1. SPME as a Method of Extraction
According to the description, the SPME technique is based on the divide of equilibrium of the analytes between the extracted matrix and extraction phase (in this case, fiber) [44]. The SPME fiber can be exposed directly to the media by direct immersion (DI) or in its head-space (HS) [42]. At the GC injection port, the analytes are thermally desorbed and then transferred to the chromatographic column using a carrier gas for their separation [45]. The universal schema of the SPME technique is shown in Figure 2. Concerning polarity of chemicals, different sorts of coatings might be implemented for extraction, such as polyacrylate coating (PA), carboxen (CAR), polydimethylsiloxane (PDMS), polydimethylsiloxane-divinylbenzene (PDMS/DVB), divinylbenzen-carboxen-polydimethylsiloxane (DVB/CAR/PDMS), or with another type of sorbents. The success of the SPME method might be connected with its timesaving feature and possible options for automatization, as well as non-solvents usage [46]. The positive aspect of the SPME usage is that it can cover multiple steps of analysis such as sampling, separation of compounds of interest from the other matrix compounds, transfer of analytes from outside to the laboratory, and transport of analytes to the instrument. Despite these advantages offered by SPME, Souza-Silva et al. [47] enumerated few limitations of SPME use in analysis of food, e.g., (1) a narrow amount of commercially available coatings, (2) the relatively low temperature to operating due to coatings’ poor thermal stability, (3) de-stability and probable swelling of the coating by organic solvents; and (4) the quiet short period of the coating physical usage.
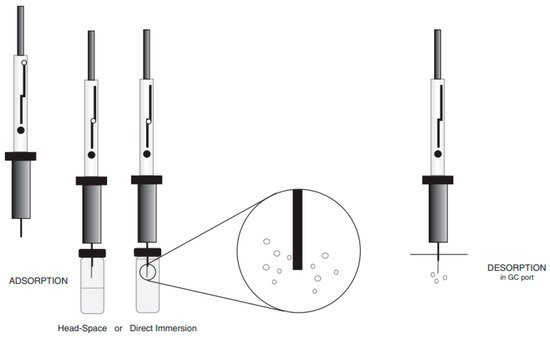
Figure 2. The scheme of the SPME technique.
The SPME was introduced into numerous applications in cannabis, environmental, forensic, and food analysis. As said by Jeleń et al. [48], the main five classes of food products that the SPME was implemented for aroma analysis are beverages, dairy, fruits/vegetables, honey, meat, seafood, and wine [49][50][51][52][53]. Usage of SPME in the determination of volatile chemicals extracted from various food samples is presented in Table 2. Furthermore, the main problems that were solved by using SPME are: studies of volatiles in different varieties of food products, profiling individual molecules reliable for food aroma, categorization of foodstuffs, also proof their authenticity, analysis of particular compounds responsible for food quality, to screen the technological process influences on aroma properties, screening chemical and biochemical processes related to transformations of aroma molecules, and to linked SPME with gas chromatography-olfactometry (GC-O) to describe the characteristic aroma of volatiles extracted by the coating of the fiber.
Over thirty years on the market, the SPME technology is still developing. Herrington et al. [54] presented the SPME arrow as a new concept of SPME device that can limit the low inter-device reproducibility, as well as a little volume of mobile phases. Vazquez et al. [55] suggested also miniaturization of the SPME device to make it portable and to allow sampling also not in a laboratory environment. Moreover, Starowicz et al. [56] demonstrated that volatile compounds analysis with SPME is a favourable tool to determine also the quality of functional food and designing the product for consumer’s needs. Xu et al. [9] found out SPME as an appropriate method to separate volatile components from the oil matrix and afterwards to determine compounds responsible for oil quality during storage. Furthermore, SPME coupled with GC/MS, and with other techniques of analysis, e.g., ATR-FTIR could be a promising possibility to prove the authenticity of food products [23]. Aceña et al. [57] admitted that in comparison to other extraction methods SPME is useful to determine more aroma-active regions of analyzed products whereas Berrou et al. [58] showed a high, 90% recovery of volatiles and semi-volatile using SPME technique. Berrou et al. [58], as well as Ruvalcaba et al. [59], compare SPME with another popular extraction method that is stir bar sorptive extraction, abbreviated as the SBSE method.
Table 2. Identification of volatiles in various food products using the SPME technique.
| Food Sample | Equilibration | Extraction | Identification | References | |||||
|---|---|---|---|---|---|---|---|---|---|
| Sample Amount [mL/g] | Agitation [yes/no] | Time [min] | Temperature [°C] | Time [min] | Temperature [°C] | ||||
| Coffee | Arabica and Robusta | 0.1 g | no | 10 | 40 | 20 | - | RI, mass spectra | [49] |
| Arabica and Robusta | 1.5 g | yes | 40 | 50 | 20 | 50 | semi-quant. with IS | [32] | |
| Arabica | 5.0 mL | yes | 1 | 25 | 15 | 25 | RI, mass spectra | [60] | |
| Alcoholic beverages | white wines | 20.0 mL | no | - | - | 15 | 50 | mass spectra | [46] |
| beer | |||||||||
| Baijiu | 10.0 mL | yes | 5 | 40 | 40 | 40 | RI, mass spectra | [20] | |
| Bee products | honey | 0.2 g | no | 2 | - | 30 | - | semi-quant. with IS | [55] |
| honey/beebread/bee pollen/beeswax | 2.0 g | yes | 50 | 40 | 15 | 50 | RI, mass spectra | [50] | |
| Nuts | hazelnuts | 1.5 g (grinding) | no | 20 | 50 | 20 | 50 | IS 1 | [39] |
| almond | 5.0 g (grinding) | no | 40 | 24 | 30 | 24 | semi-quant. with IS | [24] | |
| almond | 1.0 g (oil) | yes | 5 | 60 | 60 | 60 | retention times and mass spectra | [23] | |
| almond | 5.0 g | no | 40 | 24 | 30 | 24 | IS | [61] | |
| chestnut | 250.0 g | - | - | - | 24 | - | RI, mass spectra | [25] | |
| peanut | 3.0 g (grinding and oil extraction) | yes | 10 | 50 | 40 | 50 | semi-quant. with IS | [62] | |
| pistachio | 15.0 g (grinding) | yes | 15 | 50 | 120 | 50 | - 2 | [63] | |
| walnut | 0.5 g (grinding and oil extraction) | yes | 15 | 50 | 30 | 60 | semi-quant. with IS | [64] | |
| Meat | beef | 4 g (cooked) | no | - | - | 50 | 80 | RI, mass spectra | [20] |
| beef meatballs | 5 g | no | 20 | 60 | 40 | 60 | semi-quant. with IS | [51] | |
| fish miso | 3 g | - | - | - | 40 | 40 | RI, mass spectra, standards | [65] | |
| pork | 5 g | no | - | - | 30 | 60 | RI, mass spectra | [66] | |
| yellowfin tuna | 6 g | no | 15 | 70 | 40 | 70 | RI, mass spectra, IS | [67] | |
| Oil | peanut/soybean/rapeseed/linseed oil | 5 g | yes | 30 | 50 | 30 | 50 | RI, mass spectra | [9] |
1 IS: internal standard; 2 without quantification.
2.2. The SBSE Extraction
SBSE is supposed to be the second most mentioned technique of extraction in volatiles analysis. Same as SPME, SBSE is a green extraction technique that does not require the usage of organic solvent, but only a small amount of samples. It could be useful in the evaluation of aroma components of varied foodstuffs. SBSE has a more effective extraction capacity in comparison to the SPME method. The volume of coating used for SBSE is from 50 to 250 times higher than in SPME [68]. The magnetic bar of SBSE is mainly coated by PDMS fiber, whereas SBSE is dedicated to extracting semi- and volatile compounds in aqueous foods’ matrices. Thus, the stir bar is inserted into the medium directly, rotated and then molecules are captured by bars, and then desorbed by gas or liquid phase [8]. The other option developed by Bicchi et al. [69] is that the volatile compounds are adsorbed onto the PDMS stirring bar in the headspace of the sample for some time (headspace sorptive extraction—HSSE). The main advantage of using a coating bar in both extractions is the possibility to extract low polarity molecules. SBSE has a very low limit of detection (LoD) and this was the reason why it was applied to describe, for example, wine off-flavours [70]. The SBSE technique is also coupled with GC equipment, however, SBSE is a not-automated technique, due to that it is not common like extraction by SPME fiber.
Franc et al. [70] showed high reproducibility and repeatability, and a good correlation of SBSE in contrast to SPME and liquid-liquid extraction (LLE), in the wines’ off-flavours profiling. With SBSE, Franc et al. [70] successfully extracted and quantified eight domain volatiles, which is likewise highly responsible for the unpleasant aroma of wines for instance geosmin with characteristic muddy and musty odour. SBSE and HSSE were also exploited to develop the aroma composition of beer samples by Ruvalcaba et al. [59]. The SBSE was successfully validated to determine the volatiles profile of Sherry brandy [71], to established Spanish brandy authenticity. Moreover, this technique of extraction allowed researchers to determine safral as a main volatile compound in cured ham with saffron addition [22]. Moreover, Berrou et al. [58] optimized the method of SBSE to extract bacterial metabolites and established the 90% recovery of volatiles. Expect alcoholic beverages and meat, SBSE was similar to Guerrero et al. [72], evaluate to the determination of volatile components of vinegar samples with low detection limits. In another study, validation of SBSE with GC-MS allowed determining limonene as a major aroma compound in squeezed orange juices as well as commercial samples [73]. Usage of SBSE simultaneously with SPME could be important to determine the wide range of volatiles that could be aroma-active compounds [74].
3. Methods of Volatile Compounds Separation and Identification
The second important step of volatiles analysis is their separation and identification. SPME has been broadly employed in a sequence of several analytical techniques: capillary electrophoresis (CE), gas chromatography (GC), inductively coupled plasma MS (ICP-MS), ICP-optical emission spectrometry (ICP-OES), and mass spectrometry (MS) [75][76]. Not frequently liquid chromatography (LC) was applied to analyze volatile compounds [77]. Moreover, various detectors are applied, e.g., universal flame ionization (FID) or selective detectors, e.g., nitrogen-phosphorus (NPD), flame photometric (FPD), electron capture (ECD) detectors. However, the most frequently used detector in the analysis of volatile compounds is the mass detector (MS) [75]. MS is a powerful tool to identify compounds. By the MS it is possible to maintain screening of volatile compounds and then the comparison of obtained mass spectrums with the literature and/or available databases.
For example, Chin et al. [78] optimized the GC-MS technique to aroma evaluation of most popular beverages like wine and coffee. Additionally, Jordán et al. [79], who evaluated aroma profiles of food products, based on compounds’ molecular masses. In the latest research, Singh et al. [80] presented the usefulness of GC-MS in the determination of aroma-active regions of protein-based products. Research about the aroma of protein-rich products is nowadays an important aspect because a lot of vegan products are not always formulated with high consumer acceptability. Therefore, the knowledge in this area needs a lot of improvement. Moreover, based on GC-MS signals, Abou-el-Karam et al. [81] built the database of the volatile profile, which can be used in the food authenticity. Nicolaï et al. [82] reconstruct the aroma of citrus fruits using chemical standards and then analyzed its profile on GC-MS. Miniaturization is not only proposed in extraction practice but also as it was presented by Beck et al. [83] in the case of analytical devices. Beck et al. [83] constructed portable GC-MS equipment and compare their possibilities with benchtop devices. In their opinion usage of a portable GC-MS was satisfactory and allowed to analyze in any place. The time-consuming methods are replaced with the newer ones. Mayr et al. [84] employed Proton Transfer Reaction Mass Spectrometry to do fast screening of volatiles profile of meat from the market and therefore, avoid the spoilage of meat. Instead of a time-consuming method that needs a few days to be performed, they applied a methodology that requires only a few minutes.
The volatile profile is a complex mixture, which is why, often, the usage of GC-MS is not enough to unravel the aroma of food products. Therefore, a set of equipment is needed to recognize the active regions responsible for odour. Cialiè Rosso et al. [85] used two-dimensional GC to describe the potent odorants of hazelnuts, whereas Stilo et al. [86] prepared the quality fingerprint of virgin olive oil due to its volatiles profile. Interestingly, the GCxGC approach was established by Giri et al. [87] to evaluate the profile of aroma as well as compounds with toxicology properties. Besides the demand for advanced analytical equipment, Lubes and Goodarzi [61] highlighted the importance of the statistical analysis of obtained data. An important aspect is also the determination of food odour at the time of its consumption. In this case, the APCI-MS, named as Atmospheric-Pressure Chemical Ionization Mass Spectrometry, is often used. Elmasry et al. [88] applied APCI-MS to evaluate honey authenticity. Additionally, other techniques of analysis have been adopted to the determination of volatiles profile, e.g., ion mobility spectrometry [89], or infrared spectroscopy [90].
Several approaches have been adopted to establish volatile profiles in quantitative and semi-quantitative terms and, therefore, the results have been expressed in different ways. Sometimes authors expressed their results only as an integrated peak area, or as a per cent contribution of each compound in the total amount of volatiles. This method could be used only in the case of profiling the volatiles in food samples, to determine general differences between samples [50]. The proper study of volatiles content should be carried out using an internal standard, that might be useful in the calculation of the volatiles content in the semi-quantitative way [32]. Furthermore, the quantitative analysis could be performed after preparing the calibration curve of standards of each main volatile, as was carried out in the research of Starowicz, Koutsidis and Zieliński [33] and Cialiè Rosso et al. [85]. In both cases, either reference compounds (from the stock/synthetized in the laboratory) or stable labelled isotopes reference compounds (e.g., 2H, 13C, 15N) could be used. Moreover, if the food matrix plays an important role in aroma molecules release, Sgorbini et al. [91] propose multiple headspace extraction as the exhaustive extraction of an analyte from a sample by the HS-SPME method. It allows to sum up the peak areas of each compound after series of extraction to quantify its content.
4. Sensory Characterization
4.1. Olfactometry Study
It is well-known that food staples usually contain several hundreds of molecules responsible for their aroma. However, ~20–30 of these compounds are important to generate its aroma. Therefore, to perform an important analysis of aroma, it is important to recognize these compounds with a significant impact on the aroma. In this case, gas chromatography and olfactometry (GC-O) is the best selection [92]. The GC-O technique uses the human nose as a detector that elutes the aroma from the chromatography column. Further, when an odour is sensed, the time and characteristic aroma quality, and from time to time the intensity are written down. There are different methods to evaluate the relative odour potential of the individual aroma-active molecule, as aroma extraction dilution assay (AEDA), CharmAnalysis, Osme, and some others [93][94][95]. Interestingly, Feng et al. [96] combine AEDA with the SPME technique and achieved satisfactory results that allowed to determine the aroma with sensory impact in Japanese soy sauce. Afterwards, detection and the quantification of the odour-active molecules by analysis of GC-O, the odour activity value (OAV) (ratio between the concentration of one compound in the food and its threshold concentration) could be determined. Commonly, if one of the compounds shows an OAV value higher than 1, then this molecule might have an aroma impact on the overall odour of a food product. The presented procedure of analysis is called the molecular sensory concept. According to this procedure, Averbeck and Schieberle [28] pointed out the off-flavour formation with a significant amount of dimethyl sulphide and 2-methoxy-4-vinyl phenol in stored orange juice. Moreover, using this methodology allowed to differentiate the quality of market-available food products [97]. To proper identification of aroma, deuterated standards and/or molecules with isotopically labelled carbon could be used [98].
Mostly, results realized during the methodology for GC-O are associated with sensorial analysis. The recombination or omission analysis is the last part of the molecular sensory concept, during that test the aroma overall is judged by a professional sensory panel. This methodology allowed Kiefl and Schieberle [99] to differentiate three hazelnut cultivars, whereas Żołnierczyk and Szumny [100] established the basic composition of volatiles in edible insects, and compared it with their sensorial properties, and their possible acceptance by consumers. The chemical and sensory connection was also helpful in the elevation of new products [101]. In these cases, sensory panellists are the main “analytical tool”, who need to be specially trained.
4.2. Electonic Nose (E-Nose)
The electronic nose is not strictly used to prepare sensory characteristics of food products according to a definition it is a matter of sensory analysis with trained panellists. Therefore, it can be said that e-nose is an instrumental way to reveal some aroma discriminates of foods. An e-nose is a group of chemical sensors, associated with a pattern-recognition system that is responsible for odours transport through it. Various aromas affect different responses in the sensor system, and these reactions provide a signal pattern characteristic to a particular aroma [102]. The computer system recognizes the pattern of signals and then is able to compare the character of the aroma of varied foods’ extracts by pattern recognition system, e.g., artificial neural network. This artificial olfaction could be helpful in the characterization of hundreds of samples in a relatively short time [103]. For instance, a producer who uses coffees from around the world can analyze coffee samples by e-nose, and the coffees’ odours can be putting together on a multidimensional response map (Figure 3. The analysis of numerous coffee samples, using the best conditions, can result in a group of points for each sample, that furthermore can be grouped. As the number of samples increases, the differences among the groups ought to also grow. Mapping the sensor responses of an unknown sample on the same scheme could allow its detection, by its proximity to one of the known samples.

Figure 3. The analogy between the biological olfactory system and e-nose technology.
Electronic noses have been already applied in the food industry for quality control and detection of contaminants and off-odours. Wojnowski et al. [104] also described the advantages of portable electronic nose usage. However, the human nose is often more sensitive to odorous compounds than are instruments used to detect odours. The study of Lindinger et al. [105] about the aroma of espresso coffee presented a higher correlation of sensory panel results with aroma characteristics obtained from online proton-transfer-reaction mass spectrometry (PTR-MS). The important innovation was using a combination of HS-SPME with a mass spectrometer detector and to determine the aroma of in-cup coffee [106]. The proposed method allows to receive the fingerprint of volatiles in tested food material and predict the sensory quality of other coffee beans; however, the limitations are a high number of samples that need to be analyzed to prepare a proper model and, in the next step, the insufficient access to data mining program.
References
- Kaczmarska, K.; Taylor, M.; Piyasiri, U.; Frank, D. Flavor and Metabolite Profiles of Meat, Meat Substitutes, and Traditional Plant-Based High-Protein Food Products Available in Australia. Foods 2021, 10, 801.
- Bradford, K.D.; Desroches, D.M. The use of scents to influence consumers: The sense of using scents to make cents. J. Bus. Ethics 2009, 90, 141–153.
- Dun, Q.; Yao, L.; Deng, Z.; Li, H.; Li, J.; Fan, Y.; Zhang, B. Effects of Hot and Cold-Pressed Processes on Volatile Compounds of Peanut Oil and Corresponding Analysis of Characteristic Flavor Components. LWT Food Sci. Technol. 2019, 112, 107648.
- Genovese, A.; Caporaso, N.; Di Bari, V.; Yang, N.; Fisk, I. Effect of Olive Oil Phenolic Compounds on the Aroma Release and Persistence From O/W Emulsion Analysed In Vivo By APCI-MS. Food Res. Int. 2019, 126, 108686.
- Gan, H.H.; Yan, B.N.; Linforth, R.S.T.; Fisk, I.D. Development and Validation of an APCI-MS/GC-MS Approach for the Classification and Prediction of Cheddar Cheese Maturity. Food Chem. 2016, 190, 442–447.
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of Plant-Derived Flavor Compounds. Plant J. 2008, 54, 712–732.
- Pastorelli, S.; Valzacchi, S.; Rodriguez, A.; Simoneau, C. Solid-Phase Microextraction Method for the Determination of Hexanal in Hazelnuts as an Indicator of the Interaction of Active Packaging Materials with Food Aroma Compounds. Food Addit. Contam. 2006, 23, 1236–1241.
- Starowicz, M.; Zieliński, H. How Maillard Reaction Influences Sensorial Properties (Color, Flavor and Texture) Of Food Products? Food Rev. Int. 2019, 35, 707–725.
- Xu, L.; Yu, X.; Li, M.; Chen, J.; Wang, X. Monitoring Oxidative Stability and Changes in Key Volatile Compounds in Edible Oils during Ambient Storage through HS-SPME/GC–MS. Int. J. Food Prop. 2018, 20, S2926–S2938.
- Zhou, Y.; Fan, W.; Chu, F.; Wang, C.; Pei, D. Identification of volatile oxidation compounds as potential markers of walnut oil quality. J. Food Sci. 2018, 83, 2745–2752.
- Ahn, D.U.; Sell, J.L.; Jo, C.; Chen, X.; Wu, C.; Lee, J.I. Effects of dietary vitamin E supplementation on lipid oxidation and volatiles content of irradiated, cooked turkey meat patties with different packaging. Poult. Sci. 1998, 77, 912–920.
- Mottram, D.S.; Elmore, J.S. Sensory Evaluation: Aroma. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Trugo, L., Finglas, P.M., Eds.; Academic Press: London, UK, 2003; pp. 5174–5180.
- Fleming-Jones, M.E.; Smith, R.E. Volatile organic compounds in foods: A five year study. J. Agric. Food Chem. 2003, 51, 8120–8127.
- Yang, X.; Yang, F.; Liu, Y.; Li, J.; Song, H.-L. Identification of Key Off-Flavor Compounds in Thermally Treated Watermelon Juice via Gas Chromatography–Olfactometry–Mass Spectrometry, Aroma Recombination, and Omission Experiments. Foods 2020, 9, 227.
- Zachariah, T.J.; Leela, N.K. 11-Volatiles from herbs and spices. In Handbook of Herbs and Spices; Peter, K.V., Ed.; Woodhead Publishing: Cambridge, UK, 2006; Volume 3, pp. 177–218.
- Dosoky, N.S.; Satyal, P.; Barata, L.M.; Da Silva, J.K.R.; Setzer, W.N. Volatiles of Black Pepper Fruits (Piper nigrum L.). Molecules 2019, 24, 4244.
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738.
- Kendirci, P.; Onoǧur, T.A. Investigation of Volatile Compounds and Characterization of Flavor Profiles of Fresh Pistachio Nuts (Pistacia vera L.). Int. J. Food Prop. 2011, 14, 319–330.
- Georgiadou, M.; Gardeli, C.; Komaitis, M.; Tsitsigiannis, D.I.; Paplomatas, E.J.; Sotirakoglou, K.; Yanniotis, S. Volatile Profiles of Healthy and Aflatoxin Contaminated Pistachios. Food Res. Int. 2015, 74, 89–96.
- Wang, Z.; Sun, X.; Liu, Y.; Yang, H. Characterization of Key Aroma Compounds in Xiaoqu Liquor and Their Contributions to the Sensory Flavor. Beverages 2020, 6, 42.
- Buck, N.; Goblirsch, T.; Beauchamp, J.; Ortner, E. Key Aroma Compounds in Two Bavarian Gins. Appl. Sci. 2020, 10, 7269.
- Gómez-Sáez, E.M.; Moratalla-López, N.; Lorenzo, C.; Vergara, H.; Alonso, G.L. Determination of Saffron Volatiles by HS-SBSE-GC in Flavored Cured Ham. Molecules 2021, 26, 2073.
- Beltrán, A.; Ramos, M.; Grané, N.; Martín, M.L.; Garrigós, M.C. Monitoring the Oxidation of Almond Oils by HS-SPME–GC-MS and ATR-FTIR: Application of Volatile Compounds Determination to Cultivar Authenticity. Food Chem. 2011, 468, 126.
- Lee, J.; Xiao, L.; Zhang, G.; Ebeler, S.E.; Mitchell, A.E. Influence of Storage on Volatile Profiles in Roasted Almonds (Prunus dulcis). J. Agric. Food Chem. 2014, 62, 11236–11245.
- Krist, S.; Unterweger, H.; Bandion, F.; Buchbauer, G. Volatile Compound Analysis of SPME Headspace and Extract Samples from Roasted Italian Chestnuts (Castanea Sativa Mill.) Using GC-MS. Eur. Food Res. Technol. 2004, 219, 470–473.
- Lykomistros, D.; Fogliano, V.; Capuano, E. Drivers of preference and perception of freshness in roasted peanuts (Arachis spp.) for European consumers. J. Food Sci. 2018, 83, 1103–1115.
- Karabagias, I.K. Volatile Profile of Raw Lamb Meat Stored at 4 ± 1 °C: The Potential of Specific Aldehyde Ratios as Indicators of Lamb Meat Quality. Foods 2018, 7, 40.
- Averbeck, M.; Schieberle, P. Influence of different storage conditions on changes in the key aroma compounds of orange juice reconstituted from concentrate. Eur. Food Res. Technol. 2011, 232, 129–142.
- Nordvi, B.; Langsrud, Ø.; Egelandsdal, B.; Slinde, E.; Vogt, G.; Gutierrez, M.; Olsen, E. Characterization of volatile compounds in a fermented and dried fish product during cold storage. J. Food Sci. 2007, 72, S373–S380.
- Charry-Parra, G.; DeJesus-Echevarria, M.; Perez, F.J. Beer volatile analysis: Optimization of HS/SPME Coupled to GC/MS/FID. J. Food Sci. 2011, 76, C205–C211.
- Dumitriu, G.-D.; Teodosiu, C.; Gabur, I.; Cotea, V.V.; Peinado, R.A.; López de Lerma, N. Alternative Winemaking Techniques to Improve the Content of Phenolic and Aromatic Compounds in Wines. Agriculture 2021, 11, 233.
- Bressanello, D.; Liberto, E.; Cordero, C.; Rubiolo, P.; Pellegrino, G.; Ruosi, M.R.; Bicchi, C. Coffee aroma: Chemometric comparison of the chemical information provided by three different samplings combined with GC–MS to describe the sensory properties in cup. Food Chem. 2017, 214, 218–226.
- Starowicz, M.; Koutsidis, G.; Zieliński, H. Determination of antioxidant capacity, phenolics and volatile Maillard reaction products in rye-buckwheat biscuits supplemented with 3β-d-rutinoside. Molecules 2019, 24, 982.
- Ozolina, V.; Kunkulberga, D.; Cieslak, B.; Obiedzinski, M. Furan derivatives dynamic in rye bread processing. Procedia Food Sci. 2011, 1158–1164.
- Xu, D.; Zhang, H.; Xi, J.; Jin, Y.; Chen, Y.; Guo, L.; Jin, Z.; Xu, X. Improving bread aroma using low-temperature sourdough fermentation. Food Biosci. 2020, 37, 100704.
- Garcia, C.V.; Quek, S.-Y.; Stevenson, R.J.; Winz, R.A. Characterization of the Bound Volatile Extract from Baby Kiwi (Actinidia arguta). J. Agric. Food Chem. 2011, 59, 8358–8365.
- Ulrich, D.; Kecke, S.; Olbricht, K. What Do We Know about the Chemistry of Strawberry Aroma? J. Agric. Food Chem. 2018, 66, 3291–3301.
- Frauendorfer, F.; Schieberle, P. Identification of the Key Aroma Compounds in Cocoa Powder Based on Molecular Sensory Correlations. J. Agric. Food Chem. 2006, 54, 5521–5529.
- Nicolotti, L.; Cordero, C.; Bicchi, C.; Rubiolo, P.; Sgorbini, B.; Liberto, E. Volatile Profiling of High Quality Hazelnuts (Corylus Avellana L.): Chemical Indices of Roasting. Food Chem. 2013, 138, 1723–1733.
- Available online: www.pherobase.com (accessed on 5 July 2021).
- Available online: http://www.thegoodscentscompany.com (accessed on 5 July 2021).
- Starowicz, M. Food and Nutritional Analysis|Sample Preparation. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 3, pp. 465–470.
- Mondello, L.; Dugo, P.; Costa, R. Advances in Sample Preparation; LCGC: Palermo, Italy, 2012.
- Roberts, D.D.; Pollien, P.; Milo, C. Solid-phase microextraction method development for headspace analysis of volatile flavor compounds. J. Agric. Food Chem. 2000, 48, 2430–2437.
- Pawliszyn, J. Solid Phase Microextraction–Theory and Practice; Wiley: New York, NY, USA, 1997.
- Jeleń, H.; Szczurek, A. Solid phase microextraction for profiling volatile compounds in liquered white wines. Acta Sci. Pol. Technol. Aliment. 2010, 9, 23–32.
- Souza-Silva, É.A.; Gionfriddo, E.; Pawliszyn, J. A critical review of the state of the art of solid-phase microextraction of complex matrices II. Food Analysis. Trends Anal. Chem. 2015, 71, 236–248.
- Jelen, H.H.; Majcher, M.; Dziadas, M. Microextraction techniques in the analysis of food flavor compounds: A review. Anal. Chim. Acta 2012, 738, 13–26.
- Caporaso, N.; Whitworth, M.B.; Cui, C.; Fisk, I.D. Variability of single bean coffee volatile compounds of Arabica and Robusta roasted coffees analysed by SPME-GC-MS. Food Res. Int. 2018, 108, 628–640.
- Starowicz, M.; Hanus, P.; Lamparski, G.; Sawicki, T. Characterizing the volatile and sensory profiles, and sugar content of beeswax, beebread, bee pollen, and honey. Molecules 2021, 26, 3410.
- Sun, Y.; Zhang, Y.; Song, H. Variation of aroma components during frozen storage of cooked beef balls by SPME and SAFE coupled with GC-O-MS. J. Food Process. Preserv. 2020, 45, e15036.
- Chen, J.; Tao, L.; Zhang, T.; Zhang, J.; Wu, T.; Luan, D.; Ni, L.; Wang, X.; Zhong, J. Effect of four types of thermal processing methods on the aroma profiles of acidity regulator-treated tilapia muscles using E-nose, HS-SPME-GC-MS, and HS-GC-IMS. LWT Food Sci. Technol. 2021, 147, 111585.
- Moran, L.; Aldai, N.; Barron, L.J.R. Elucidating the combined effect of sample preparation and solid-phase microextraction conditions on the volatile composition of cooked meat analyzed by capillary gas chromatography coupled with mass spectrometry. Food Chem. 2021, 352, 129380.
- Herrington, J.S.; Gómez-Ríos, G.A.; Myers, C.; Stidsen, G.; Bell, D.S. Hunting molecules in complex matrices with SPME arrows: A review. Separations 2020, 7, 12.
- Vazquez, L.; Celeiro, M.; Sergazina, M.; Dagnac, T.; Llompart, M. Optimization of a miniaturized solid-phase microextraction method followed by gas chromatography mass spectrometry for the determination of twenty four volatile and semivolatile compounds in honey from Galicia (NW Spain) and foreign countries. Sustain. Chem. Pharm. 2021, 21, 100451.
- Starowicz, M.; Lelujka, E.; Ciska, E.; Lamparski, G.; Sawicki, T.; Wronkowska, M. The application of Lamiaceae Lindl. promotes aroma compounds formation, sensory properties, and antioxidant activity of oat and buckwheat-based cookies. Molecules 2020, 25, 5626.
- Aceña, L.; Vera, L.; Guasch, J.; Busto, O.; Mestres, M. Comparative study of two extraction techniques to obtain representative aroma extracts for being analysed by gas chromatography-olfactometry: Application to roasted pistachio aroma. J. Chromatogr. A 2010, 1217, 7781–7787.
- Berrou, K.; Dunyach-Remy, C.; Lavigne, J.-P.; Roig, B.; Cadiere, A. Comparison of stir bar sorptive extraction and solid phase microextraction of volatile and semi-volatile metabolite profile of Staphylococcus aureus. Molecules 2020, 25, 55.
- Ruvalcaba, J.E.; Durán-Guerrero, E.; Barroso, C.G.; Castro, R. Development of head space sorptive extraction method for the determination of volatile compounds in beer and comparison with stir bar sorptive extraction. Foods 2020, 9, 255.
- Zapata, J.; Londoño, V.; Naranjo, M.; Osorio, J.; Lopez, C.; Quintero, M. Characterization of aroma compounds present in an. industrial recovery concentrate of coffee flavor. CyTA-J. Food 2018, 16, 367–372.
- Xiao, L.; Lee, J.; Zhang, G.; Ebeler, S.E.; Wickramasinghe, N.; Seiber, J.; Mitchell, A.E. HS-SPME GC/MS Characterization of Volatiles in Raw and Dry-Roasted Almonds (Prunus dulcis). Food Chem. 2014, 151, 31–39.
- Liu, X.J.; Jin, Q.Z.; Liu, Y.F.; Huang, J.H.; Wang, X.G.; Mao, W.Y.; Wang, S.S. Changes in volatile compounds of peanut oil during the roasting process for production of aromatic roasted peanut oil. J. Food Sci. 2011, 76, 404–412.
- Lubes, G.; Goodarzi, M. Analysis of volatile compounds by advanced analytical techniques and multivariate chemometrics. Chem. Rev. 2017, 117, 6399–6422.
- Lee, J.; Vázquez-Araújo, L.; Adhikari, K.; Warmund, M.; Elmore, J. Volatile Compounds in Light, Medium, and Dark Black Walnut and Their Influence on the Sensory Aromatic Profile. J. Food Sci. 2011, 76, C199–C204.
- Giri, A.; Osako, K.; Ohshima, T. SPME technique for analyzing headspace volatiles in fish miso, a Japanese fish meat-based fermented product. Biosci. Biotechnol. Biochem. 2010, 74, 1770–1776.
- Da, D.; Nian, Y.; Shi, J.; Li, Y.; Zhao, D.; Zhang, G.; Li, C. Characterization of specific volatile components in braised pork with different tastes by SPME-GC/MS and electronic nose. J. Food Process. Preserv. 2021, 45, e15492.
- Zhang, Y.; Ma, X.; Dai, Z. Comparison of nonvolatile and volatile compounds in raw, cooked, and canned yellowfin tuna (Thunnus albacores). J. Food Process. Preserv. 2019, 43, e14111.
- Vállez-Gomis, V.; Grau, J.; Benedé, J.L.; Giokas, D.L.; Chisvert, A.; Salvador, A. Fundamentals and applications of stir bar sorptive dispersive microextraction: A tutorial review. Anal. Chim. Acta 2021, 1153, 338271.
- Bicchi, C.; Cordero, C.; Iori, C.; Rubiolo, P.; Sandra, P. Headspace Sorptive Extraction (HSSE) in the headspace analysis of aromatic and medicinal plants. HRC J. High Resolut. Chromatogr. 2000, 23, 539–546.
- Franc, C.; David, F.; de Revel, G. Multi-residue off-flavour profiling in wine using stir bar sorptive extraction–thermal desorption–Gas Chromatography–Mass Spectrometry. J. Chromatogr. A 2009, 1216, 3318–3327.
- Delgado, R.; Durán, E.; Castro, R.; Natera, R.; Barroso, C.G. Development of a stir bar sorptive extraction method coupled to gas chromatography-mass spectrometry for the analysis of volatile compounds in Sherry brandy. Anal. Chim. Acta 2010, 672, 130–136.
- Guerrero, E.D.; Marín, R.N.; Mejías, R.C.; Barroso, C.G. Optimisation of stir bar sorptive extraction applied to the determination of volatile compounds in vinegars. J. Chromatogr. A 2006, 1104, 47–53.
- Herrera, C.; Castro, R.; García-Barroso, C.; Durán-Guerrero, E. Development of a stir bar sorptive extraction method for the determination of volatile compounds in orange juices. J. Sep. Sci. 2016, 39, 3586–3593.
- Wang, L.; Gao, M.; Liu, Z.; Chen, S.; Xu, Y. Three Extraction Methods in Combination with GC×GC-TOFMS for the Detailed Investigation of Volatiles in Chinese Herbaceous Aroma-Type Baijiu. Molecules 2020, 25, 4429.
- Rodriguez-Campos, J.; Escalona-Buendía, H.B.; Orozco-Avila, I.; Lugo-Cervantes, E.; Jaramillo-Flores, M.E. Dynamics of volatile and non-volatile compounds in cocoa (Theobroma cacao L.) during fermentation and drying processes using principal components analysis. Food Res. Int. 2011, 44, 250–258.
- Vas, G.; Vékey, K. Solid-Phase Microextraction: A Powerful Sample Preparation Tool Prior to Mass Spectrometric Analysis. J. Mass Spectrom. 2004, 39, 233–254.
- Zambonin, C.; Aresta, A. Recent Applications of Solid Phase Microextraction Coupled to Liquid Chromatography. Separations 2021, 8, 34.
- Chin, S.T.; Eyres, G.T.; Marriott, P.J. Application of integrated comprehensive/multidimensional gas chromatography with mass spectrometry and olfactometry for aroma analysis in wine and coffee. Food Chem. 2015, 185, 355–361.
- Jordán, M.J.; Margaría, C.A.; Shaw, P.E.; Goodner, K.L. Aroma active components in aqueous kiwi fruit essence and kiwi fruit puree by GC-MS and multidimensional GC/GC-O. J. Agric. Food Chem. 2002, 50, 5386–5390.
- Singh, A.; Shi, Y.; Magreault, P.; Kitts, D.D.; Jarzebski, M.; Siejak, P.; Pratap-Singh, A. A rapid gas-chromatography/mass-spectrometry technique for determining odour activity values of volatile compounds in plant proteins: Soy, and allergen-free pea and brown rice protein. Molecules 2021, 26, 4104.
- Abou-el-Karam, S.; Ratel, J.; Kondjoyan, N.; Truan, C.; Engel, E. Marker discovery in volatolomics based on systematic alignment of GC-MS signals: Application to food authentication. Anal. Chim. Acta 2017, 991, 58–67.
- Nicolaï, B.M.; Micholt, E.; Scheerlinck, N.; Vandendriessche, T.; Hertog, M.L.A.T.M.; Ferguson, I.; Lammertyn, J. Real time aroma reconstruction using odour primaries. Sens. Actuators B Chem. 2016, 227, 561–572.
- Beck, J.J.; Willett, D.S.; Gee, W.S.; Mahoney, N.E.; Higbee, B.S. Differentiation of Volatile Profiles from Stockpiled Almonds at Varying Relative Humidity Levels Using Benchtop and Portable GC-MS. J. Agric. Food Chem. 2016, 64, 9286–9292.
- Mayr, D.; Margesin, R.; Klingsbichel, E.; Hartungen, E.; Jenewein, D.; Schinner, F.; Märk, T.D. Rapid Detection of Meat Spoilage by Measuring Volatile Organic Compounds by Using Proton Transfer Reaction Mass Spectrometry. Appl. Environ. Microbiol. 2003, 69, 4697–4705.
- Cialiè Rosso, M.; Liberto, E.; Spigolon, N.; Fontana, M.; Somenzi, M.; Bicchi, C.; Cordero, C. Evolution of Potent Odorants within the Volatile Metabolome of High-Quality Hazelnuts (Corylus Avellana L.): Evaluation by Comprehensive Two-Dimensional Gas Chromatography Coupled with Mass Spectrometry. Anal. Bioanal. Chem. 2018, 410, 3491–3506.
- Stilo, F.; Cordero, C.; Sgorbini, B.; Bicchi, C.; Liberto, E. Highly Informative Fingerprinting of Extra-Virgin Olive Oil Volatiles: The Role of High Concentration-Capacity Sampling in Combination with Comprehensive Two-Dimensional Gas Chromatography. Separations 2019, 6, 34.
- Giri, A.; Khummueng, W.; Mercier, F.; Kondjoyan, N.; Tournayre, P.; Meurillon, M.; Ratel, J.; Engel, E. Relevance of two-dimensional gas chromatography and high resolution olfactometry for the parallel determination of heat-induced toxicants and odorants in cooked food. J. Chromatogr. A 2015, 1388, 217–226.
- Elmasry, G.; Morsy, N.; Al-Rejaie, S.; Ayed, C.; Linforth, R.; Fisk, I.D. Real-Time Quality Authentication of Honey Using Atmospheric-Pressure Chemical Ionization Mass Spectrometry (APCI-MS). Int. J. Food Sci. Technol. 2019, 54, 2983–2997.
- Rodríguez-Maecker, R.; Vyhmeister, E.; Meisen, S.; Rosales Martinez, A.; Kuklya, A.; Telgheder, U. Identification of terpenes and essential oils by means of static headspace gas chromatography-ion mobility spectrometry. Anal. Bioanal. Chem. 2017, 409, 6595–6603.
- Jiao, L.; Guo, Y.; Chen, J.; Zhao, X.; Dong, D. Detecting volatile compounds in food by open-path Fourier-transform infrared spectroscopy. Food Res. Int. 2019, 119, 968–973.
- Sgorbini, B.; Cagliero, C.; Liberto, E.; Rubiolo, P.; Bicchi, C.; Cordero, C. Strategies for Accurate Quantitation of Volatiles from Foods and Plant-Origin Materials: A Challenging Task. J. Agric. Food Chem. 2019, 67, 1619–1630.
- Delahunty, C.M.; Eyres, G.; Dufour, J.P. Gas chromatography-olfactometry. J. Sep. Sci. 2006, 29, 2107–2125.
- Schieberle, P.; Grosch, W. Evaluation of the flavour of wheat and rye bread crusts by aroma extract dilution analysis. Z. Lebensm.-Unters. Forsch. 1987, 185, 111–113.
- Acree, T.; Barnard, J.; Cunningham, D.G. A Procedure for the Sensory Analysis of Gas Chromatographic Effluents. Food Chem. 1984, 14, 273–286.
- Miranda-López, R.; Libbey, L.M.; Watson, B.T.; McDaniel, M.R. Identification of Additional Odor-Active Compounds in Pinot noir Wines. Am. J. Enol. Vitic. 1992, 43, 90–92.
- Feng, Y.; Cai, Y.; Sun-Waterhouse, D.; Cui, C.; Su, G.; Lin, L.; Zhao, M. Approaches of aroma extraction dilution analysis (AEDA) for headspace solid phase microextraction and gas chromatography–olfactometry (HS-SPME–GC–O): Altering sample amount, diluting the sample or adjusting split ratio? Food Chem. 2015, 187, 44–52.
- Franitza, L.; Granvogl, M.; Schieberle, P. Characterization of the Key Aroma Compounds in Two Commercial Rums by Means of the Sensomics Approach. J. Agric. Food Chem. 2015, 64, 637–645.
- Alim, A.; Song, H.; Liu, Y.; Zou, T.; Zhang, Y.; Zhang, S.; Raza, A. Research of beef-meaty aroma compounds from yeast extract using carbon module labeling (CAMOLA) technique. LWT Food Sci. Technol. 2019, 112, 108239.
- Kiefl, J.; Schieberle, P. Evaluation of Process Parameters Governing the Aroma Generation in Three Hazelnut Cultivars (Corylus avellana L.) by Correlating Quantitative Key Odorant Profiling with Sensory Evaluation. J. Agric. Food Chem. 2013, 61, 5236–5244.
- Żołnierczyk, A.K.; Szumny, A. Sensory and Chemical Characteristic of Two Insect Species: Tenebrio molitor and Zophobas morio Larvae Affected by Roasting Processes. Molecules 2021, 26, 2697.
- Faccia, M.; Gambacorta, G.; Martemucci, G.; Difonzo, G.; D’Alessandro, A.G. Chemical-Sensory Traits of Fresh Cheese Made by Enzymatic Coagulation of Donkey Milk. Foods 2020, 9, 16.
- Karakaya, D.; Ulucan, O.; Turkan, M. Electronic Nose and Its Applications: A Survey. Int. J. Comput. Methods 2020, 17, 179–209.
- Di Pizio, A.; Behr, J.; Krautwurst, D. Toward the Digitalization of Olfaction. In The Senses: A Comprehensive Reference, 2nd ed.; Fritzsch, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 758–768.
- Wojnowski, W.; Majchrzak, T.; Dymerski, T.; Gebicki, J.; Namiesnik, J. Portable electronic nose based on electro-chemical sensors for food quality assessment. Sensors 2017, 17, 2715.
- Lindinger, C.; Labbe, D.; Pollien, P.; Rytz, A.; Juillerat, M.A.; Yeretzian, C.; Blank, I. When machine tastes coffee: Instrumental approach to predict the sensory profile of espresso coffee. Anal. Chem. 2008, 80, 1574–1581.
- Liberto, E.; Bressanello, D.; Strocchi, G.; Cordero, C.; Ruosi, M.R.; Pellegrino, G.; Bicchi, C.; Sgorbini, B. HS-SPME-MS-enose coupled with chemometrics as an analytical decision maker to predict in-cup coffee sensory quality in routine controls: Possibilities and limits. Molecules 2019, 24, 4515.
More
