HMF, an indispensable member of the furan-based platform compound, known as the “sleeping giant”, is a bridge between renewable biomass and industrial bulk chemicals. In recent years, the catalytic transformation of biomass to HMF has been widely studied and envisaged to be hopeful in achieving sustainable biorefineries. The synthesis of HMF from biomass requires the acid hydrolysis of biomass to hexose, and then dehydration of hexose, to obtain HMF. In the second step of dehydration, starting from ketohexose (fructose) is more efficient than starting from aldohexose (glucose).
- biomass
- 2
- 5-furandicarboxylic acid
- 5-hydroxymethylfurfural
- biocatalysis
1. Synthesis of HMF from Fructose
HMF is mainly converted from natural cellulose, glucose, or fructose. Among these processes, the most studied and generally the easiest is HMF from fructose. When the dehydration reaction starts from ketohexoses (such as fructose), it is more selective and efficient [1]. Although from a stoichiometric point of view, the reaction looks quite simple, that is, the formal elimination of three water molecules from fructose, the design of catalysts that achieve high-yield HMF production is made difficult by the lack of understanding of the mechanistic aspects of fructose conversion to HMF [2][3].
Solid acid catalysts are very effective for the dehydration of fructose to HMF. Hou et al. investigated an efficient reaction system using sulfonated graphene oxide (SGO) as a solid acid catalyst for the production of HMF from fructose, where the HMF yield could reach 94% [4]. Songo et al. investigated the TiO
2
2C as the catalyst, dimethyl sulfoxide (DMSO) as the solvent, and a reaction temperature of 120 °C, the catalyst yield can reach as high as 90% after one hour of catalysis [5]. Wang et al., using a sulfonated carbonaceous material as a solid acid catalyst for the dehydration of fructose to HMF in DMSO, achieved 96.1% conversion of fructose, and an HMF yield of 93.4% [6]. Moreover, several mineral acids such as H
2
4
3
4 have been used in the catalyzed dehydration of fructose to yield HMF [7]. Ava-Biochem utilized the simple thermal route, heating fructose in the presence of aqueous H
2
4 to produce HMF, although low yields were obtained [8]. Thus far, the selectivity and yield of reactions implemented in aqueous reaction systems are not comparable to those observed in aprotic high-boiling organic solvents such as DMSO, where the solvent also acts as the catalyst. Zhao et al. developed a sulfonated carbon sphere solid acid catalyst that produced 90% of HMF in DMSO as a solvent for 1.5 h at 160 °C with 100% conversion of fructose [9]. Guo et al. investigated a lignin-derived carbonaceous catalyst to convert fructose into HMF under microwave irradiation in a mixture of ionic liquid and DMSO at 110 °C for 10 min with 98% conversion of fructose and an HMF yield of 84% [10]. Hu et al. explored the use of a magnetic lignin-derived carbonaceous acid catalyst for the catalytic conversion of 100% fructose into 81.1% HMF in DMSO as a solvent [11]. Ionic liquids (ILs) are also used as solvents, which are expensive, and it is very troublesome to recover HMF from high-boiling IL systems. Water formed during the dehydration reaction often deactivates ILs, and high-boiling point solvents such as DMF and DMSO are unable to resolve the drawbacks related to the separation issues over different bifunctional acid catalysts [12].
Marullo et al. studied the dehydration of sucrose and fructose to HMF in the presence of Amberlyst 15, using eight deep eutectic solvents (DESs) as solvent media, differing by their hydrogen bond acceptors (HBAs) and hydrogen bond donors (HBDs). By optimizing the reaction conditions, they obtained a yield of HMF of 78% at 60 °C and 69% at 80 °C for fructose [3].
To further explore the novel and cost-efficient technologies for selective dehydration of fructose to HMF, photocatalysis is a promising candidate for inhibiting the side reactions at high temperature [13]. Fu et al. reported that Brønsted acidic Ag
@
2
4 NWs exhibit excellent photo-Fenton catalytic activity for the dehydration of fructose to HMF under visible light irradiation [14].
2. Dehydration of Glucose to HMF
Glucose is the desirable source of HMF, attributed to the lower cost compared to fructose [1]. Glucose is one of the richest monosaccharides in biomass, accessible by chemical or enzymatic hydrolysis from cellulose, starch, or sugar. Moreover, a variety of chemical products can be achieved from glucose which positions it in a critical position as a basic raw material/building block. Aldohexoses (such as glucose) can only enolize to a low degree, which is considered the limiting step in the production of HMF from glucose [1].
Up to now, most studies regarding the acid-catalyzed conversion of fructose and, to lesser extent, glucose to HMF carried out their investigations in aqueous reaction media [1]. Imidazolium-based ILs represent the most extensively studied ones for the dehydration of glucose to HMF. Zhao et al. reported that CrCl
3−
3− was to affect a formal hydride transfer by forming hydrogen bonds with the hydroxyl groups of glucose, causing glucose to isomerize to fructose which is easily dehydrated to HMF [15]. This can be explained by glucose only being able to enolize to a low degree, which is considered the limiting step in the production of HMF from glucose. Therefore, the challenge for the production of HMF from glucose is to find a catalytic system that can selectively isomerize glucose to fructose in tandem with the dehydration reaction.
Dessbesell proposed converting starch into glucose through enzymatic hydrolysis and then using niobium phosphate to catalyze the dehydration of glucose to produce HMF, which is low cost and high in efficiency [16]. Wang et al. reported a high-yield (62%) reaction system for the catalytic conversion of glucose to HMF. The reaction system is divided into an organic phase and an aqueous phase. The organic phase consists of an alkylphenol compound (2-sec-butylphenol), and the aqueous phase consists of a Lewis acid metal chloride (e.g., AlCl
3) and Brønsted acid (e.g., HCl). The conversion of glucose to HMF in this biphasic reactor system in the presence of Lewis acid salts through a tandem reaction involves glucose being isomerized into fructose and the dehydration of fructose [17].
A cost-efficient and convenient method featuring the integration of acid and enzymatic catalysis has been investigated for the selective conversion of glucose into HMF, which provides a new strategy for HMF production from glucose. Huang et al. reported an HMF yield of 63% from glucose in a biphasic system by a two-step process consisting of the isomerization of glucose to fructose using borate ions and glucose isomerase, followed by the acid-catalyzed dehydration of fructose to HMF using HCl as a catalyst [18]. Nikolla et al. reported that the application of Lewis acidic Sn-Beta zeolite along with aqueous HCl could convert glucose to HMF at 180 °C in a biphasic system with about 60% HMF selectivity. However, in the context of green chemical pathways, the corrosivity of HCl is a limiting factor [19]. Recently, Abu-Omar et al. used AlCl
3
2O as the catalyst and THF as the extracting solvent for the production of HMF from glucose in a biphasic system with an HMF yield of 61% [10][20]. Feng et al. discovered that a metal catalyst supported by MCM-41 had an excellent performance in converting carbohydrates into HMF [21]. A one-pot/two-step hybrid catalytic pathway was recently reported by Gimbernat et al. for HMF production from glucose in a triphasic compartmentalized reactor [22]. The preparation of HMF from glucose is a complicated process. In order to achieve high yields, future research needs to pay attention to the research on the reaction mechanism and reaction kinetics of HMF from glucose; find low-cost, green, and efficient catalytic systems and solvent systems; and establish high-efficiency and low-energy consumption product separation technology to reduce costs.
3. Synthesis of HMF from Cellulose
The cellulosic fraction of biomass is an abundant renewable feedstock and the most widely distributed natural polymeric material on earth. Cellulose is a linear polymer consisting of several units of glucose joined together by β-1,4-glycosidic bonds [23]. In recent years, an increased effort has been conducted to utilize this cellulosic biomass fraction for the production of fuels and chemicals as renewable alternatives to petroleum-based resources [24]. Compared with glucose dehydration, the direct conversion of cellulose to HMF is more attractive and challenging. The production of HMF from cellulose is still not operative at an industrial level [25]. Generally speaking, the conversion of cellulose to HMF requires three steps: the hydrolysis of cellulose into monosaccharides, the isomerization of aldose-type sugars to ketose-type sugars, and the subsequent dehydration of ketose-type sugars to HMF [15] (
Figure 1).
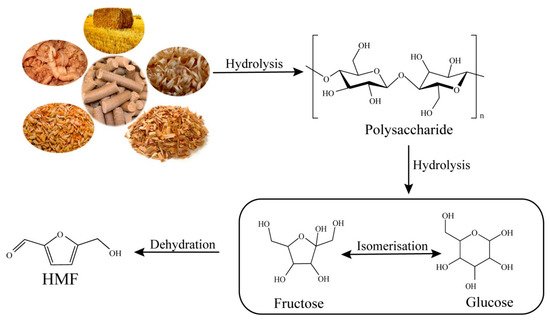
Figure 13.
3
3
3
3
3
3
3 was the most efficient catalyst among these transition metal chlorides for HMF production and resulted in both the highest yield of 83.3% and selectivity of 87.5% in a NaCl-aqueous/butanol biphasic system [26]. Zhang et al. reported the CuCl
2
2-catalyzed transformation of cellulose, and 58% HMF was achieved in IL([EMIM]Cl) [27]. Kim et al. also reported the formation of 58% HMF from cellulose using CrCl
2
3 as the catalyst in 1-ethyl-3-methylimidazolium chloride ([EMIM]Cl) [28]. It was reported that using
N
N
3 and 10% HCl with 60% [EMIM]Cl as the catalyst produced HMF and furfural with a yield of 48% and 34%, respectively, at 160 °C after 2 h from corn stover [29]. Due to the complex structure of lignocellulose, its conversion is difficult. Researchers are seeking efficient methods to allow cellulose to develop in the direction of mild conditions, easy operation, low cost, fast speed, environmental protection, and green production, and to realize the organic connection between the cellulose preparation platform compound and the existing energy chemical industry.
