The nucleocapsid of negative strand RNA virus (NSV) is a linear protein-RNA complex that is the template for the viral RNA-dependent RNA-polymerase. The structure of the nucleocapsid protein from all NSVs shares a core motif that is suitable for assembling the linear nucleocapsid. The unique structure of the nucleocapsid allows it to encapsidate the viral RNA genome for virion assembly, and at the same time serve as the template for viral RNA synthesis.
- nucleocapsid
- negative strand RNA virus
- 5H+3H motif
- linear assembly
- protein-RNA complex
- template
- viral RNA synthesis
- RNA-dependen RNA polymerase
- codon usage bias
- gene junction
1. Definition
1. Definition
Negative strand RNA viruses (NSVs) include many important human pathogens, such as influenza virus, Ebola virus, and rabies virus. One of the unique characteristics that NSVs share is the assembly of the nucleocapsid and its role in viral RNA synthesis. In NSVs, the single strand RNA genome is encapsidated in the linear nucleocapsid throughout the viral replication cycle. Subunits of the nucleocapsid protein are parallelly aligned along the RNA genome that is sandwiched between two domains composed of conserved helix motifs. The viral RNA-dependent-RNA polymerase (vRdRp) must recognize the protein–RNA complex of the nucleocapsid and unveil the protected genomic RNA in order to initiate viral RNA synthesis. In addition, vRdRp must continuously translocate along the protein–RNA complex during elongation in viral RNA synthesis. This unique mechanism of viral RNA synthesis suggests that the nucleocapsid may play a regulatory role during NSV replication.
2. Introduction
Negative strand RN
All viruses (NSVs) include many important human pathogens, such as influenzaassemble a nucleocapsid. The capsid consists of viral proteins and encloses the nucleotide genome of the virus, Ebola virus, and ra. The word “capsid” is originated from the Latin capsa (biox). Thes virus. One of the unique characteristics that NSVs share is the assembly of the primary function of the capsid (also known as “protein coat” or “protein shell”) is to carry and protect the viral genome during transmission between cells. The term “nucleocapsid” refers to the capsid protein–nucleotide complex. For efficiency, the viral nucleocapsid and its role in viral RNA synthesis. In NSVs, the single strand RNA genome is encapsidated in the li is assembled by organization of capsid protein subunits following a geometric symmetry, including the icosahedral symmetry for spherical viruses and the helical symmetry for filamentous viruses (read Chapter 3 in Fields Virology for details) [1]. For negar tive strand RNA viruses (NSVs), the nucleocapsid throughout has a unique structure that is pertinent to its functions in the viralus replication cycle. SubunitsThis review will discuss the assembly of the NSV nucleocapsid protein are pas and the structure–function relationship.
Accorallelly aldigned along the RNA genome that is sandwiched between two domng to International Committee on Taxonomy of Viruses (ICTV), NSVs belong to Phylum Negarnaviricota, Realm: Riboviria [2][3]. Since 2003, the s composed of conserved helix motifs. The viral RNA-dependent-RNA polymerase (vRdRp) must recogniztructure of the nucleocapsid or the capsid protein has been determined for at least 21 genera in Negarnaviricota (Table t1). The protein–RNA complex of the nucleocapsid and unveil the protected genomic RNA in order to initiate viral RNA synthesis. In addition, vRdRp must continuously translocate along the protein–RNA complex during elongation indetermined structures have confirmed a genetic relationship among members of different NSV families. Common folds of the NSV capsid proteins and principles of nucleocapsid assembly have emerged, and the functional role of the nucleocapsid in the unique NSV viral RNA synthesis. This unique mechanism of viral RNA synthesis suggests that the has also become clearer. It is because of this role that additional examination of the structure of NSV nucleocapsid may play a regulatory role during s has now become essential for further understanding of NSV replicationn and pathogenesis.
2. Introduction
Reported nucleocapsid structure of members from Phylum
| Family | Subfamily | Genus | Name | (PDB Code) |
|---|
| Subphylum:Haploviricotina;Class:Monjiviricetes;Order:Mononegavirales | ||||
| Bornaviridae | Mammalian 1 orthobornavirus | Borna disease virus (BoDV) | (1N93) [4] | |
The two largest NSV orders contain the majority of known capsid protein structures are
and
. Beyond these, there are a small number of structures from
represented by the segmented
. Structures from the order
are included in this review because of structural similarities, even though these are positive single strand RNA viruses.
The structures of
capsid proteins from 11 members in the order
were superimposed using the program Fr-TM-align [36] (Table 2). Large variance in capsid protein Size is observed in known structures with MWs ranging from 41 kDa in BoDV up to 83.3 kDa in EBOV; however, the capsid cores maintain structural homology. Similarities among these structures are essentially the same as previously observed [34][37]. It is well expected that viruses in the same family have a high structural similarity in their capsid proteins, such as VSV and RABV; RSV and hMPV; PIV5, MeV, NiV, and NDV; EBOV and MARV (Figure 1A). However, the observed similarity between NDV and MARV appears to be an exception (Table 2). The nucleocapsid structure of MARV is very close to that of EBOV. The apparent more favorable alignment for MARV could be due to that less residues are included in the structure of MARV nucleocapsid protein.
Structural Comparison of
Nucleocapsids.
| RABV | RSV | hMPV | PIV5 | MeV | NiV | NDV | EBOV | MARV | BoDV |
|---|
| VSV | 2.63/96 * | 5.07/79 | 5.17/81 | 5.34/78 | 5.30/79 | 4.73/83 | 5.20/75 | 5.18/82 | 4.20/85 | 5.09/78 |
| RABV | 5.01/76 | 4.93/78 | 5.04/73 | |||||||
| 5.53/83 | ||||||||||
| MARV | ||||||||||
| 5.47/84 |
For
NSVs, homology is only present among members in the same family (Table 3 and Table 4). However, the fold of the capsid proteins shares some common features with those from members in
. The most apparent similarity is that the encapsidated genomic RNA is also sandwiched between the N- and C-terminal lobes (Figure 1B).
Structural Comparison of
Nucleocapsids.
| LACV | LEAV | SBV | TSWV | TOSV | SFTSV | |
|---|---|---|---|---|---|---|
| BUNV | 2.11/98 | 1.87/100 | 2.07/100 | 3.68/87 | - | - |
| Filoviridae | Ebolavirus | Ebola virus (EBOV) | ||||
| TOSV | ||||||
| 2.65/94 |
Structural Comparison of Other Nucleocapsids.
| HTNV | HAZV | KUPV | ERVEV | IFBV | IFDV | ISAV | PepMV |
|---|
| ANDV | 1.94/95 | - | - | - | - | - | - | - | |||||||
| LACV | 2.07/94 | 5.06/76 | 4.27/81 | 2.09/93 | 3.64/85 | -5.11/73 | 4.38/78 | 5.01/82 | 5.03/75 | ||||||
| - | |||||||||||||||
| CCHFV | 1.77/100 | † | 1.90/99 | 1.56/91 | - | - | - | - | (4Z9P) [5] | ||||||
| RSV | 1.40/100 | 4.35/91 | 4.35/91 | 4.90/93 | 4.36/91 | LEAV | 1.66/99 | 3.96/89 | - | - | |||||
| HAZV | 4.39/87 | 4.85/86 | 4.83/80 | ||||||||||||
| 1.56/97 | 1.32/89 | - | - | - | - | Marburgvirus | Marburg virus (MARV) | (5F5M) [6] | |||||||
| hMPV | 4.10/94 | 4.20/94 | 4.79/92 | 4.24/94 | 4.48/89 | 4.11/90 | 4.36/78 | ||||||||
| SBV | 3.52/88 | - | - | ||||||||||||
| KUPV | 1.27/87 | - | - | - | - | Paramyxoviridae | Avulaviridae | Avian orthoavulavirus 1 | PIV5 | Newcastle disease virus (NDV) | (6JC3) [7] | ||||
| 2.47/97 | 3.59/100 | 1.82/98 | 4.87/89 | 3.77/94 | 4.51/83 | ||||||||||
| RVFV | 1.88/91 | 1.83/90 | |||||||||||||
| IFAV | Orthoparamyxovirdae | Henipavirus | Nipah virus (NiV) | MeV | (4CO6) | [8] | |||||||||
| 3.26/97 | 2.32/98 | 4.43/87 | 3.97/94 | 4.41/81 | |||||||||||
| 2.29/95 | 3.21/94 | 3.49/84 | - | 3.02/93 | 3.36/78 | Morbillivirus | Measles virus (MeV) | NiV | (4UFT) [9] | ||||||
| 3.38/99 | 4.12/92 | 3.62/91 | 5.19/80 | Rubulaviridae | Orrthorubulavirus | parainfluenza virus 5 (PIV5) | (4XJN) [10] | ||||||||
| - | NDV | ||||||||||||||
| IFBV | 4.79/89 | 3.92/96 | 4.76/84 | Pneumoviridae | Metapneumovirus | ||||||||||
| EBOV | Human metapneumovirus (hMPV) | (5FVC) [11] | |||||||||||||
| Orthopneumovirus | respiratory syncytial virus (RSV) | (2WJ8) [12] | |||||||||||||
| Rhabdoviridae | Lyssavirus | Rabies virus (RABV) | (2GTT) [13] | ||||||||||||
| Vesiculovirus | Vesicular stomatitis virus (VSV) | (2GIC) [14] | |||||||||||||
| Subphylum:Polyploviricotina;Class:Ellioviricetes;Order:Bunyavirales | |||||||||||||||
| Arenaviridae | Mammarenavirus | Lassa Virus (LASV) | (3T5Q) [15] | ||||||||||||
| Hantaviridae | Mammantaviridae | Orthohantavirus | Andes virus (ANDV) | (5E04) [16] | |||||||||||
| Hantaan virus (HTNV) | (6I2N) [17] | ||||||||||||||
| 1.87/99 | Nairoviridae | ||||||||||||||
| IFDV | 3.58/76 | - | |||||||||||||
| PapMV | Orthonairovirus | Crimean-Congo Hemorrhagic Fever Virus (CCHFV) | (4AKL) [18] | ||||||||||||
| 2.06/93 | Hazara virus (HAZV) | (4XZE) [19] | |||||||||||||
| Kupe virus (KUPV) | (4XZC) [19] | ||||||||||||||
| Erve virus (ERVEV) | (4XZ8) [19] | ||||||||||||||
| Peribunyaviridae | Orthobunyavirus | Bunyamwera virus (BUNV) | (4IJS) [20] | ||||||||||||
| La Crosse virus (LACV) | (4BHH) [21] | ||||||||||||||
| Leanyer orthobunyavirus (LEAV) | (4J1G) [22] | ||||||||||||||
| Schmallenberg virus (SBV) | (4JNG) [23] | ||||||||||||||
| Phenuiviridae | Phlebovirus | Rift Valley Fever Virus (RVFV) | (4H5P) [24] | ||||||||||||
| Toscana virus (TOSV) | (4CSF) [25] | ||||||||||||||
| Severe fever with thrombocytopenia syndrome virus (SFTSV) | 4J4R) [26] | ||||||||||||||
| Tospoviridae | Orthotospovirus | Tomato spotted wilt tospovirus (TSWV) | (5IP3) | ||||||||||||
Further examination reveals that the 5-H and 3-H motifs share the same topology, but the orientations of the helices are often different. For members in
and
, the N-terminal lobe contains the same 5-helices and may be superimposed with that from members in
(Table 5). For the C-terminal lobe, on the other hand, the first two helices in the 3-H motif could be superimposed with those from members in
while the third α-helix takes a reverse orientation. For
members in
and
, the helices in the N-terminal 5-H motif have large differences in their orientation despite the same topology. Their N-terminal lobes could not be superimposed with those from members in
. The same situation is found in the C-terminal 3-H motif. However, the fold in the C-terminal lobe from members in
and
is homologous to that from members in
, except for the orientation of the last α-helix (Table 5).
Structural Comparison of Representative Nucleocapsid Lobes.
| VSV-N ≠ | CCHFV-N | HTNV-C | |
|---|---|---|---|
| BUNV-N | 4.77/82 | 3.77/82 | |
| BUNV-C | 2.88/87 | ||
| PepMV-N | 3.28/61 | ||
| [ | |||
| 27 | |||
| ] | |||
| Subphylum: | |||
| Polyploviricotina | |||
| ; | |||
| Class: | |||
| Insthoviricetes | |||
| ; | |||
| Order: | |||
| Articulavirales | |||
| Orthomyxoviridae | |||
| Alphainfluenzavirus | |||
| Influenza A virus (IFAV) | |||
| (2IQH) | |||
| [ | 28 | ] | |
| Betainfluenzavirus | Influenza B virus (IFBV) | (3TJ0) [29] | |
| Deltainfluenzavirus | Influenza D virus (IFDV) | (5N2U) [30] | |
| Isavirus | Infectious Salmon Anemia Virus (ISAV) | (4EWC) [31] | |
| Class:Tymovirales *;Order:Alphaflexiviridae | |||
| Potexvirus | Papaya mosaic virus (PapMV) | (4DOX) [32] | |
| Pepino mosaic virus (PepMV) | (5FN1) [33] | ||
3. The Capsid Protein Fold
The Phylum
has been divided into two Subphyla;
and
The distinction between the two Subphyla is the nonsegmented or segmented nature of the viral genome. The NSVs are then further classified phylogenetically ultimately yielding a separation by virion morphology. Although these viruses can differ greatly in their global structures, structural and functional similarities are observed when comparing their capsid proteins.
The capsid proteins (N) of NSVs share a common fold. As shown for vesicular stomatitis virus (VSV) in Figure 1A, the N-terminal lobe and the C-terminal lobe are connected by a single polypeptide chain [14]. The core of the capsid protein consists of five α-helices in the N-terminal lobe (5-H motif) and three α-helices in the C-terminal lobe (3-H motif) [34]. When the protein subunit is assembled into the nucleocapsid, the encapsidated RNA is situated between the two lobes. The observation of a conserved structural motif present in all nucleocapsid proteins in NSVs is analogous to that a β-barrel fold is observed in the capsid proteins of spherical viruses [35].
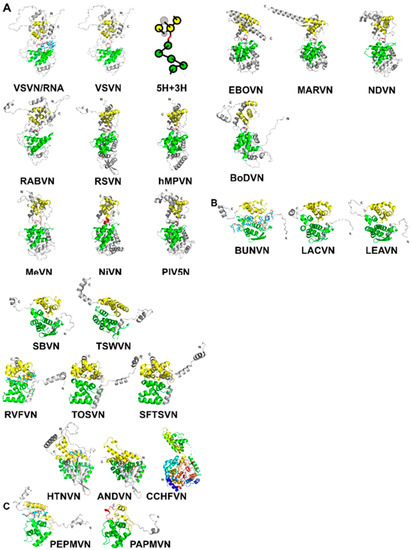
Figure 1. (A) A ribbon drawing of VSV nucleocapsid protein (VSVN) in complex with 9 nucleotides (sticks) encapsidated. The 5H in the N-lobe is colored green, and 3H in the C-lobe is colored yellow. Termini are labeled as N and C, respectively. The linker between the two lobes is colored red. The same ribbon drawing without RNA is shown to the right. Ribbons drawings in this and the following figures are prepared with PyMol [40]. A cartoon is drawn to illustrate the 5H+3H motif. Ten other related N structures in Table 2 are also shown using the same color scheme. (B) Eleven N structures in Polyploviricotina are shown, with those from BUNV (BUNVN), RVFV (RVFVN), and HTNV (HTNVN) in complex with encapsidated nucleotides (sticks). The N-domain in the N core is colored green and the C-domain in the N core is colored yellow. Structures of closely relayed N proteins according to Table 3 and Table 5 are grouped together. The structure from CCHFV (CCHFVN) is colored rainbow from the N-terminus (blue) to the C-terminus (red) because no clear N- and C-domains could be identified. (C) Two structures from the +ss RNA viruses are shown, with that from PepMV (PepMVN) in complex with encapsidated RNA. The color scheme is the same as in (B).
Finally, the size of the RNA cleft in a N subunit may vary widely in these viruses. The C-terminal lobe from members in
has much less separation from the N-terminal lobe so that the cleft between the two lobes is much narrower than that from members in
. This narrowing of the RNA cleft results in a very shallow pocket in which the RNA resides in a fissure rather than within a deep cavitation [21]. Differences in these structural features may allow more a flexibility in supramolecular nucleocapsid morphology to package the segmented genomes that are incorporated stochastically during virion assembly [38][39].
For members in
, direct structural comparison of the nucleocapsid proteins with other families is not informative; two terminal lobes are visible, but the division is not completely clear because no information of RNA encapsidation is available [28].
In addition to NSVs, the structure of nucleocapsid proteins from members in

References
- Knipe, D.; Howley, P.; Fields, B.N.; Griffin, D.E. Fields’ Virilogy: Principles of Virus Structure; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2001; pp. 53–86. [Google Scholar]
- Lefkowitz, E.J.; Dempsey, D.M.; Hendrickson, R.C.; Orton, R.J.; Siddell, S.G.; Smith, D.B. Virus taxonomy: The database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic Acids Res. 2018, 46, D708–D717. [Google Scholar] [CrossRef] [PubMed]
- Amarasinghe, G.K.; Ayllón, M.A.; Bào, Y.; Basler, C.F.; Bavari, S.; Blasdell, K.R.; Briese, T.; Brown, P.A.; Bukreyev, A.; Balkema-Buschmann, A.; et al. Taxonomy of the order Mononegavirales: Update 2019. Arch. Virol. 2019, 164, 1967–1980. [Google Scholar] [CrossRef] [PubMed]
- Amarasinghe, G.K.; Ayllón, M.A.; Bào, Y.; Basler, C.F.; Bavari, S.; Blasdell, K.R.; Briese, T.; Brown, P.A.; Bukreyev, A.; Balkema-Buschmann, A.; et al. Crystal structure of the borna disease virus nucleoprotein. Structure 2003, 11, 1219–1226. [Google Scholar] [CrossRef]
- Dong, S.; Yang, P.; Li, G.; Liu, B.; Wang, W.; Liu, X.; Xia, B.; Yang, C.; Lou, Z.; Guo, Y.; et al. Insight into the Ebola virus nucleocapsid assembly mechanism: Crystal structure of Ebola virus nucleoprotein core domain at 1.8 A resolution. Protein Cell 2015, 6, 351–362. [Google Scholar] [CrossRef]
- Liu, B.; Dong, S.; Li, G.; Wang, W.; Liu, X.; Wang, Y.; Yang, C.; Rao, Z.; Guo, Y. Structural insight into nucleoprotein conformation change chaperoned by VP35 peptide in marburg virus. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Song, X.; Shan, H.; Zhu, Y.; Hu, S.; Xue, L.; Chen, Y.; Ding, W.; Niu, T.; Gu, J.; Ouyang, S.; et al. Self-capping of nucleoprotein filaments protects the Newcastle disease virus genome. eLife 2019, 8. [Google Scholar] [CrossRef]
- Yabukarski, F.; Lawrence, P.; Tarbouriech, N.; Bourhis, J.M.; Delaforge, E.; Jensen, M.R.; Ruigrok, R.W.; Blackledge, M.; Volchkov, V.; Jamin, M. Structure of Nipah virus unassembled nucleoprotein in complex with its viral chaperone. Nat. Struct. Mol. Biol. 2014, 21, 754–759. [Google Scholar] [CrossRef]
- Gutsche, I.; Desfosses, A.; Effantin, G.; Ling, W.L.; Haupt, M.; Ruigrok, R.W.; Sachse, C.; Schoehn, G. Structural virology: Near-atomic cryo-EM structure of the helical measles virus nucleocapsid. Science 2015, 348, 704–707. [Google Scholar] [CrossRef]
- Alayyoubi, M.; Leser, G.P.; Kors, C.A.; Lamb, R.A. Structure of the paramyxovirus parainfluenza virus 5 nucleoprotein-RNA complex. Proc. Natl. Acad. Sci. USA 2015, 112, E1792–E1799. [Google Scholar] [CrossRef]
- Renner, M.; Bertinelli, M.; Leyrat, C.; Paesen, G.C.; Saraiva de Oliveira, L.F.; Huiskonen, J.T.; Grimes, J.M. Nucleocapsid assembly in pneumoviruses is regulated by conformational switching of the N protein. eLife 2016, 5, e12627. [Google Scholar] [CrossRef]
- Tawar, R.G.; Duquerroy, S.; Vonrhein, C.; Varela, P.F.; Damier-Piolle, L.; Castagné, N.; MacLellan, K.; Bedouelle, H.; Bricogne, G.; Bhella, D.; et al. Crystal structure of a nucleocapsid-like nucleoprotein-RNA complex of respiratory syncytial virus. Science 2009, 326, 1279–1283. [Google Scholar] [CrossRef] [PubMed]
- Albertini, A.A.; Wernimont, A.K.; Muziol, T.; Ravelli, R.B.; Clapier, C.R.; Schoehn, G.; Weissenhorn, W.; Ruigrok, R.W. Crystal structure of the rabies virus nucleoprotein-RNA complex. Science 2006, 313, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Green, T.J.; Zhang, X.; Wertz, G.W.; Luo, M. Structure of the vesicular stomatitis virus nucleoprotein-RNA complex. Science 2006, 313, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Hastie, K.M.; Liu, T.; Li, S.; King, L.B.; Ngo, N.; Zandonatti, M.A.; Woods, V.L., Jr.; de la Torre, J.C.; Saphire, E.O. Crystal structure of the Lassa virus nucleoprotein-RNA complex reveals a gating mechanism for RNA binding. Proc. Natl. Acad. Sci. USA 2011, 108, 19365–19370. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, W.; Sun, Y.; Ma, C.; Wang, X.; Wang, X.; Liu, P.; Shen, S.; Li, B.; Lin, J.; et al. Crystal structure of the core region of hantavirus nucleocapsid protein reveals the mechanism for ribonucleoprotein complex formation. J. Virol. 2016, 90, 1048–1061. [Google Scholar] [CrossRef]
- Arragain, B.; Reguera, J.; Desfosses, A.; Gutsche, I.; Schoehn, G.; Malet, H. High resolution cryo-EM structure of the helical RNA-bound Hantaan virus nucleocapsid reveals its assembly mechanisms. eLife 2019, 8. [Google Scholar] [CrossRef]
- Carter, S.D.; Surtees, R.; Walter, C.T.; Ariza, A.; Bergeron, É.; Nichol, S.T.; Hiscox, J.A.; Edwards, T.A.; Barr, J.N. Structure, function, and evolution of the Crimean-Congo hemorrhagic fever virus nucleocapsid protein. J. Virol. 2012, 86, 10914–10923. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Wang, X.; Dong, H.; Ma, C.; Wang, J.; Liu, B.; Mao, Y.; Wang, Y.; Li, T.; et al. Structural and functional diversity of nairovirus-encoded nucleoproteins. J. Virol. 2015, 89, 11740–11749. [Google Scholar] [CrossRef]
- Li, B.; Wang, Q.; Pan, X.; Fernández de Castro, I.; Sun, Y.; Guo, Y.; Tao, X.; Risco, C.; Sui, S.F.; Lou, Z. Bunyamwera virus possesses a distinct nucleocapsid protein to facilitate genome encapsidation. Proc. Natl. Acad. Sci. USA 2013, 110, 9048–9053. [Google Scholar] [CrossRef]
- Reguera, J.; Malet, H.; Weber, F.; Cusack, S. Structural basis for encapsidation of genomic RNA by La Crosse Orthobunyavirus nucleoprotein. Proc. Natl. Acad. Sci. USA 2013, 110, 7246–7251. [Google Scholar] [CrossRef]
- Niu, F.; Shaw, N.; Wang, Y.E.; Jiao, L.; Ding, W.; Li, X.; Zhu, P.; Upur, H.; Ouyang, S.; Cheng, G.; et al. Structure of the Leanyer orthobunyavirus nucleoprotein-RNA complex reveals unique architecture for RNA encapsidation. Proc. Natl. Acad. Sci. USA 2013, 110, 9054–9059. [Google Scholar] [CrossRef]
- Dong, H.; Li, P.; Bottcher, B.; Elliott, R.M.; Dong, C. Crystal structure of Schmallenberg orthobunyavirus nucleoprotein-RNA complex reveals a novel RNA sequestration mechanism. Rna 2013, 19, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Raymond, D.D.; Piper, M.E.; Gerrard, S.R.; Skiniotis, G.; Smith, J.L. Phleboviruses encapsidate their genomes by sequestering RNA bases. Proc. Natl. Acad. Sci. USA 2012, 109, 19008–19213. [Google Scholar] [CrossRef]
- Olal, D.; Dick, A.; Woods, V.L.; Jr Liu, T.; Li, S.; Devignot, S.; Weber, F.; Saphire, E.O.; Daumke, O. Structural insights into RNA encapsidation and helical assembly of the Toscana virus nucleoprotein. Nucleic Acids Res. 2014, 42, 6025–6037. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Ouyang, S.; Liang, M.; Niu, F.; Shaw, N.; Wu, W.; Ding, W.; Jin, C.; Peng, Y.; Zhu, Y.; et al. Structure of severe fever with thrombocytopenia syndrome virus nucleocapsid protein in complex with suramin reveals therapeutic potential. J. Virol. 2013, 87, 6829–6839. [Google Scholar] [CrossRef]
- Komoda, K.; Narita, M.; Yamashita, K.; Tanaka, I.; Yao, M. Asymmetric trimeric ring structure of the nucleocapsid protein of tospovirus. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Krug, R.M.; Tao, Y.J. The mechanism by which influenza A virus nucleoprotein forms oligomers and binds RNA. Nature 2006, 444, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.K.; Lam, M.K.; Zhang, H.; Liu, J.; Au, S.W.; Chan, P.K.; Wang, J.; Shaw, P.C. Structural basis for RNA binding and homo-oligomer formation by influenza B virus nucleoprotein. J. Virol. 2012, 86, 6758–6767. [Google Scholar] [CrossRef] [PubMed]
- Donchet, A.; Oliva, J.; Labaronne, A.; Tengo, L.; Miloudi, M.; CA Gerard, F.; Mas, C.; Schoehn, G.; WH Ruigrok, R.; Ducatez, M.; et al. The structure of the nucleoprotein of Influenza D shows that all Orthomyxoviridae nucleoproteins have a similar NPCORE, with or without a NPTAIL for nuclear transport. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Zheng, W.; Olson, J.; Vakharia, V.; Tao, Y.J. The crystal structure and RNA-binding of an orthomyxovirus nucleoprotein. PLoS Pathog. 2013, 9, e1003624. [Google Scholar] [CrossRef]
- Yang, S.; Wang, T.; Bohon, J.; Gagné, M.È.; Bolduc, M.; Leclerc, D.; Li, H. Crystal structure of the coat protein of the flexible filamentous papaya mosaic virus. J. Mol. Biol. 2012, 422, 263–273. [Google Scholar] [CrossRef]
- Agirrezabala, X.; Méndez-López, E.; Lasso, G.; Sánchez-Pina, M.A.; Aranda, M.; Valle, M. The near-atomic cryoEM structure of a flexible filamentous plant virus shows homology of its coat protein with nucleoproteins of animal viruses. eLife 2015, 4, e11795. [Google Scholar] [CrossRef] [PubMed]
- Green, T.J.; Cox, R.; Tsao, J.; Rowse, M.; Qiu, S.; Luo, M. Common mechanism for RNA encapsidation by negative-strand RNA viruses. J. Virol. 2014, 88, 3766–3775. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.S.; Liljas, L. Structural folds of viral proteins. Adv. Protein Chem. 2003, 64, 125–196. [Google Scholar] [CrossRef]
- Pandit, S.B.; Skolnick, J. Fr-TM-align: A new protein structural alignment method based on fragment alignments and the TM-score. BMC Bioinform. 2008, 9, 531. [Google Scholar] [CrossRef]
- Luo, M.; Green, T.J.; Zhang, X.; Tsao, J.; Qiu, S. Structural comparisons of the nucleoprotein from three negative strand RNA virus families. J. Virol. 2007, 4, 72–78. [Google Scholar] [CrossRef]
- Wichgers Schreur, P.J.; Kormelink, R.; Kortekaas, J. Genome packaging of the Bunyavirales. Curr. Opin. Virol. 2018, 33, 151–155. [Google Scholar] [CrossRef]
- Sherman, M.B.; Freiberg, A.N.; Holbrook, M.R.; Watowich, S.J. Single-particle cryo-electron microscopy of Rift Valley fever virus. Virology 2009, 387, 11–15. [Google Scholar] [CrossRef]
- DeLano, W.L. PyMOL Molecular Graphic System, version 1.3; Schrödinger LLC: New York, NY, USA, 2015. [Google Scholar]
. They contain similar N- and C-terminal lobes that encapsidate the genomic nucleotide between them (Figure 1C). Some of the bases in the RNA strand are stacked and face the exterior of the nucleocapsid, whereas some face the interior. The N-terminal domain of their N proteins is homologous to that of NSV N proteins (Table 5). This suggests that the strategy to assemble a linear nucleocapsid is common in both negative and positive single strand RNA viruses, and the same structural features in the capsid protein are essential for the encapsidation of the linear nucleotide in the nucleocapsid.
References
- Ming Luo; James Ross Terrell; Shelby Ashlyn McManus; Nucleocapsid Structure of Negative Strand RNA Virus. Viruses 2020, 12, 835, 10.3390/v12080835.Knipe, D.; Howley, P.; Fields, B.N.; Griffin, D.E. Fields’ Virilogy: Principles of Virus Structure; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2001; pp. 53–86.
- Lefkowitz, E.J.; Dempsey, D.M.; Hendrickson, R.C.; Orton, R.J.; Siddell, S.G.; Smith, D.B. Virus taxonomy: The database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic Acids Res. 2018, 46, D708–D717, doi:10.1093/nar/gkx932.
- Amarasinghe, G.K.; Ayllón, M.A.; Bào, Y.; Basler, C.F.; Bavari, S.; Blasdell, K.R.; Briese, T.; Brown, P.A.; Bukreyev, A.; Balkema-BuschmannA.; et al. Taxonomy of the order Mononegavirales: Update 2019. Arch. Virol. 2019, 164, 1967–1980, doi:10.1007/s00705-019-04247-4.
- Amarasinghe, G.K.; Ayllón, M.A.; Bào, Y.; Basler, C.F.; Bavari, S.; Blasdell, K.R.; Briese, T.; Brown, P.A.; Bukreyev, A.; Balkema-Buschmann, A.; et al. Crystal structure of the borna disease virus nucleoprotein. Structure 2003, 11, 1219–1226, doi:10.1016/j.str.2003.08.011.
- Dong, S.; Yang, P.; Li, G.; Liu, B.; Wang, W.; Liu, X.; Xia, B.; Yang, C.; Lou, Z.; Guo, Y.; et al. Insight into the Ebola virus nucleocapsid assembly mechanism: Crystal structure of Ebola virus nucleoprotein core domain at 1.8 A resolution. Protein Cell 2015, 6, 351–362, doi:10.1007/s13238-015-0163-3.
- Liu, B.; Dong, S.; Li, G.; Wang, W.; Liu, X.; Wang, Y.; Yang, C.; Rao, Z.; Guo, Y. Structural insight into nucleoprotein conformation change chaperoned by VP35 peptide in marburg virus. J. Virol. 2017, 91, doi:10.1128/JVI.00825-17.
- Song, X., Shan, H., Zhu, Y., Hu, S., Xue, L., Chen, Y., Ding, W., Niu, T., Gu, J., Ouyang, S.; et al. Self-capping of nucleoprotein filaments protects the Newcastle disease virus genome. eLife 2019, 8, doi:10.7554/eLife.45057.
- Yabukarski, F.; Lawrence, P.; Tarbouriech, N.; Bourhis, J.M.; Delaforge, E.; Jensen, M.R.; Ruigrok, R.W.; Blackledge, M.; Volchkov, V.; Jamin, M. Structure of Nipah virus unassembled nucleoprotein in complex with its viral chaperone. Nat. Struct. Mol. Biol. 2014, 21, 754–759, doi:10.1038/nsmb.2868.
- Gutsche, I.; Desfosses, A.; Effantin, G.; Ling, W.L.; Haupt, M.; Ruigrok, R.W.; Sachse, C.; Schoehn, G. Structural virology: Near-atomic cryo-EM structure of the helical measles virus nucleocapsid. Science 2015, 348, 704–707, doi:10.1126/science.aaa5137.
- Alayyoubi, M.; Leser, G.P.; Kors, C.A.; Lamb, R.A. Structure of the paramyxovirus parainfluenza virus 5 nucleoprotein-RNA complex. Proc. Natl. Acad. Sci. USA 2015, 112, E1792–E1799, doi:10.1073/pnas.1503941112.
- Renner, M.; Bertinelli, M.; Leyrat, C.; Paesen, G.C.; Saraiva de Oliveira, L.F.; Huiskonen, J.T.; Grimes, J.M. Nucleocapsid assembly in pneumoviruses is regulated by conformational switching of the N protein. eLife 2016, 5, e12627, doi:10.7554/eLife.12627.
- Tawar, R.G.; Duquerroy, S.; Vonrhein, C.; Varela, P.F.; Damier-Piolle, L.; Castagné; N; MacLellan, K.; Bedouelle, H.; Bricogne, G.; Bhella, D.; et al. Crystal structure of a nucleocapsid-like nucleoprotein-RNA complex of respiratory syncytial virus. Science 2009, 326, 1279–1283, 1048, doi:10.1126/science.1177634.
- Albertini, A.A.; Wernimont, A.K.; Muziol, T.; Ravelli, R.B.; Clapier, C.R.; Schoehn, G.; Weissenhorn, W.; Ruigrok, R.W. Crystal structure of the rabies virus nucleoprotein-RNA complex. Science 2006, 313, 360–363, doi:10.1126/science.1125280.
- Green, T.J.; Zhang, X.; Wertz, G.W.; Luo, M. Structure of the vesicular stomatitis virus nucleoprotein-RNA complex. Science 2006, 313, 357–360, doi:10.1126/science.1126953.
- Hastie, K.M.; Liu, T.; Li, S.; King, L.B.; Ngo, N.; Zandonatti, M.A.; Woods, V.L., Jr.; de la Torre, J.C.; Saphire, E.O. Crystal structure of the Lassa virus nucleoprotein-RNA complex reveals a gating mechanism for RNA binding. Proc. Natl. Acad. Sci. USA 2011, 108, 19365–19370, 1091, doi:10.1073/pnas.1108515108.
- Guo, Y.; Wang, W.; Sun, Y.; Ma, C.; Wang, X.; Wang, X.; Liu, P.; Shen, S.; Li, B.; Lin, J.; et al. Crystal structure of the core region of hantavirus nucleocapsid protein reveals the mechanism for ribonucleoprotein complex formation. J. Virol. 2016, 90, 1048–1061, doi:10.1128/JVI.02523-15.
- Arragain, B.; Reguera, J.; Desfosses, A.; Gutsche, I.; Schoehn, G.; Malet, H. High resolution cryo-EM structure of the helical RNA-bound Hantaan virus nucleocapsid reveals its assembly mechanisms. eLife 2019, 8, doi:10.7554/eLife.43075.
- Carter, S.D.; Surtees, R.; Walter, C.T.; Ariza, A.; Bergeron, É.; Nichol, S.T.; Hiscox, J.A.; Edwards, T.A.; Barr, J.N. Structure, function, and evolution of the Crimean-Congo hemorrhagic fever virus nucleocapsid protein. J. Virol. 2012, 86, 10914–10923, doi:10.1128/JVI.01555-12.
- Wang, W.; Liu, X.; Wang, X.; Dong, H.; Ma, C.; Wang, J.; Liu, B.; Mao, Y.; Wang, Y., Li, T.; et al. Structural and functional diversity of nairovirus-encoded nucleoproteins. J. Virol. 2015, 89, 11740–11749, doi:10.1128/JVI.01680-15.
- Li, B.; Wang, Q.; Pan, X.; Fernández de Castro, I.; Sun, Y.; Guo, Y.; Tao, X.; Risco, C.; Sui, S.F.; Lou, Z. Bunyamwera virus possesses a distinct nucleocapsid protein to facilitate genome encapsidation. Proc. Natl. Acad. Sci. USA 2013, 110, 9048–9053, doi:10.1073/pnas.1222552110.
- Reguera, J.; Malet, H.; Weber, F.; Cusack, S. Structural basis for encapsidation of genomic RNA by La Crosse Orthobunyavirus nucleoprotein. Proc. Natl. Acad. Sci. USA 2013, 110, 7246–7251, doi:10.1073/pnas.1302298110.
- Niu, F.; Shaw, N.; Wang, Y.E.; Jiao, L.; Ding, W.; Li, X.; Zhu, P.; Upur, H.; Ouyang, S.; Cheng, G.; et al. Structure of the Leanyer orthobunyavirus nucleoprotein-RNA complex reveals unique architecture for RNA encapsidation. Proc. Natl. Acad. Sci. USA 2013, 110, 9054–9059, doi:10.1073/pnas.1300035110.
- Dong, H.; Li, P.; Bottcher, B.; Elliott, R.M.; Dong, C. Crystal structure of Schmallenberg orthobunyavirus nucleoprotein-RNA complex reveals a novel RNA sequestration mechanism. Rna 2013, 19, 1129–1136, doi:10.1261/rna.039057.113.
- Raymond, D.D.; Piper, M.E.; Gerrard, S.R.; Skiniotis, G.; Smith, J.L. Phleboviruses encapsidate their genomes by sequestering RNA bases. Proc. Natl. Acad. Sci. USA 2012, 109, 19008–19213, doi:10.1073/pnas.1213553109.
- Olal, D.; Dick, A.; Woods, V.L.; Jr Liu, T.; Li, S.; Devignot, S.; Weber, F.; Saphire, E.O.; Daumke, O. Structural insights into RNA encapsidation and helical assembly of the Toscana virus nucleoprotein. Nucleic Acids Res. 2014, 42, 6025–6037, doi:10.1093/nar/gku229.
- Jiao, L.; Ouyang, S.; Liang, M.; Niu, F.; Shaw, N.; Wu, W.; Ding, W.; Jin, C.; Peng, Y.; Zhu, Y.; et al. Structure of severe fever with thrombocytopenia syndrome virus nucleocapsid protein in complex with suramin reveals therapeutic potential. J. Virol. 2013, 87, 6829–6839, doi:10.1128/JVI.00672-13.
- Komoda, K.; Narita, M.; Yamashita, K.; Tanaka, I.; Yao, M. Asymmetric trimeric ring structure of the nucleocapsid protein of tospovirus. J. Virol. 2017, 91, doi:10.1128/JVI.01002-17.
- Ye, Q.; Krug, R.M.; Tao, Y.J. The mechanism by which influenza A virus nucleoprotein forms oligomers and binds RNA. Nature 2006, 444, 1078–1082, doi:10.1038/nature05379.
- Ng, A.K.; Lam, M.K.; Zhang, H.; Liu, J.; Au, S.W.; Chan, P.K.; Wang, J.; Shaw, P.C. Structural basis for RNA binding and homo-oligomer formation by influenza B virus nucleoprotein. J. Virol. 2012, 86, 6758–6767, doi:10.1128/JVI.00073-12.
- Donchet, A.; Oliva, J.; Labaronne, A.; Tengo, L.; Miloudi, M.; CA Gerard, F.; Mas, C.; Schoehn, G.; WH Ruigrok, R.; Ducatez, M.; et al. The structure of the nucleoprotein of Influenza D shows that all Orthomyxoviridae nucleoproteins have a similar NPCORE, with or without a NPTAIL for nuclear transport. Sci. Rep. 2019, 9, 600, doi:10.1038/s41598-018-37306-y.
- Zheng, W.; Olson, J.; Vakharia, V.; Tao, Y.J. The crystal structure and RNA-binding of an orthomyxovirus nucleoprotein. PLoS Pathog. 2013, 9, e1003624, doi:10.1371/journal.ppat.1003624.
- Yang, S.; Wang, T.; Bohon, J.; Gagné; M.È.; Bolduc, M.; Leclerc, D.; Li, H. Crystal structure of the coat protein of the flexible filamentous papaya mosaic virus. J. Mol. Biol. 2012, 422, 263–273, doi:10.1016/j.jmb.2012.05.032.
- Agirrezabala, X.; Méndez-López, E.; Lasso, G.; Sánchez-Pina, M.A.; Aranda, M.; Valle, M. The near-atomic cryoEM structure of a flexible filamentous plant virus shows homology of its coat protein with nucleoproteins of animal viruses. eLife 2015, 4, e11795, doi:10.7554/eLife.11795.
- Green, T.J.; Cox, R.; Tsao, J.; Rowse, M.; Qiu, S.; Luo, M. Common mechanism for RNA encapsidation by negative-strand RNA viruses. J. Virol. 2014, 88, 3766–3775, doi:10.1128/JVI.03483-13.
- Chapman, M.S.; Liljas, L. Structural folds of viral proteins. Adv. Protein Chem. 2003, 64, 125–196, doi:10.1016/s0065-323301004-0.
- Pandit, S.B.; Skolnick, J. Fr-TM-align: A new protein structural alignment method based on fragment alignments and the TM-score. BMC Bioinform. 2008, 9, 531, doi:10.1186/1471-2105-9-531.
- Luo, M.; Green, T.J.; Zhang, X.; Tsao, J.; Qiu, S. Structural comparisons of the nucleoprotein from three negative strand RNA virus families. J. Virol. 2007, 4, 72–78, doi:10.1186/1743-422X-4-72.
- Wichgers Schreur, P.J.; Kormelink, R.; Kortekaas, J. Genome packaging of the Bunyavirales. Curr. Opin. Virol. 2018, 33, 151–155, doi:10.1016/j.coviro.2018.08.011.
- Sherman, M.B.; Freiberg, A.N.; Holbrook, M.R.; Watowich, S.J. Single-particle cryo-electron microscopy of Rift Valley fever virus. Virology 2009, 387, 11–15, doi:10.1016/j.virol.2009.02.038.
- DeLano, W.L. PyMOL Molecular Graphic System, version 1.3; Schrödinger LLC: New York, NY, USA, 2015.
