Tin-based perovskite solar cells exist in p-i-n or n-i-p configurations alternating the position of the electron transport (n) and hole transport layer (p). Therefore, during fabrication of these layered devices, it is very common to encounter energy level mismatches and defects at the interface. The simplest trick to improve the performance of tin-based perovskite solar cells is to add an interfacial layer to minimise the energy mismatch and defects at interfaces.
- lead-free
- tin-based
- perovskite solar cells
1. Introduction
The absorption layers of traditional lead-based perovskite solar cells possess an APbX 3 configuration, where A refers to a metal cation or organic cation such as monovalent cesium cation (Cs + ), methylammonium (CH 3NH 3, MA + ), formamidinium ( NH 2CHNH 2, FA + ) and so on; X is the halogen anion, such as iodide (I − ), bromide (Br − ), chloride (Cl − ), or a combination of the above halogens [3][1]. Since the last decade, there are intriguing research studies worldwide on lead-based perovskite solar cells. There exists a wide range of variety and flexibility in perovskite solar cells like fine tuning the perovskite composition [4,5[2][3][4][5],6,7], types of additives [8,9,10][6][7][8], structure [11,12][9][10], employing different electron transport or hole transport layers [13,14][11][12]. There is a significant development in PCE and stability of perovskite solar cells and many commercial companies across the world are investing heavily in the manufacture of these 3rd generation solar cells. A few to mentions are Oxford PV GmbH (Brandenburg, Germany); Saule Technology (Wroclaw, Poland); Microquanta Semiconductor (Hangzhou, China); Solaronix (Aubonne, Switzerland); Swiftsolar (Sancarlos, CA, USA), etc. However, the highly toxic lead metal present in these solar cells still remains a major environmental concern [15][13] as the recycling of such a heavy metal is complicated and expensive. Therefore, researchers have been looking for metal ions that can replace lead in perovskite materials. The radius must not be too different from that of lead ions (Pb 2+ ), otherwise the replacement will cause lattice distortion and form defects. In the periodic table, Sn, located in the same main group as Pb and presenting a close atomic radius, has attracted huge attention from the scientific community. The ionic radius of Pb 2+ is 119 pm and that of Sn 2 + is 118 pm, which allows the simple replacement of Pb by Sn in tin-based perovskite solar cells [16][14]. However, tin suffers from the notorious problem of easily being oxidized from Sn 2+ to Sn 4+ on exposure to ambient conditions [17,18,19,20][15][16][17][18]. This stability concern remains a challenge for long lasting stability of tin-based perovskite cells.
Studies showed that tetravalent tin ion (Sn 4+ ) is self-doped during the crystallization process of perovskite, and it was concluded that the homogeneous distribution of a small amount of Sn 4+ could increase the conductivity of perovskite solar cells [21][19]. However, an excessive amount of Sn 4+ could increase the defects of tin-based perovskite, which could degrade the performance of perovskite solar cells [17,22,23][15][20][21]. Since the first report of pure tin-based perovskite solar cells in 2014 [17][15], research on pure tin-based perovskite solar cells has become more and more popular with scientific researchers. FASnI 3 (FA=CN 2H 5) tin-based perovskite is one of many tin-based perovskites, which is an upgraded version of the MASnI 3 (MA=CH 3NH 3) perovskite type. The former has better thermal stability than the latter [22,23][20][21] due to the higher thermal stability of CN 2H 5 compared to CH 3NH 3. Tin-based perovskite solar cells are achieving good efficiency performance and are the closest candidate among the other Pb-free alternatives to compete with lead-based perovskite. Tin-perovskites are generally having low energy bandgaps, such as MASnI 3 (1.30 eV), CsSnI 3 (1.30 eV) and FASnI 3 (1.41 eV) with bandgaps lower than MAPbI 3 (1.59 eV) maximizing the absorption of photons from sunlight [24,25,26][22][23][24].
One of the most promising approachs to improve the stability of Sn-based perovskite solar cells is the addition of dopants to the tin perovskite material to enhance the homogeneous deposition and to enhance the optoelectronic properties of the absorber layer. Song et al. reported the use of an excess of SnI 2 in CsSnI 3 with a PCE of 4.81% [25][23]. It is demonstrated that the excess of SnI 2 was beneficial to form a uniform film and suppress Sn 4+ vacancies, thereby reducing the p-type conductivity of tin-based perovskite materials. It was also reported that the combination of SnF 2 and pyrazine solves the problem of perovskite phase aggregation and improves the oxidation resistance of the film [26,27][24][25]. In 2016, Chen et al., employed quantum rods (QR) of CsSnI 3 to enhance the PCEs of CsSnI 3 perovskite solar cells to 12.96% [28][26].
2. Crystal Structure Engineering
Low-dimensional FASnI 3 perovskite is a hot spot in current research. The added advantages of low-dimensional tin-based perovskite are its excellent stability, adjustable band gap and excellent photoelectric properties. In 2017, Liao et al., introduced phenylethyl ammonium iodide (PEAI) into a FASnI 3 perovskite. PEA could divide the body type perovskite into a 2D structure. This 2D structure was more stable than the 3D structure [54][27]. The change of perovskite structure with different PEAI levels is shown in Figure 31 a. The same year, Ke et al., demonstrated the addition of ethylene diammonium (en) to FASnI 3 perovskite. The 3D FASnI 3 type perovskite had an adjustable structure, mainly because {en}FASnI 3 could generate many Schottky defects in the crystal structure. Schottky defects provide to ability to change the bandgap of tin-based perovskite [55][28]. The crystal diagram is shown in Figure 31 b. Ran et al. , used a multi-channel diffusion two-step film formation method. In this process, phenylethyl ammonium iodide (PEAI) was evaporated to the surface of FAI and then SnI 2 is evaporated to form a 2D-3D heterojunction perovskite material. This 2D-3D structure could improve the quality and the photoelectric performance of the film. The fabricated device employing this 2D-3D tin-based perovskite structure achieved a PCE of 6.98% [56][29]. The 2D-3D heterojunction fabrication flow chart is shown in Figure 3 1 c. Ng and co-workers added PEAI and Ge in FASnI 3, where PEAI mainly plays a role of blocking water and Ge could reduce the reduce density of trap states in the perovskite [57][30]. The roles and positions of Ge 2+ and PEA + in perovskite crystals are shown in Figure 31 d. Wang and co-workers fabricated a 2D-quasi-2D-3D Sn perovskite film with the pseudohalogen ammonium thiocyanate (NH 4SCN). The film had good oxidation resistance that enhanced the stability of the resulting perovskite film [58][31]. Structure characterization of the control film and structure characterization of film with HSP (2D-quasi-2D-3D structure) are shown in Figure 3 1 e.
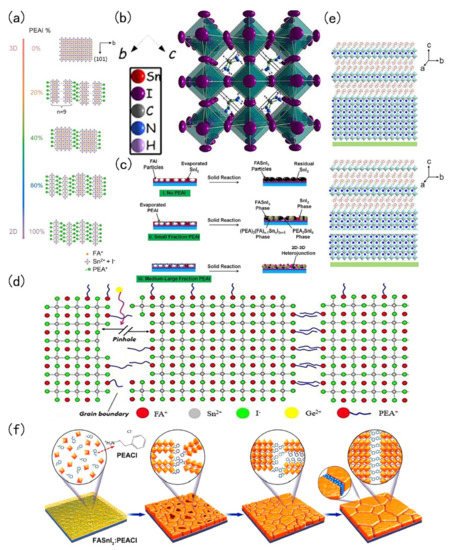
In 2018, Xu et al., employed 5-ammonium valeric acid (5-AVA) and NH 4Cl to make quasi-2D perovskites. The perovskite crystals had a vertical orientation that minimises recombination. They found that NH 4Cl plays an important catalytic role in the crystal growth direction. Such vertically oriented grains were conducive to the migration of the charge carrier, thereby improving the performance of the solar cell [60][33]. Tsai et al., found that different proportions of 2-hydroxyethylammonium (HEA + ) ion mixed with FA + could change the dimensions of perovskite crystals. Below 100%, it formed a 3D orthogonal structure; at 100%, it was a 2D orthogonal structure [61][34]. In 2020, Li and co-workers encapsulated the perovskite layer, where they introduced butylethylammonium (BEA) and synthesized perovskite of (BEA)(FA) n−1 SnnI 3n+1 (n = 1–3) with a low-dimension Dion-Jacobson phase. The advantage of the tin-based perovskite of this material and low-dimensional tin-based perovskite was that its band gap was less affected by lattice distortion. Moreover, the low-dimensional tin-based perovskite was stable against attack by water and oxygen. Due to the introduction of BEA the confinement effect of quantum wells was also greatly reduced, and the migration length of carriers reaches 340 nm (holes) and 450 nm (electrons) [62][35]. In the same year, Li et al., synthesized a vertically oriented 2D tin-based perovskite. They introduced phenylethylammonium chloride (PEACl) to grow a vertically oriented 2D tin-based perovskite. Usual parallel oriented 2D perovskite has good stability but negatively affects the electrical properties due to the fact large-long alkyl chain cations will hinder the carrier transportation [63][36]. On the contrary, the vertically oriented 2D tin-based perovskite FASnI 3: PEACl demonstrated an improved stability with the photoelectric performance reaching 9%, but the growth of this 2D tin-based perovskite was highly temperature sensitive at only 100 ℃, and then the film was pure vertical 2D tin-based perovskite [59][32].
3. Interfacial Layer Engineering
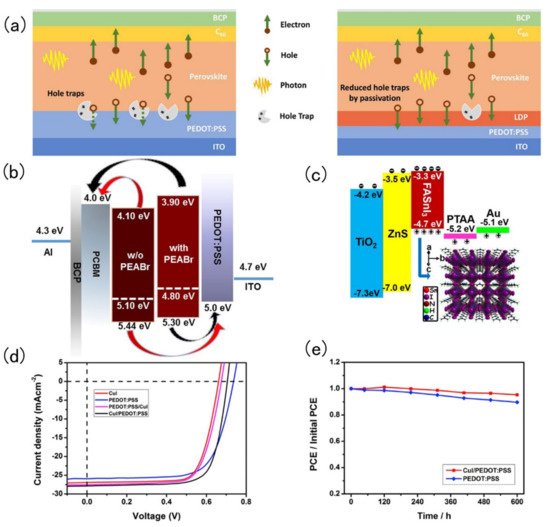
Tin-based perovskite solar cells exist in p-i-n or n-i-p configurations alternating the position of the electron transport (n) and hole transport layer (p). Therefore, during fabrication of these layered devices, it is very common to encounter energy level mismatches and defects at the interface. The simplest trick to improve the performance of tin-based perovskite solar cells is to add an interfacial layer to minimise the energy mismatch and defects at interfaces. Chen and co-workers introduced a low dimension perovskite interlayer. The passivation of defects in perovskite crystals by LDP is shown in Figure 42 a. Phenylethyl ammonium bromide (PEABr) was added to a Sn-perovskite absorber layer, then the hole transport layer could separate 3D perovskite crystals into 2D perovskite crystals. The resulting 2D perovskite could greatly reduce the accumulation and recombination of the charge carriers. The manufactured device reaches a PCE of 7.05% [64][37]. In 2019, Liao and co-workers spin coated PEABr on top of a FASnI 3 perovskite layer to form an ultra-thin perovskite layer. After PEABr treatment, the energy level of perovskite, hole transport layer and electron transport layer match well, which was conducive to the better transport of holes and electrons [65][38]. PEABr changes the perovskite band structure as shown in Figure 42 b. Ke and his collaborators spin-coated a ZnS layer on the TiO 2 layer. In this way, the band structure between the electron transport layer and the perovskite layer could be adjusted, and the open circuit voltage could be significantly increased, but the short-circuit current and filling factor were not reduced, and the device achieved a PCE of 5.27% [66][39]. The energy band diagram of the FASnI 3 solar cells and crystal structure of the perovskite absorber are shown in Figure2 4 c. Hu and his collaborators fabricated SnO 2 and TiO 2 dual-electron transport layer FASnI 3 perovskite solar cells. The advantage of this dual-electron transport layer was that it could hinder the formation of defects at the interface and in the perovskite [67][40]. Song and his collaborators designed a double-layer hole transport layer (CuI and PEDOT: PSS). The 2D double-layer had more outstanding hole-transport capabilities than a single-layer one and it could also improve the quality of perovskite film formation [68][41]. J-V curve of devices based on various HTLs is shown in Figure 42 d. CuI and a PEDOT: PSS double hole transport layer structure change the solar cell stability as shown in Figure 42 e.
4. Conclusions and Perspectives
This review briefly introduces the important research progress made in improving the stability and performance of tin-based perovskites. The review focuses on role of introducing additives such as inorganic additives like SnF 2, NH 4H 2PO 2, N 2H 5Cl, organic additives, viz. ethylene diammonium diiodide (EDAI 2), 5-ammonium valeric acid iodide (5-AVAI) 3-phenyl-2-propen-1-amine (PPA) to study their effects on film properties and device performance. Low-dimensional tin-based perovskites are very mysterious to many researchers and hence it is crucial to understand their properties through in-depth research. This article introduces some tin-based perovskites such as 2D, 3D, 2D-3D, etc. 2D perovskites are more stable than 3D perovskites, but the photoelectric performance is disadvantaged, so low-dimensional perovskite may be a future research hot spot. There will always be some practical issues in the direct combination of the materials of the tin-based perovskite solar cells.
References
- Yang, W.F.; Igbari, F.; Lou, Y.H.; Wang, Z.K.; Liao, L.S. Tin halide perovskites: Progress and challenges. Adv. Energy Mater. 2019, 10, 1902584.
- Li, M.; Wang, Z.K.; Zhuo, M.P.; Hu, Y.; Hu, K.H.; Ye, Q.Q.; Jain, S.M.; Yang, Y.G.; Gao, X.Y.; Liao, L.S. Pb–Sn–Cu ternary organometallic halide perovskite solar cells. Adv. Mater. 2018, 30, e1800258.
- Singh, T.; Miyasaka, T. Stabilizing the efficiency beyond 20% with a mixed cation perovskite solar cell fabricated in ambient air under controlled humidity. Adv. Energy Mater. 2018, 8, 1700677.
- Zeng, Q.; Zhang, X.; Feng, X.; Lu, S.; Chen, Z.; Yong, X.; Redfern, S.A.T.; Wei, H.; Wang, H.; Shen, H.; et al. Polymer-passivated inorganic cesium lead mixed-halide perovskites for stable and efficient solar cells with high open-circuit voltage over 1.3 V. Adv. Mater. 2018, 30, 1705393.
- Valerio, L.; Rosa, A.; Rodriguez, V.; Enriquez, C.; Telles, A.; Ramirez, Y.; Rivera, D.; Hierro, J.; Bustamante, L.; Tong, X.; et al. Characterization and analysis of FAxCs(1−x) Pb(IyBr(1−y))3 perovskite solar cells with thickness controlled transport layers for performance optimization. AIP Adv. 2019, 9, 105006.
- Bai, S.; Da, P.; Li, C.; Wang, Z.; Yuan, Z.; Fu, F.; Kawecki, M.; Liu, X.; Sakai, N.; Wang, J.T.; et al. Planar perovskite solar cells with long-term stability using ionic liquid additives. Nature 2019, 571, 245–250.
- Li, M.; Yang, Y.G.; Wang, Z.K.; Kang, T.; Wang, Q.; Turren-Cruz, S.H.; Gao, X.Y.; Hsu, C.S.; Liao, L.S.; Abate, A. Perovskite grains embraced in a soft fullerene network make highly efficient flexible solar cells with superior mechanical stability. Adv. Mater. 2019, 31, e1901519.
- Li, M.; Zuo, W.W.; Wang, Q.; Wang, K.L.; Zhuo, M.P.; Köbler, H.; Halbig, C.E.; Eigler, S.; Yang, Y.G.; Gao, X.Y.; et al. Ultrathin nanosheets of oxo-functionalized graphene inhibit the ion migration in perovskite solar cells. Adv. Energy Mater. 2019, 10, 1902653.
- Zhang, T.; Long, M.; Qin, M.; Lu, X.; Chen, S.; Xie, F.; Gong, L.; Chen, J.; Chu, M.; Miao, Q.; et al. Stable and efficient 3D-2D perovskite-perovskite planar heterojunction solar cell without organic hole transport layer. Joule 2018, 2, 2706–2721.
- Hartono, N.T.P.; Sun, S.; Gélvez-Rueda, M.C.; Pierone, P.J.; Erodici, M.P.; Yoo, J.; Wei, F.; Bawendi, M.; Grozema, F.C.; Sher, M.-J.; et al. The effect of structural dimensionality on carrier mobility in lead-halide perovskites. J. Mater. Chem. A 2019, 7, 23949–23957.
- Li, M.; Wang, Z.-K.; Kang, T.; Yang, Y.; Gao, X.; Hsu, C.-S.; Li, Y.; Liao, L.-S. Graphdiyne-modified cross-linkable fullerene as an efficient electron-transporting layer in organometal halide perovskite solar cells. Nano Energy 2018, 43, 47–54.
- Yang, J.; Zhang, Q.; Xu, J.; Liu, H.; Qin, R.; Zhai, H.; Chen, S.; Yuan, M. All-inorganic perovskite solar cells based on CsPbIBr2 and metal oxide transport layers with improved stability. Nanomaterials 2019, 9, 1666.
- Li, J.; Cao, H.L.; Jiao, W.B.; Wang, Q.; Wei, M.; Cantone, I.; Lu, J.; Abate, A. Biological impact of lead from halide perovskites reveals the risk of introducing a safe threshold. Nat. Commun. 2020, 11, 310.
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomie distances in halides and chaleogenides. Acta Cryst. 1976, 32, 751.
- Noel, N.K.; Stranks, S.D.; Abate, A.; Wehrenfennig, C.; Guarnera, S.; Haghighirad, A.-A.; Sadhanala, A.; Eperon, G.E.; Pathak, S.K.; Johnston, M.B.; et al. Lead-free organic–inorganic tin halide perovskites for photovoltaic applications. Energy Environ. Sci. 2014, 7, 3061–3068.
- Mare, S.D.; Menossi, D.; Salavei, A.; Artegiani, E.; Piccinelli, F.; Kumar, A.; Mariotto, G.; Romeo, A. SnS thin film solar cells: Perspectives and limitations. Coatings 2017, 7, 34.
- Mare, S.D.; Salavei, A.; Menossi, D.; Piccinelli, F.; Bernardi, P.; Artegiani, E.; Kumar, A.; Mariotto, G.; Romeo, A. A study of SnS recrystallization by post deposition treatment. In Proceedings of the IEEE 43rd Photovoltaic Specialists Conference 2016, Portland, OR, USA, 5–10 June 2016; p. 16468525.
- Mare, S.D.; Salavei, A.; Menossi, D.; Piccinelli, F.; Artegiani, E.; Kumar, A.; Mariotto, G.; Bernardi, P.; Romeo, A. Effects of temperature and post deposition annealing on SnS polycrystalline thin film growth. In Proceedings of the 32nd European Photovoltaic Solar Energy Conference and Exhibition 2016, Munich, Germany, 20–24 June 2016; pp. 263–266.
- Takahashi, Y.; Obara, R.; Lin, Z.Z.; Takahashi, Y.; Naito, T.; Inabe, T.; Ishibashi, S.; Terakura, K. Charge-transport in tin-iodide perovskite CH3NH3SnI3: Origin of high conductivity. Dalton Trans. 2011, 40, 5563–5568.
- Koh, T.M.; Krishnamoorthy, T.; Yantara, N.; Shi, C.; Leong, W.L.; Boix, P.P.; Grimsdale, A.C.; Mhaisalkar, S.G.; Mathews, N. Formamidinium tin-based perovskite with low Eg for photovoltaic applications. J. Mater. Chem. A 2015, 3, 14996–15000.
- Shi, T.; Zhang, H.-S.; Meng, W.; Teng, Q.; Liu, M.; Yang, X.; Yan, Y.; Yip, H.-L.; Zhao, Y.-J. Effects of organic cations on the defect physics of tin halide perovskites. J. Mater. Chem. A 2017, 5, 15124–15129.
- Bansode, U.; Naphade, R.; Game, O.; Agarkar, S.; Ogale, S. Hybrid Perovskite films by a new variant of pulsed excimer laser deposition: A room-temperature dry process. J. Phys. Chem. C 2015, 119, 9177–9185.
- Song, T.-B.; Yokoyama, T.; Aramaki, S.; Kanatzidis, M.G. Performance enhancement of lead-free tin-based perovskite solar cells with reducing atmosphere-assisted dispersible additive. ACS Energy Lett. 2017, 2, 897–903.
- Lee, S.J.; Shin, S.S.; Kim, Y.C.; Kim, D.; Ahn, T.K.; Noh, J.H.; Seo, J.; Seok, S.I. Fabrication of efficient formamidinium tin iodide perovskite solar cells through SnF2-pyrazine complex. J. Am. Chem. Soc. 2016, 138, 3974–3977.
- Yokoyama, T.; Song, T.-B.; Cao, D.H.; Stoumpos, C.C.; Aramaki, S.; Kanatzidis, M.G. The origin of lower hole carrier concentration in methylammonium tin halide films grown by a vapor-assisted solution process. ACS Energy Lett. 2017, 2, 22–28.
- Chen, L.J.; Lee, C.R.; Chuang, Y.J.; Wu, Z.H.; Chen, C. Synthesis and optical properties of lead-free cesium tin halide perovskite quantum rods with high-performance solar cell application. J. Phys. Chem. Lett. 2016, 7, 5028–5035.
- Liao, Y.; Liu, H.; Zhou, W.; Yang, D.; Shang, Y.; Shi, Z.; Li, B.; Jiang, X.; Zhang, L.; Quan, L.N.; et al. Highly oriented low-dimensional tin halide perovskites with enhanced stability and photovoltaic performance. J. Am. Chem. Soc. 2017, 139, 6693–6699.
- Ke, W.; Stoumpos, C.C.; Zhu, M.; Mao, L.; Spanopoulos, I.; Liu, J.; Kontsevoi, O.Y.; Chen, M.; Sarma, D.; Zhang, Y.; et al. Enhanced photovoltaic performance and stability with a new type of hollow 3D perovskite FASnI3. Sci. Adv. 2017, 3, e1701293.
- Ran, C.; Xi, J.; Gao, W.; Yuan, F.; Lei, T.; Jiao, B.; Hou, X.; Wu, Z. Bilateral interface engineering toward efficient 2D–3D bulk heterojunction tin halide lead-free perovskite solar cells. ACS Energy Lett. 2018, 3, 713–721.
- Ng, C.H.; Hamada, K.; Kapil, G.; Kamarudin, M.A.; Wang, Z.; Iikubo, S.; Shen, Q.; Yoshino, K.; Minemoto, T.; Hayase, S. Reducing Traps Density and Carriers Concentration by Ge Additive for An Efficient Quasi 2D/3D Perovskite Solar Cell. J. Mater. Chem. A 2020, 8, 2962–2968.
- Wang, F.; Jiang, X.; Chen, H.; Shang, Y.; Liu, H.; Wei, J.; Zhou, W.; He, H.; Liu, W.; Ning, Z. 2D-quasi-2D-3D hierarchy structure for tin perovskite solar cells with enhanced efficiency and stability. Joule 2018, 2, 2732–2743.
- Li, M.; Zuo, W.-W.; Yang, Y.-G.; Aldamasy, M.H.; Wang, Q.; Cruz, S.H.T.; Feng, S.-L.; Saliba, M.; Wang, Z.-K.; Abate, A. Tin halide perovskite films made of highly oriented 2D crystals enable more efficient and stable lead-free perovskite solar cells. ACS Energy Lett. 2020, 5, 1923–1929.
- Xu, H.; Jiang, Y.; He, T.; Li, S.; Wang, H.; Chen, Y.; Yuan, M.; Chen, J. Orientation regulation of tin-based reduced-dimensional perovskites for highly efficient and stable photovoltaics. Adv. Funct. Mater. 2019, 29, 1907696.
- Tsai, C.-M.; Lin, Y.-P.; Pola, M.K.; Narra, S.; Jokar, E.; Yang, Y.-W.; Diau, E.W.-G. Control of crystal structures and optical properties with hybrid formamidinium and 2-hydroxyethylammonium cations for mesoscopic carbon-electrode tin-based perovskite solar cells. ACS Energy Lett. 2018, 3, 2077–2085.
- Li, P.; Liu, X.; Zhang, Y.; Liang, C.; Chen, G.; Li, F.; Su, M.; Xing, G.; Tao, X.; Song, Y. Low-dimensional dion–jacobson-phase lead-free perovskites for high-performance photovoltaics with improved stability. Angew. Chem. Int. Ed. Engl. 2020, 59, 6909–6914.
- Chen, A.Z.; Shiu, M.; Ma, J.H.; Alpert, M.R.; Zhang, D.; Foley, B.J.; Smilgies, D.M.; Lee, S.H.; Choi, J.J. Origin of vertical orientation in two-dimensional metal halide perovskites and its effect on photovoltaic performance. Nat. Commun. 2018, 9, 1336.
- Chen, K.; Wu, P.; Yang, W.; Su, R.; Luo, D.; Yang, X.; Tu, Y.; Zhu, R.; Gong, Q. Low-dimensional perovskite interlayer for highly efficient lead-free formamidinium tin iodide perovskite solar cells. Nano Energy 2018, 49, 411–418.
- Liao, M.; Yu, B.B.; Jin, Z.; Chen, W.; Zhu, Y.; Zhang, X.; Yao, W.; Duan, T.; Djerdj, I.; He, Z. Efficient and stable FASnI3 perovskite solar cells with effective interface modulation by low-dimensional perovskite layer. ChemSusChem 2019, 12, 5007–5014.
- Ke, W.; Stoumpos, C.C.; Logsdon, J.L.; Wasielewski, M.R.; Yan, Y.; Fang, G.; Kanatzidis, M.G. TiO2-ZnS cascade electron transport layer for efficient formamidinium tin iodide perovskite solar cells. J. Am. Chem. Soc. 2016, 138, 14998–15003.
- Hu, M.; Zhang, L.; She, S.; Wu, J.; Zhou, X.; Li, X.; Wang, D.; Miao, J.; Mi, G.; Chen, H.; et al. Electron transporting bilayer of SnO2 and TiO2 nanocolloid enables highly efficient planar perovskite solar cells. Solar RRL 2019, 4, 1900331.
- Song, J.; Hu, W.; Li, Z.; Wang, X.-F.; Tian, W. A double hole-transport layer strategy toward efficient mixed tin-lead iodide perovskite solar cell. Sol. Energy Mater. Sol. Cells 2020, 207, 110351.
