There are two species of camels: one-humped camels (Camelus dromedarius) and two-humped camels (Camelus bactrianus). Camel milk (CM) is an essential food source in arid and semi-arid areas, as cow milk is not always available in these areas due to a lack of adaptation mechanisms. Despite these conditions, CM has been considered as one of the best alternatives to feed adults and infants, as well as for the production of many dairy products such as yogurt, cheese, cream, and butter.
- camel milk
- milk fat globules
- cholesterol
- fatty acids
- phospholipids
- health-promoting benefits
1. Introduction
Understanding the relationship between components of milk fats, diet, and health is now known to be one of the key concepts to a better lifestyle, disease prevention, and well-being promotion. Milk consumption for all mammals except cows has increased by 17% in the last 50 years in all countries [1]. Camels are a cultural, economic, and health-promoting icon [2] with an estimated worldwide population of over 35 million [3]. There are two species of camels: one-humped camels ( Camelus dromedarius ) and two-humped camels ( Camelus bactrianus ) [4]. Camel milk (CM) is an essential food source in arid and semi-arid areas, as cow milk is not always available in these areas due to a lack of adaptation mechanisms. Despite these conditions, CM has been considered as one of the best alternatives to feed adults and infants [5[5][6],6], as well as for the production of many dairy products such as yogurt, cheese, cream, and butter [7]. Besides its nutritive value, CM has also long been recognized for its health benefits by nomadic people for centuries, and recent studies have revealed its potential value in the treatment of a variety of human diseases such as asthma, tuberculosis, jaundice, and gastrointestinal diseases [8]. However, despite these interesting properties and applications, it has not received as much attention as cow milk.
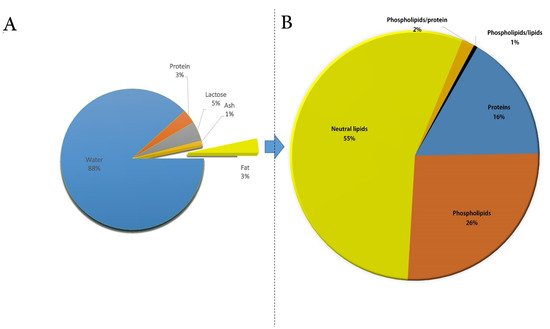
Reported data collected from 121 references published between 1905 and 2019 showed that the mean and standard deviation of components in CM were 12.2 ± 1.62% total solids, 3.28 ± 0.59 protein, 4.47 ± 0.66 lactose, 0.81 ± 0.19 ash, and 3.68 ± 1.00 fat ( Figure 1 A) [9]. CM fat exists as milk fat globules (MFGs) in the water, with a size ranging from 1.1 to 2.1 mm, which is lower than those of buffalo (3.9–7.7 mm), cow (1.6–4.9 mm), and goat milk (1.1–3.9 mm) [10], justifying its faster digestion rate in comparison to other mammalian milk. MFGs are covered with a layer of a surface-active substance called milk fat globule membrane (MFGM), and they have many nutritional and health roles. Similar to other milk types, the composition of CM fat constantly changes as a result of many environmental and physiological influences [11,12,13][11][12][13]. Such chemical changes affect its physical properties and its dairy products [12,14][12][14]. Fat is an important component of CM, including a complex mixture of natural fats (i.e., triglycerides, phospholipids, cholesterol, and other elements), representing one of the sources of energy, in addition to fat-soluble vitamins. Moreover, CM fat is an excellent source of essential fatty acids (EFAs) [12,15][12][15] and may meet the daily nutritional requirements of countries whose traditional diet is high in carbohydrates. Indeed, the consumption of human milk in these countries commonly leads to a low level of EFAs, such as alpha-linolenic acid (ALA) and linoleic acid (LA) [16,17][16][17]. Another characteristic lipid profile of CM includes its good source of polyunsaturated FAs (PUFAs) i.e., ALA, eicosapentaenoic acid (EPA), and arachidonic acid (AA) [18[18][19][20],19,20], compared to other mammalian species of milk, posing it as a better fat source for individuals at risk of lipid-related cardiovascular diseases [21,22][21][22]. It should be noted that CM cholesterol level is contradictory if compared to cow milk [11,14,23,24][11][14][23][24]. Despite some contradictory reports, several researchers have demonstrated that CM (both fresh and fermented milk) reduced the development of hypercholesterolemia in rats [25,26,27,28][25][26][27][28].
CM fat was reported to act as an emulsifier more than human or cow milk because it contains almost 1% of its total lipids as phospholipids (PLs) [6]. Furthermore, it was reported that the amount of plasmalogen and sphingomyelin (SM) were similar in camel and human milk [6]. For this reason, it can be useful in newborn nutrition if the PLs are purified and concentrated and used to complement human milk fortifiers or produce novel milk replacements.
On the basis of the aforementioned information, it is evident that the CM fat components are quite comparable to human and cow milk. Nevertheless, the majority of studies have focused on the fat composition of cow and human milk, with less reported regarding CM. The objective of the present review is to highlight the reports currently available on the detailed composition and nutritional value of CM fat for improved utilization in dairy industries with focus on the recent findings of its applications.
2. Composition and Functional Properties of CM Fat Globule and Membrane
Milk is a lipid-in-water emulsion that plays an important role in human nutrition. MFGs are a combination of proteins and lipids with nutraceutical characteristics linked to the MFGM, which preserves them and prevents coalescence.
The complex MFGM architecture ensures stable dispersion of MFGs in milk through polar lipids and glycoproteins present in the membrane, inducing electrostatic and steric repulsion, in addition to its nutritional and health effects inside the body. According to Lindmark Månsson [46][30], fats account for 30% of the membrane and can be divided into three types (i.e., phospholipids (25%), cerebrosides (3%), and cholesterol (2%)), while proteins make up the remaining 70% of the membrane. Recently, increasing attention has been paid to the components of MFGM, especially to their protein components [47][31]. MFGM proteins, which account for 1–4% of the total milk protein, differ depending on the breed [48][32]. The proteins of camel MFGM are mainly involved in protein processing, bio-synthesis of fat, and actin cytoskeleton organization [47,49][31][33]. The main MFGM proteins are FAs synthase, xanthine oxidase, butyrophilin, lactadherin, and adipophilin [49][33]. During the secretion of the MFGs [50][34], the localization of the proteins differs: some are related to the inner monolayer membrane, while others to the outer bilayer membrane [51,52][35][36]. Camel MFGM proteins have been investigated in order to explain MFGM structure and how the proteins vary between the different species [47,48,49][31][32][33].
Finally, it has been suggested that MFGM proteins may be exploited to make products rich in MFGM fraction to boost the immune system, control cholesterol metabolism, and provide the body with beneficial polar lipids [57][37]. Moreover, the MFGM protects the triglycerides from lipolysis and auto-oxidation before the actual digestion [58][38]. As a result, MFGM protein is used in the dairy industry, particularly in infant formula supplements. In addition, it has numerous health-promoting properties, such as antiviral [59][39], anticancer [60][40], and anti-inflammatory effects [61][41]. Thus, future research will most likely focus on a deeper comprehension of MFGM proteins in CM as well as their possible biological roles.
From a technological point of view, the camel MFG behavior during drying has been recently studied by Zouari et al. [76][42]. The microstructure of drying the whole CM revealed less surface roughness compared to that of partially skimmed cow milk. Furthermore, the lower distribution size and the high crystallization temperature of CM fat led to the encapsulation of most CM fat globules with proteins near the surface of the powder.
3. Composition and Distributions of TAGs in the CM
The determination of CM fat physicochemical and nutritional properties provides insight into the structure of TAGs, which is defined by the types, quantities, and distribution of FAs. Differences in diet, season, lactation stage, and animal species can lead to changes in the amount and composition of milk TAGs. Only one study reported on the composition of milk TAGs for both types of camel milk [12]. Previous studies of TAGs in milk fat have shown that there are a large number of species-related TAGs, as well as the associated positional isomers, which makes the determination of TAG composition rather challenging [12,15][12][15]. For example, there were few variations among the species; for example, C. bactrianus milk fat had more TAGs than C. dromedarius milk fat. The species influence has been attributed to the empirically observed positive association between TAG saturation and relative strength of [TAG + H] + in a variety of molecular systems, most notably in the relative splint magnitude of a sequence of linolyte and arachidonate TAGs [109][43]. In addition, TAGs composition in CM significantly differs from that of other ruminants [110][44]. Several studies [12,14,20,85,91][12][14][20][45][46] showed that major TAGs in CM fat are CN46, CN48, CN50, and CN52 ( Table 41 ) and that CM fat is free of SC-SFAs and characterized by low MC-SFAs levels, which may be attributed to the lower levels of caprylic and capric acids in the lipids. As a result, TAGs in this milk fat are often made up of LC-FAs with high equivalent carbon atom numbers. In addition, Ref.[14] found that TAGs composition had a similar trend in buffalo, cow, and sheep milk, which were found to be richer in CN32 and CN40 fats than in CM.
| Studies | Samples | Extraction Method | Analysis Method | Major TAGs/Concentration |
|---|---|---|---|---|
| [20] | C. dromedarius (n = 5) | Folch | RP-HPLC/-APCI-MS | PPL (13.67%), POM (12.78%), PPO (11.77%), POO (10.67%), PPM (6.76%) |
| [46] | C. dromedarius (n = 20) | Folch | HPLC/ESI-MS | MPO (6.71%), PPO (5.72%), SPO (5.30%) |
| [14] | C. dromedarius (n = 20) | Rose–Gottlieb | GC | CN52 (20.37%), CN48 (21.60%), CN46 (12.48%), CN50 (25.79%) |
| [45] | C. dromedarius | Folch | UPLC/Q-TOF–MS | OPM/PPaP/PaMS (10.96%), OPaP/OMO/PLP (8.91%), OPP/OSM/PSPa (8.80%) |
| [12] | C. dromedarius and C. bactrianus | Mojonnier | UPC2/Q-TOF–MS | C. dromedarius: MPaPa/LMM/OMMy (5.36%), MPO (11.73%), SPP/MSS (7.15%), PaPaO (8.37%), PSS (7.88%) C. bactrianus: MMO/MyMS/LaPO (7.25%), MSP (8.52%), LaLS/MHH (7.04%), SPP/MSS (8.22%), PSS (5.91%) |
Structure of fatty acids: M, C14:0; My, C14:1; P, C16:0; Pa, C16:1; L, C18:2; O, C18:1; S, C18:0; Reverse-phase high-performance liquid chromatography–atmospheric pressure chemical ionization mass spectrometr, (RP-HPLC/APCI-MS); Electrospray ionization-mass spectrometry, (ESI-MS); Gas chromatography, (GC); Ultra performance liquid chromatography–electrospray ionization–Quadrupole time-of-flight mass spectrometery, (UPLC/Q-TOF–MS); Ultra-performance convergence chromatography, (UPC2).
Odd-chain FAs are widely present in CM fat and constitute about 13.5% of TAGs [91][46], allowing for several nutritional and medical applications [111][47]. The majority of TAGs in the mammary glands of camels were found to be made up of both SFAs and UFAs [91][46], likely due to the preservation of fat fluidity at physiological temperatures. According to a previous study by Haddad et al. [91][46] , CM contains a large amount of saturated and unsaturated LC-TAG molecular species. From an industry point of view, these saturated LC-TAG molecular species improve the crystallization of butter products [112][48], and it would be very important to examine whether its inclusion in butter manufacture would improve quality without affecting other sensory characters. In contrast, the high degree of unsaturated LC-TAG molecular species is negatively associated with firmness in bovine milk butter [113][49].
To date, relatively few studies have reported TAG distribution and composition in CM compared to extensive reports focused on cow and human milk. The acyltransferase specificity and activity are responsible for the non-random FA distribution. Thus, by comparing experimental to theoretical TAG distributions, researchers have shown the non-random distribution of FAs in CM [91][46]. FA distribution of CM TGA follows a certain pattern with the highest concentration of SFAs detected at the sn -2 position, whereas UFAs are primarily at sn -1 and sn -3 positions. The majority of palmitic acid is present at the sn -1,3 locations [12,15][12][15]. Thus, there are relatively low contents of palmitic acid at the sn -2 positions, contrary to most natural fats. As a result, the distribution of the three main FAs in natural fats significantly differs from that in human milk and standard vegetable oils [94][50], whereas the presence of palmitic acid at the TAG sn -2 location has been linked to the enhanced fat and calcium absorption, intestinal comfort, and the growth of intestinal microorganisms [114,115][51][52]. Other properties of CM TAG distributions are primarily responsible for the rheological qualities of milk fat products.
4. CM Butter and Its Production Challenges
CM butter is one of the dairy products made from CM. In various regions around the world, such as Algerian Sahara [133][53], northern Kenya [134][54], and Sinai Peninsula [102][55], it is produced by a traditional churning process as in the case of other animal butter. Ghee (filtered butter) is produced from CM, a popular Indian commodity, though the end product has a lower yield than buffalo or cow milk [135][56]. Moreover, the possibility of making butter from CM was also reported by [102,136][55][57]. To produce 80% of butter yield, vigorous hot shaking (22–23 °C) was needed [102][55]. In 2021, Mtibaa et al. studied the influence of ripening and churning circumstances on the turn ability of CM cream and the physical characteristics of CM butter [137][58]. It was found that CM butter could only be produced when churned at 21 °C, independent of the ripening temperature. In arid areas, this commodity is essential for nutrition, and there is an increasing demand for it all over the world for its health benefits.
Butter flavor and aroma, as well as its rheological properties, have a large impact on consumer acceptance. Its properties are determined by the types of FAs found in butter. SC-FAs play a significant part in butter flavor. The lower content of butyric acid in CM butter resulted in a less intense flavor relative to cow milk butter [138][59]. Another lacking chemical in camel butter is B-carotene, and thus the obtained butter is whiter in color compared to the cow milk-derived butter [102,104][55][60]. CM butter, on the other hand, contained a significant amount of MUFAs (C16:1 and C18:1) (32.2% of total FAs), which is nutritionally advantageous [138][59]. The butter characteristics are determined by the kinds of FAs present. Hard butter is made from fats with a high content of high melting point FAs, whereas soft butter is made from fats with a low quantity of low melting point FAs [139][61]. The rheological qualities of butter are largely determined by the TAG structure, mainly by the distribution of FAs at the sn -1, sn -2, and sn -3 positions, as well as by the ripening temperature [137][58]. The content of total solids and fat of C. dromedarius milk butter generally ranges from 64 to 65% and 49 to 58%, respectively [133,138][53][59]. Due to the high content of SFAs in CM cream, the churning temperature (15–35 °C) was found to be higher than that of cow milk cream (10–15 °C) [67][62]. As a result, the ripening and churning conditions had a significant impact on the melting characteristics and rheological behavior of CM butter [137][58]. The acid degree value, melting point, and refractive index for C. dromedarius were at 6.7, 43.2 °C, and 1.4, respectively [138][59]. Such values confirm that C. dromedarius milk butter is less prone to rancidity because of the lower acid degree value. The high level of LC-FAs (C14-C18) and low percentage of SC-FAs (C4-C6) in C. dromedarius milk butter may have contributed to its high melting point, and high refractive index value.
The selected technology for the manufacturing of CM butter is a major challenge. Indeed, traditional churning presents some criticisms because it shows a slight tendency to cream up, due to the lack of the agglutinin protein in the CM [62][63]. The euglobulin protein is the key factor responsible for speeding the cream layer development in cow milk. Consequently, CM displays a very slow creaming rate at all temperatures with respect to cow milk [55][64]. Camel MFGs and proteins are closely related, which is an inherent property of the CM fat [140][65]. Its smaller size MFGs [6,45,66][6][66][67] leads to low butter yield compared to cow milk. Additionally, the high melting point of CM fat (41–42 °C) makes it difficult to churn the cream at the temperatures used for cow milk churning (8–12 °C) [102][55]. As a result, more force is needed to separate the fat globule membrane from the fat and to allow for the globules to adhere to each other, forming butter as water-in-oil emulsion [134][54]. The traditional churning method associated with higher churning force has recently been documented, resulting in less churning time and higher butterfat recovery from CM, compared to the common conventional mixing method [138][59]. Furthermore, it has a relatively low flavor content when compared to buffalo or cow milk, which would discourage consumer preferences [138][59]. The low flavor is mostly attributed to its low butyric acid content (less than 0.5%) compared to that in cow milk (about 5%) [11]. Moreover, it is difficult to distinguish butter color from other CM components during production, owing to its low β-carotene level [102,104][55][60]. The poor CM creaming production is also due to many other factors, such as the electrical charges on the globules, the ionic distribution, and the interfacial tension between milk serum and fat globules [55][64]. For this reason, producing CM butter by means of the same technique employed for cow milk butter may be difficult. As a result, the systematic use of already proven technology for cow milk butter is not always appropriate for CM butter, and adjustments based on more basic study on the behavior of milk fat components during processing are required. Moreover, transferring research results, which are now available, to an industrial scale is still insufficient, especially for the CM butter product, which requires additional technical and economic evaluations.
References
- Khalesi, M.; Salami, M.; Moslehishad, M.; Winterburn, J.; Moosavi-Movahedi, A.A. Biomolecular content of camel milk: A traditional superfood towards future healthcare industry. Trends Food Sci. Technol. 2017, 62, 49–58.
- Suliman, G.M.; Alowaimer, A.N.; Hussein, E.O.; Ali, H.S.; Abdelnour, S.A.; El-Hack, M.E.A.; Swelum, A.A. Chemical Composition and Quality Characteristics of Meat in Three One-Humped Camel (Camelus dromedarius) Breeds as Affected by Muscle Type and Post-Mortem Storage Period. Animals 2019, 9, 834.
- FAO. Proceedings of the Gateway to Dairy Production and Products; Food and Agriculture Organisation of the United Nations (FAOSTAT): Rome, Italy, 2019; Available online: http://www.fao.org/dairy-production-products/en/ (accessed on 3 September 2021).
- Al-Sayyed, H.F. Historical Background and Population of Camels. In Handbook of Research on Health and Environmental Benefits of Camel Products; IGI Global: Hershey, PA, USA, 2020; pp. 1–14.
- El-Agamy, E.I. Camel milk. In Handbook of Milk of Non-Bovine Mammals; Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 297–344.
- Garcia, C.; Lutz, N.W.; Confort-Gouny, S.; Cozzone, P.J.; Armand, M.; Bernard, M. Phospholipid fingerprints of milk from different mammalians determined by 31P NMR: Towards specific interest in human health. Food Chem. 2012, 135, 1777–1783.
- Konuspayeva, G.; Faye, B. Recent Advances in Camel Milk Processing. Animals 2021, 11, 1045.
- Swelum, A.A.; El-Saadony, M.T.; Abdo, M.; Ombarak, R.A.; Hussein, E.O.S.; Suliman, G.; Alhimaidi, A.R.; Ammari, A.A.; Ba-Awadh, H.; Taha, A.E. Nutritional, antimicrobial and medicinal properties of Camel’s milk: A review. Saudi J. Biol. Sci. 2021, 28, 3126–3136.
- Konuspayeva, G.S. Camel milk composition and nutritional value. In Handbook of Research on Health and Environmental Benefits of Camel Products; IGI Global: Hershey, PA, USA, 2020; pp. 15–40.
- Meena, S.; Rajput, Y.; Sharma, R. Comparative fat digestibility of goat, camel, cow and buffalo milk. Int. Dairy J. 2014, 35, 153–156.
- Konuspayeva, G.; Lemarie, É.; Faye, B.; Loiseau, G.; Montet, D. Fatty acid and cholesterol composition of camel’s (Camelus bactrianus, Camelus dromedarius and hybrids) milk in Kazakhstan. Dairy Sci. Techn. 2008, 88, 327–340.
- Bakry, I.A.; Ali, A.H.; Abdeen, E.-S.; Ghazal, A.F.; Wei, W.; Wang, X. Comparative characterisation of fat fractions extracted from Egyptian and Chinese camel milk. Int. Dairy J. 2020, 105, 104691.
- He, J.; Xiao, Y.; Orgoldol, K.; Ming, L.; Yi, L.; Ji, R. Effects of Geographic Region on the Composition of Bactrian Camel Milk in Mongolia. Animals 2019, 9, 890.
- Smiddy, M.A.; Huppertz, T.; van Ruth, S.M. Triacylglycerol and melting profiles of milk fat from several species. Int. Dairy J. 2012, 24, 64–69.
- Haddad, I.; Mozzon, M.; Strabbioli, R.; Frega, N.G. Stereospecific analysis of triacylglycerols in camel (Camelus dromedarius) milk fat. Int. Dairy J. 2010, 20, 863–867.
- Bakry, I.A.; Korma, S.A.; Wei, W.; Nafea, A.E.; Mahdi, A.A.; Ziedan, N.I.; Wang, X. Changes in the fatty acid content of Egyptian human milk across the lactation stages and in comparison with Chinese human milk. Eur. Food Res. Technol. 2021, 247, 1035–1048.
- Nyuar, K.; Min, Y.; Ghebremeskel, K.; Khalil, A.; Elbashir, M.; Cawford, M. Milk of northern Sudanese mothers whose traditional diet is high in carbohydrate contains low docosahexaenoic acid. Acta Paediatr. 2010, 99, 1824–1827.
- Cardak, A.D.; Yetismeyen, A.; Bruckner, H. Quantitative comparison of camel, goat and cow milk fatty acids. Milchwissenschaft 2003, 58, 34–36.
- Maqsood, S.; Al-Dowaila, A.; Mudgil, P.; Kamal, H.; Jobe, B.; Hassan, H.M. Comparative characterization of protein and lipid fractions from camel and cow milk, their functionality, antioxidant and antihypertensive properties upon simulated gastro-intestinal digestion. Food Chem. 2018, 279, 328–338.
- Zou, X.; Huang, J.; Jin, Q.; Guo, Z.; Liu, Y.; Cheong, L.-Z.; Xu, X.; Wang, X. Lipid Composition Analysis of Milk Fats from Different Mammalian Species: Potential for Use as Human Milk Fat Substitutes. J. Agric. Food Chem. 2013, 61, 7070–7080.
- Nikkhah, A. Science of Camel and Yak Milks: Human Nutrition and Health Perspectives. Food Nutr. Sci. 2011, 2, 667–673.
- Mann, J. Diet and risk of coronary heart disease and type 2 diabetes. Lancet 2002, 360, 783–789.
- Faye, B.; Bengoumi, M.; Al-Massaud, A.; Konuspayeva, G. Comparative milk and serum cholesterol content in dairy cow and camel. J. King Saud Univ. Sci. 2015, 27, 168–175.
- Gorban, A.M.S.; Izzeldin, O.M. Study on cholesteryl ester fatty acids in camel and cow milk lipid. Int. J. Food Sci. Technol. 1999, 34, 229–234.
- Alabdulkarim, B. Effect of camel milk on blood glucose, cholesterol, triglyceride and liver enzymes activities in female albino rats. World Appl. Sci. J. 2012, 17, 1394–1397.
- Sulieman, A.M.E.; Elayan, A.A.; Saleh, F. The Hypocholesterolemic Effect of Gariss and Gariss Containing Bifidobacteria in Rats Fed on a Cholesterol-Enriched Diet. Asian J. Biochem. 2008, 3, 43–47.
- Sboui, A.; Djegham, M.; Khorchani, T.; Hammadi, M.; Barhoumi, K.; Belhadj, O. Effect of camel milk on blood glucose, cholesterol and total proteins variations in alloxan-induced diabetic dogs. Int. J. Diab. Metabol. 2010, 18, 5–11.
- Meena, S.; Rajput, Y.S.; Sharma, R.; Singh, R. Effect of goat and camel milk vis a vis cow milk on cholesterol homeostasis in hypercholesterolemic rats. Small Rumin. Res. 2018, 171, 8–12.
- Karray, N.L.; Danthine, S.; Blecker, C.; Attia, H. Contribution to the study of camel milk fat globule membrane. Int. J. Food Sci. Nutr. 2006, 57, 382–390.
- Månsson, H.L. Fatty acids in bovine milk fat. Food Nutr. Res. 2008, 52.
- Yang, Y.; Zheng, N.; Zhao, X.; Zhang, Y.; Han, R.; Ma, L.; Zhao, S.; Li, S.; Guo, T.; Wang, J. Proteomic characterization and comparison of mammalian milk fat globule proteomes by iTRAQ analysis. J. Proteom. 2015, 116, 34–43.
- Sabha, B.H.; Masood, A.; Alanazi, I.O.; Alfadda, A.A.; Almehdar, H.A.; Benabdelkamel, H.; Redwan, E.M. Comparative Analysis of Milk Fat Globular Membrane (MFGM) Proteome between Saudi Arabia Camelus dromedary Safra and Wadha Breeds. Molecules 2020, 25, 2146.
- Saadaoui, B.; Henry, C.; Khorchani, T.; Mars, M.; Martin, P.; Cebo, C. Proteomics of the milk fat globule membrane from C amelus dromedarius. Proteomics 2013, 13, 1180–1184.
- Evers, J.M. The milkfat globule membrane—compositional and structural changes post secretion by the mammary secretory cell. Int. Dairy J. 2004, 14, 661–674.
- Lopez, C. Milk fat globules enveloped by their biological membrane: Unique colloidal assemblies with a specific composition and structure. Curr. Opin. Colloid Interface Sci. 2011, 16, 391–404.
- Lee, H.; Padhi, E.; Hasegawa, Y.; Larke, J.; Parenti, M.; Wang, A.; Hernell, O.; Lönnerdal, B.; Slupsky, C. Compositional Dynamics of the Milk Fat Globule and Its Role in Infant Development. Front. Pediatr. 2018, 6, 313.
- Manoni, M.; Di Lorenzo, C.; Ottoboni, M.; Tretola, M.; Pinotti, L. Comparative Proteomics of Milk Fat Globule Membrane (MFGM) Proteome across Species and Lactation Stages and the Potentials of MFGM Fractions in Infant Formula Preparation. Foods 2020, 9, 1251.
- Bauer, E.; Jakob, S.; Mosenthin, R. Principles of Physiology of Lipid Digestion. Asian-Australas. J. Anim. Sci. 2005, 18, 282–295.
- El Fakharany, E.; El-Baky, N.A.; Linjawi, M.H.; AlJaddawi, A.A.; Saleem, T.H.; Nassar, A.Y.; Osman, A.; Redwan, E.M. Influence of camel milk on the hepatitis C virus burden of infected patients. Exp. Ther. Med. 2017, 13, 1313–1320.
- Imam, A.; Drushella, M.M.; Taylor, C.R.; Tökés, Z.A. Preferential expression of a Mr 155,000 milk-fat-globule membrane glycoprotein on luminal epithelium of lobules in human breast. Cancer Res. 1986, 46, 6374–6379.
- Khatoon, H.; Ikram, R.; Anser, H.; Naeem, S.; Khan, S.S.; Fatima, S.; Sultana, N.; Sarfaraz, S. Investigation of anti-inflammatory and analgesic activities of camel milk in animal models. Pak. J. Pharm. Sci. 2019, 32, 1879–1883.
- Zouari, A.; Schuck, P.; Gaucheron, F.; Triki, M.; Delaplace, G.; Gauzelin-Gaiani, C.; Lopez, C.; Attia, H.; Ayadi, M.A. Microstructure and chemical composition of camel and cow milk powders’ surface. LWT 2019, 117, 108693.
- Hansen, H.S.; Jensen, B. Essential function of linoleic acid esterified in acylglucosylceramide and acylceramide in maintaining the epidermal water permeability barrier. Evidence from feeding studies with oleate, linoleate, arachidonate, columbinate and α-linolenate. Biochim. Biophys. Acta (BBA)—Lipids Lipid Metab. 1985, 834, 357–363.
- Ali, A.; Zou, X.; Huang, J.; Abed, S.M.; Tao, G.; Jin, Q.; Wang, X. Profiling of phospholipids molecular species from different mammalian milk powders by using ultra-performance liquid chromatography-electrospray ionization-quadrupole-time of flight-mass spectrometry. J. Food Compos. Anal. 2017, 62, 143–154.
- Ali, A.H.; El-Wahed, E.M.A.; Abed, S.M.; Korma, S.A.; Wei, W.; Wang, X. Analysis of triacylglycerols molecular species composition, total fatty acids, and sn-2 fatty acids positional distribution in different types of milk powders. J. Food Meas. Charact. 2019, 13, 2613–2625.
- Haddad, I.; Mozzon, M.; Strabbioli, R.; Frega, N.G. Electrospray ionization tandem mass spectrometry analysis of triacylglycerols molecular species in camel milk (Camelus dromedarius). Int. Dairy J. 2011, 21, 119–127.
- Jenkins, B.; West, J.A.; Koulman, A. A Review of Odd-Chain Fatty Acid Metabolism and the Role of Pentadecanoic Acid (C15:0) and Heptadecanoic Acid (C17:0) in Health and Disease. Molecules 2015, 20, 2425–2444.
- Gresti, J.; Bugaut, M.; Maniongui, C.; Bezard, J. Composition of Molecular Species of Triacylglycerols in Bovine Milk Fat. J. Dairy Sci. 1993, 76, 1850–1869.
- Bornaz, S.; Fanni, J.; Parmentier, M. Butter texture: The prevalent triglycerides. J. Am. Oil Chem. Soc. 1993, 70, 1075–1079.
- Wei, W.; Jin, Q.; Wang, X. Human milk fat substitutes: Past achievements and current trends. Prog. Lipid Res. 2019, 74, 69–86.
- Miles, E.A.; Calder, P.C. The influence of the position of palmitate in infant formula triacylglycerols on health outcomes. Nutr. Res. 2017, 44, 1–8.
- Bar-Yoseph, F.; Lifshitz, Y.; Cohen, T. Review of sn-2 palmitate oil implications for infant health. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 139–143.
- Mourad, K.; Nour-Eddine, K. Physicochemical and microbiological study of “shmen”, a traditional butter made from camel milk in the Sahara (Algeria): Isolation and identification of lactic acid bacteria and yeasts. Grasas Aceites 2006, 57.
- Yagil, R. Camels and Camel Milk: FAO Animal Production and Health; Publications Division, Food and Agriculture Organization of the United Nations: Rome, Italy, 1982; p. 26.
- Farah, Z.; Streiff, T.; Bachmann, M. Manufacture and characterization of camel milk butter. Milchwissenschaft 1989, 44, 412–414.
- Parmar, N.B. Characterization of Ghee Prepared from Camel Milk and Evaluation of Its Shelf Life During Storage. Ph.D. Thesis, Anand Agricultural University, Anand, India, 2013.
- Knoess, K.H.; Makhudum, A.J.; Rafiq, M.; Hafeez, M. Milk production potential of the dromedary with special reference to the province of Punjab, Pakistan. World Anim. Rev. 1986, 57, 11–21.
- Mtibaa, I.; Zouari, A.; Attia, H.; Ayadi, M.A.; Danthine, S. Effects of Physical Ripening Conditions and Churning Temperature on the Butter-Making Process and the Physical Characteristics of Camel Milk Butter. Food Bioprocess. Technol. 2021, 14, 1518–1528.
- Berhe, T.; Seifu, E.; Kurtu, M.Y. Physicochemical properties of butter made from camel milk. Int. Dairy J. 2013, 31, 51–54.
- Abu-Lehia, I.H. Composition of camel milk. Milchwissenschaft 1987, 42, 368–371.
- Bylund, G. Dairy processing handbook: Tetra Pak Processing Systems AB; Sweden, AB: Lund, Sweden, 1995; pp. 13–36.
- Habtegebriel, H.; Wawire, M.; Gaukel, V.; Taboada, M.L. Comparison of the viscosity of camel milk with model milk systems in relation to their atomization properties. J. Food Sci. 2020, 85, 3459–3466.
- Mulder, H.; Walstra, P. The Milk Fat Globule; Commonwealth Agricultural Bureaux Farnham Royal: Wallingford, UK, 1974; ISBN 0851982891.
- Farah, Z.; Rüegg, M. The Creaming Properties and Size Distribution of Fat Globules in Camel Milk. J. Dairy Sci. 1991, 74, 2901–2904.
- Khan, K.U.; Appanna, T.C. Carotene and vitamin A in camel milk. Indian J. Nutr. Diet. 1967, 4, 17–20.
- Walter, L.; Shrestha, P.; Fry, R.; Leury, B.; Logan, A. Lipid metabolic differences in cows producing small or large milk fat globules: Fatty acid origin and degree of saturation. J. Dairy Sci. 2020, 103, 1920–1930.
- El-Zeini, H.M. Microstructure, rheological and geometrical properties of fat globules of milk from different animal species. Polish J. Food Nutr. Sci. 2006, 15, 147–153.
