Non-viral materials, such as polymers and lipids, have been developed to create ‘synthetic artificial viruses’ for the delivery of genes into cells in vitro and in vivo. Owing to their limitations in overcoming extra- and intracellular obstacles, these synthetic materials can exhibit poor delivery efficiency relative to viral vectors. However, considering their biocompatibility, high loading capacity, facile fabrication, and potential large-scale production, non-viral vectors have shown great progress in the delivery of gene cargos, especially small interfering RNA (siRNA) molecules.
- gene therapy
- non-viral delivery systems
- myocardial infarction
- nanoparticles
1. Introduction
Gene therapies utilize the nucleic acids as drugs to introduce a new gene or alter disease-causing gene expression in cells, providing advantages such as facilitating specific manipulation, long-term therapy, the replacement of damaged cells and the potential permanent fixation of abnormal genes to eliminate diseases [1][6]. It was reported recently that CVD were the third frequent indication for clinical trials of gene therapy and was targeted by 5.0% of all gene therapy trials carried out globally from 1988 to 2022 [2][7]. The successful clinical translation of gene therapy depends on sophisticated delivery technologies to improve the stability, target affinity and internalization of nucleic acids. Viral vectors have constituted a major part of the clinical trials of cardiac gene therapies to date. Viral vectors were used to transduce the sarcoplasmic/endoplasmic reticulum calcium ATPase 2 (SERCA2a), adenylyl cyclase 6 (Ad5.hAC6), and inhibitor-1 (I-1c) genes into the myocardium to promote the function of cardiomyocytes (CMs). The Ad5.hAC6 gene moved to phase 3, while the SERCA2a gene failed in phase 2b due to ineffectiveness. To target the pathways of angiogenesis, the VEGF, VEGF-D, and FGF-4 genes were also delivered using viral vectors, while a non-viral cationic polymer carrier was used in the in vitro transfection of endothelial progenitor cells for transplantation. Two trials directly delivered SDF-1 and VEGF-165 plasmid DNA to stimulate the homing of stem cells into the myocardium and promote angiogenesis, respectively [3][8].
As viruses have essentially evolved to deliver gene cargos, they are typically very efficient and, as shown above, constitute a major part of current clinical trials for heart diseases [4][5][28,29]. However, safety concerns have greatly limited the application of viral vectors for gene delivery. The random insertion of genes into the genomes of repopulating cells via viral vectors could cause mutagenesis and oncogenesis [6][30]. Viral vectors may express immunogenic epitopes, inducing dangerous immune responses [7][31]. The lack of cell/tissue targeting has also hampered the exploitation of viral vectors, especially for in vivo gene delivery [8][32]. Other challenges with viral gene delivery include the limited packaging capacity of gene cargos and the high cost of vector production [9][33]. Non-viral materials, such as polymers and lipids, have been developed to create ‘synthetic artificial viruses’ for the delivery of genes into cells in vitro and in vivo [10][34]. Owing to their limitations in overcoming extra- and intracellular obstacles, these synthetic materials can exhibit poor delivery efficiency relative to viral vectors [5][29]. However, considering their biocompatibility, high loading capacity, facile fabrication, and potential large-scale production, non-viral vectors have shown great progress in the delivery of gene cargos, especially small interfering RNA (siRNA) molecules. In 2018, the first FDA approved small interfering RNA (siRNA) therapeutics demonstrated the promise of non-viral lipid carriers for RNA interference (RNAi) therapy [11][12][35,36]. Following this, the release of two siRNA-based drugs and the recently approved mRNA vaccine delivered through lipid nanoparticles (LNPs) further proved the potential of non-viral nanomaterials for gene therapy. The nanomaterials could be functionalized with small molecules or biomolecules to modify their physicochemical properties such as charge density, hydrophobicity, degradability, and affinity of binding to specific cells [13][37]. Owing to their controllable physicochemical parameters, nanomaterials are promising for targeted, sustained release, and environmentally responsive gene delivery.
Owing to the development of material sciences and nucleic acid chemistry, numerous non-viral nanomaterials have been created and applied in various disease circumstances. Among the 48 clinical trials involving siRNA-based therapy, most are aimed to manage cancer in tissues such as the liver, eye, and skin [14][15][38,39]. RNA (including siRNA, microRNA (miRNA), messenger RNA (mRNA), short hairpin RNA (shRNA)) therapeutics targeting the cardiac tissue, and CVD still remain to be developed. Ingenious delivery platforms were urgently needed to broaden clinical exploitations of gene therapy in CVD. Non-viral nanoparticulate systems designed for gene delivery generally encounter systemic and local hurdles: (1) the mononuclear phagocytic system (MPS) barrier, kidney filtration, and protein corona shielding in circulation; (2) extravasation, penetration, and retention in the tissue; (3) internalization and release in cells ( Figure 1 ) [16][17][40,41].
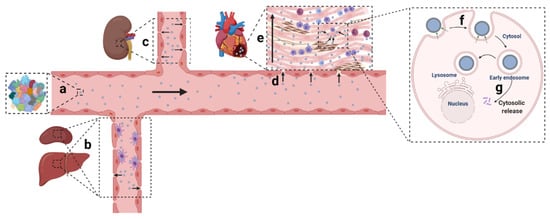
2. Non-Viral Delivery Systems with High Transfection Efficiency for Cardiac Cells
The nature of the cargo determines the basic requirements for delivery systems. Instability in the presence of nucleases is one of the major concerns for gene-based drugs, especially for small RNA molecules, which are more sensitive to enzymatic degradation. The anionic phosphate backbone is another obstacle to be overcome, as the repulsive electrostatic interaction between nucleic acids and cell membranes impedes gene molecules’ free diffusion into cells. In addition, the short half-lives of gene drugs (e.g., 5 min for siRNA molecules following intravenous injection) also demonstrate the need for a delivery system to improve the pharmacokinetic properties [18][19][64,65].
To meet the requirements mentioned above, various types of delivery vehicles—mainly polymeric materials and liposomes—have been studied for compacting or loading gene cargos into nanoscale particles for delivery in cardiac tissue. The particles protect the integrity of nucleic acids in the presence of nucleases and also optimize their pharmacokinetics by altering their sizes and surface properties. Reineke et al. designed a series of poly(glycoamidoamine) (PGAA) materials by incorporating a carbohydrate comonomer within a PEI-like backbone [20][21][66,67]. All the PGAAs showed minimal cytotoxicity, and the tartarate-incorporated T4 glycopolymer complexed with NF-κB oligodeoxynucleotide (ODN) decoys showed 87% penetration of the myocardium in mouse hearts and a nearly complete reduction in Cox-2—a well-known NF-κB dependent gene in the heart—with a dose of 10.0 μg [22][68]. Micelles, liposomes, and silica particles were also used as delivery systems in the heart [23][24][25][26][60,69,70,71].
To improve the internalization efficiency in cardiac cells, the modification of basic vehicles using additional components has become a common strategy. Cell penetrating peptides (CPPs) are a major category of ligands used for this purpose, especially TAT and oligo-arginine (R9). The methods for incorporating CPPs into delivery vehicles are diverse, such as complexing with gene cargos [27][72], conjugating to DNA or RNA molecules [28][73], modifying the surface or scaffolds of liposomes or polymers [29][30][74,75], and decorating other complicated systems [31][76]. All the results show that, compared to unmodified vehicles, CPPs did enhance the internalization of delivery systems in CMs, while simple cationic particles, such as those made from polyamidoamine (PAMAM) and PEI, failed to achieve efficient delivery [32][33][77,78]. It is also interesting to note that the modification of R9 showed better performance than that of TAT in transfecting CMs in vitro and, most likely, delivering gene cargos in cardiac tissue in vivo [33][78]. Youngsook Lee et al. also demonstrated the effectiveness of arginine-grafted polymer for plasmid human erythropoietin gene delivery in a rat MI model [34][79]. It is possible that the sequence and structure of pure oligo-arginine satisfy the preference of CMs, making it a potent element with which to construct delivery systems for hearts. The transferrin receptor, also called CD71, mediates the transport of ferric ions into cells via transferrin (Tf). The group of Hirokazu Matsumoto found that the siRNA-conjugated anti-CD71 Fab’ fragment resulted in efficient gene silencing in the liver, heart and calf muscles in healthy mice after intravenous administration, and the silencing of myostatin in a peripheral artery disease (PAD) model of mice with femoral artery ligation led to the recovery of leg functions [35][80]. Since CMs are rich in transferrin receptors involved in myoglobin synthesis [36][81], the anti-CD71 antibody could be a potential ligand of nanoparticles for the efficient delivery of gene cargos to the CMs.
To guide delivery systems into a certain type of cell, cell-type-specific ligands have been incorporated into vehicles, including carbohydrates, peptides, proteins, etc. GlcNAc is a saccharide ligand specific to CMs screened out from a carbohydrate library, and its conjugation facilitated the successful delivery of a liposome system and a polyketal system into CMs [37][38][82,83]. As for peptide ligands, at least three candidates have been identified from phage display. PCM is a ligand that targets primary CMs [39][84], and Bull et al. used this peptide in their polymeric systems to deliver siRNA to CMs in vitro [28][29][73,74]. Molecules that can recognize the membrane proteins of CMs constitute another type of potential ligand. For example, PGE-2-conjugated siRNA was used in Bull’s study to induce receptor-mediated endocytosis in H9C2 cells [40][85]. Delivery systems guided by specific ligands do possess superior binding/internalization abilities in CMs compared to unmodified systems, while CPP-guided delivery systems still have a higher delivery efficiency in general. Consequently, combining the two types of ligands may become a useful method for developing delivery systems in cardiac tissue, which, in fact, has shown some synergic effects in transfecting CMs in vitro and in vivo [41][28][29][42][56,73,74,86].
3. Non-Viral Delivery Systems Capable of Targeting Cardiac Tissue
Targeted delivery to the heart through systemic administration is a minimally invasive treatment method. Strategies developed for targeting other tissues could be utilized for the heart in which vectors are usually added with targeting components, facilitated by external sources, integrated with biological systems possessing innate natural properties or enhanced targeting via blocking MPS ( Figure 2 ). The targeting methodologies for a damaged heart are summarized in this section.
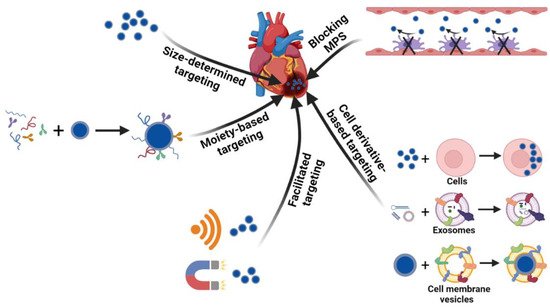
In addition to the size affecting the accumulation, nanoparticles conjugated with targeting moieties have shown enhanced final delivery efficiency. The targeting ligands recognize cardiac tissue through a specific interaction with certain receptors or markers in the myocardium and promote the accumulation of the delivery vehicles in the heart. Theoretically, nanoparticles can be modified to target all types of cells or special components in the extracellular environment in the heart. Cardiomyocytes are the most studied targets. Ligands targeted to cardiomyocytes have generally been found by phage display. Through in vivo phage display, several peptides such as CSTSMLKAC, CRPPR, and I-1 were screened out for cardiomyocyte targeting, all of which show an increase in accumulation and efficiency in the injured heart compared to nanoparticles without peptide conjugation [43][44][45][57,92,93]. Another candidate, called ischemic myocardium-targeted peptide (IMTP), was conjugated to a cystamine bisacrylamide-diamino hexane polymer with modification of the R9 peptide (IMTP-CD-9R), and the intravenous injection of IMTP-CD-9R/HO-1 plasmid complexes induced high HO-1 expression in ischemic injured left ventricle tissue, demonstrating the targeting efficacy of IMTP [42][86]. Instead of peptides screened from phage display, Kohane et al. utilized an Ang II sequence on liposome vehicles to recognize the Ang II receptors on cardiac cells, which resulted in a higher accumulation of liposomes than the scrambled sequence [46][94]. The myosin expressed by damaged cardiomyocytes could also be used as a target for nanoparticles [41][47][56,89].
As natural materials, cells or cell derivatives hold promise for targeted delivery due to advantages such as high biocompatibility, homing capability, and inherent therapeutic functions for the treatment of diseases. Circulating cells such as red blood cells, stem cells, and leukocytes were recently leveraged for drug delivery due to their long circulation times and tissue-homing properties in vivo [48][49][108,109]. A recent study by Molly M. Stevens group showed that neutrophils loaded with liposomes ex vivo could transport nanoparticles to inflamed skeletal muscle and ischemic hearts in mice, demonstrating neutrophils to be suitable carriers of nanoparticles to target an injured heart due to their inherent homing to inflammatory tissue [50][110]. To target migrating monocytes in circulation to mediate cardiac targeting of nanoparticles, Patrick C. H. Hsieh’s group developed a platelet-like proteoliposome (PLP) in which liposomes were incorporated with proteins isolated from platelets [51][111]. The specificity of the PLP was proven by the strong binding affinity for monocytes but not endothelial cells in vitro. Following intravenous injection, the PLP showed higher accumulation in the heart after 72 h of injection, and the loading of the anti-inflammatory drug resulted in a better therapeutic outcome than the liposomes without protein incorporation. Exosomes can be genetically engineered with targeted peptides for cardiac targeting. Ji-Young Kang et al. developed a cardiac-targeting peptide-modified exosome derived from the transgenic engineering of HEK293 cells. The co-delivery of curcumin and miR-144-3p in this carrier showed cardioprotective effects both in vitro and in vivo for MI treatment [52][112]. Cell membranes are another class of naturally derived materials possessing innate properties inherited from source cells. By mimicking the ability of mesenchymal stem cells (MSC) to target an injured heart, Chi Yao et al. utilized MSC membranes to coat miR-21-loaded silica nanoparticles, which enhanced the accumulation and sustainable release of miR-21 in the damaged heart, resulting in the inhibition of cardiomyocyte apoptosis and preservation of cardiac function against MI injury [53][113].
As the major hurdle for systemic targeted delivery is the MPS [54][55][48,114], previous studies have usually prevented nanoparticles from interacting with immune cells to evade the MPS by coating the nanoparticles with stealth shells or allowing them to ‘hitchhike’ on the red blood cells (RBCs) or other circulatory cells. Recent studies have offered a different strategy—blocking the MPS. The group of Petr I. Nikitin utilized the RBC antibody (IgG2a-34-3C) to bind circulatory erythrocytes to stimulate erythrophagocytosis, which intensified the clearance of the organism’s own intact blood cells. The ingestion of RBCs transiently blocked the phagocytosis of nanoparticles by the MPS and enhanced the circulation time and tumor accumulation of nanoparticles up to about 32-fold and at least 14-fold, respectively, compared to the direct injection of nanoparticles without the MPS-cytoblockade [56][115]. The MPS-blocking strategy could also be utilized for cardiac targeting. Another study showed that exosomes loaded with siClathrin silenced the expression of Clathrin in macrophages, which inhibited the endocytosis of macrophages and blocked the clearance of later injected exosomes by the MPS. Compared to the direct administration of therapeutic exosomes encapsulated with miR-21, the prior injection of exosomes blocking the MPS enhanced miR-21 accumulation in the heart by two-fold and produced a much better therapeutic effect on cardiac function in the doxorubicin-induced cardiotoxicity mouse model [57][116].
4. Modulating Different Cells for MI Treatment
The heart is mainly composed of cardiomyocytes, endothelial cells, fibroblasts, and tissue resident macrophages, which constitute about 33%, 40%, 11~27%, and 4~5% of the total cardiac cells, respectively [58][59][60][61][62][117,118,119,120,121], all of which play important roles in cardiac function. Based on the understanding of molecular mechanisms during the MI, nucleic-acid delivery systems are being developed to target different cells in the heart for cardiac repair/regeneration. Non-viral systems can be exploited to deliver gene cargos to not only one type of cell but also different cells concurrently or successively based on the demands of modulation at different stages of injury. For instance, the group of Kim utilized the PEI1.8-DA carrier to simultaneously deliver siSHP-1 and VEGF plasmids, which target the cardiomyocytes and endothelial cells, respectively, for the treatment of MI in rats [63][122]. Jinli Wang et al. constructed miR-101a-loaded MSC exosomes for MI repair. Here, miR-101a was utilized for the inhibition of fibrosis, and the MSC exosomes were leveraged for the polarization of macrophages from M1 to M2. The therapeutic co-effects from the gene cargo and the carrier increased cardiac function in the MI model of mice [64][123]. Using ethanolamine (EA)-modified poly(glycidyl methacrylate) (PGEA) to fabricate heparin-cored nanoparticles (Hep@PGEA), Jing-Jun Nie et al. sequentially delivered miR-499 and VEGF DNA for the inhibition of cardiomyocyte apoptosis in the early stages of MI and to promote angiogenesis at later stages. This chronological treatment restored the heart’s function and suppressed cardiac hypertrophy [65][124]. Given the significance of CMs, endothelial cells, immune cells and fibroblasts, non-viral delivery systems leveraged to manipulate gene targets in different cardiac cells are summarized in this section ( Figure 3 ).
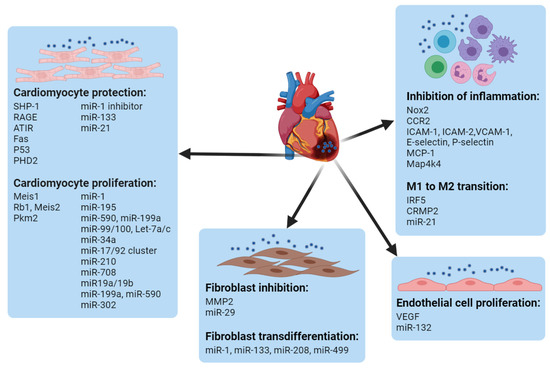
miRNA molecules can also regulate endothelial cell proliferation. Teng Ma et al. encapsulated miR-132 into exosomes derived from MSC. After the local injection of the miR-132-loaded exosomes to the infarcted heart, neovascularization in the peri-infarct zone was enhanced, and the heart’s functions were preserved [66][166]. miR-24 was found to be upregulated in endothelial cells after MI induction, for which the group of Costanza Emanueli locally delivered an miR-24 decoy using an adenovirus to inhibit miR-24 expression, which increased angiogenesis and blood perfusion in the peri-infarct myocardium, reduced the infarct size, induced fibroblast apoptosis, and improved overall cardiac function [67][167].
Immune cells play significant roles in the modulation of cardiac repair by affecting the functions of cardiomyocytes, endothelial cells, and fibroblasts [68][168]. The innate immune cells, including macrophages and neutrophils, are usually heterogeneous and plastic and can be roughly divided into pro-inflammatory type 1 and anti-inflammatory type 2, both of which are necessary for cardiac repair [69][70][71][72][169,170,171,172]. The crosstalk between innate and adaptive immune cells, e.g., T cells, is also critical for the mending of an injured heart [73][74][75][173,174,175]. Given the complex and multiple roles of immune cells, the modulation of immune systems may require precise spatial and temporal regulation.
The formation of fibroblasts occurs in the later stage of MI, which hampers the function of the heart. After MI, matrix metalloproteinase 2 (MMP2) is overexpressed, mainly in fibroblasts, by as much as 3000-fold in infarct tissue by 8 weeks [76][77][186,187]. The group of Jason A. Burdick developed an MMP-responsive hydrogel loaded with siMMP2 to silence MMP expression in cardiac fibroblasts in vitro. The intramyocardial injection of the shear-thinning hydrogel increased the infarct thickness and improved the cardiac functions in an MI model [78][188]. Depressing the fibroblast stage would have benefits for repair following MI, for which purpose Rui-Quan Li et al. developed a star PGMA cationic polymer to load miR-29 to inhibit the formation of fibroblasts. The polymer facilitated the systemic targeting of miR-29 to the injured heart, which reduced the fibrosis and improved the cardiac function following MI [79][189].
