Pharmaceutical care represents a concept introduced after 1990. According to this, the pharmacists’ activity focuses on patients and aims to provide adequate therapies that lead to safe therapeutic results, as well as to improve the quality of life. Thus, the traditional activity of preparation and development of drugs has been gradually replaced by pharmaceutical services that mostly focus on the patients’ needs and the particularities of their pathologies.
- pharmaceutical care
- pharmaceutical services
- pharmaceutical practice
- public health
- COVID-19
- pharmacist
- pharmacy
- healthcare
1. Introduction
Pharmaceutical care represents a concept introduced after 1990. According to this, the pharmacists’ activity focuses on patients and aims to provide adequate therapies that lead to safe therapeutic results, as well as to improve the quality of life [7][1]. Thus, the traditional activity of preparation and development of drugs has been gradually replaced by pharmaceutical services that mostly focus on the patients’ needs and the particularities of their pathologies [8,9][2][3]. But, with the onset of this pandemic, the activities of all professional categories, including the pharmaceutical activities, have been disturbed and this crisis deeply and continuously marked the practice of pharmacists [10][4]. Pharmacists are considered the most accessible professionals from the health sector and they support the medical practice [11][5]. Bragazzi et al. summarized the multiple roles of community or hospital pharmacists, such as experts in drugs and medicines, healthcare providers and stakeholders, educators, counsellors, mentors, managers, leaders, business developers, researchers, etc. [12][6].
Pharmacists are a valuable human resource, being at the center of their communities. Based on their professional education and their scientific activity, they can provide correct and complete medical information to the population [13,14][7][8]. Since the beginning of the pandemic, pharmacists remained in the first line in order to help their patients and to prevent and combat the infection’s effects. During this period the pharmaceutical practice diversified, some authors considering that it passed into a “new era” [12,15][6][9].
The aim of this study was to identify the impact of the authorities’ decisions on pharmaceutical practice, the involvement of the professional associations, and the responsibilities of pharmacy owners and management. On the other hand, we performed a global assessment on the pharmaceutical care services provided by the community, clinical, or hospital pharmacists.
To accomplish this study, we conducted a narrative review of the main articles identified in Pubmed, by using specific keywords: “COVID-19” or “Coronavirus” and “pharmaceutical care”, “pharmaceutical services”, “pharmacy(ies)” or “pharmacist”. Subsequently, the information was structured in some main subheadings, starting from the decision-makers to the final link of the pharmaceutical care, the pharmacist.
2. The Impact of the Authorities’ Decisions on Pharmaceutical Care
Although the authorities in every country established lockdown, the working hours of pharmacies were not restricted. During the state of emergency, pharmacies were advised to stay open according to their normal schedule or even to extend their work hours. For example, in the UK, according to NHS guidelines, pharmacies can be closed for a maximum of 2.5 h per day [24][16]. However, in Croatia, the Crisis headquarter reduced the working hours of pharmacies to a maximum of 7 h/day (from 8 AM to 5 PM), apart from on-duty pharmacies, while in Serbia, only the schedule of pharmacies located in malls were affected [23][17]. A study performed in Romania showed that pharmacies most frequently adjusted their working hours depending on the requirements and conditions generated by the state of emergency [37][14].
Under these circumstances, the pharmacies were confronted with many shortages, some of them temporary, others long-term. Therefore, pharmacists had to manage the stock of these drugs very carefully in order to ensure especially the treatment of patients with rheumatoid arthritis, systemic lupus erythematosus, and other autoimmune disorders [15,39,44][9][18][19].
The authorities from many countries took different decisions to reduce drug shortages, such as: (i) the substitution of drugs with similar ones or dose adjustments were allowed for pharmacists from different countries (e.g., Australia, Belgium, Croatia, Germany, the Netherlands, Portugal, UK, etc.); (ii) in Denmark, health authorities demanded pharmacies to report the stock of critical medicine daily; (iii) in Croatia, France, Malta, Portugal, and Spain, community pharmacies could dispense some drugs used in hospitals, and some studies showed that the patients were satisfied with this service provided by the community pharmacy [35,38,42][11][15][20]; (iv) limiting the drugs dispensed from the pharmacy was another legislative measure to prevent shortages. In Australia, the number of medical specialists that could initiate the treatment with hydroxychloroquine was restricted, or the quantity released off-label was of maximum one box/month/patient or one prescription/month/patient [14][8]. The Estonian Health Ministry limited the quantity of paracetamol to 2 boxes/patient [42][20]. The Saudi-Arabian Health Ministry limited the quantity of dietary supplements that boost the immune system to only one pack per customer to prevent the emergence of a black market for such items [45][21]; (v) in Rwanda, the Ministry of Trade and Industry applied fines to pharmaceutical companies for increasing drug prices. In order to reduce price speculation and drug shortages, the authorities sent pharmacies a list of drugs in high demand, including their prices [43][22]. On the other hand, the healthcare insurance company from Croatia allowed pharmacies to dispense drugs with higher prices than the regulated ones, the difference being supported by health insurance. Also, these measures were enforced to solve drug shortages and limit the return of patients in pharmacies [35][11].
“Mask 19” is a code used in pharmacies from different countries (Austria, Belgium, France, Greece, Italy, the Netherlands, Norway, Portugal, Spain, etc.) by victims of domestic violence. During the lockdown period, the victims of domestic violence were forced to spend substantial amounts of time together with their abusers. In this context, the incidence of domestic abuse was greater and, for these victims, the pharmacy represented “a community hub and a safe haven“ offering the best way to report their abusers. In a private discussion with the pharmacist, the victims pronounced “Mask 19” a simple code that does not attract much attention even if they are not alone. For these reasons, since the beginning of the pandemic, the authorities established that pharmacies represent a safe and trusted environment for domestic violence victims [35,53,54,55,56][11][23][24][25][26]. Furthermore, Her Majesty’s Government from the UK elaborated guidance for pharmacies in order to offer support for victims of domestic abuse to access immediate help from the police or other authorities [57,58][27][28].
In order to facilitate the financing of pharmacies, the Polish authorities decreased the time of fund reimbursement to pharmacies and allowed pharmacists to prescribe subsidized drugs for themselves and their closest relatives [48][29].
3. Pharmaceutical Care in Community Pharmacies
For the success against the COVID-19 pandemic, the authorities included the community pharmacists in the public health team [74][30]. A study conducted in Saudi Arabia confirmed that all questioned pharmacists were prepared for this role [75][31]. Every decision of each pharmacist has to reduce the number of patient visits to the pharmacy or doctors and to control the risk of infection [68][32].
In addition, Bragazzi et al. highlighted the important role of pharmacists in screening, triage, detection, reporting of potential COVID-19 cases, active surveillance, and early alerts to drug shortages, telepharmacy services, combating fake news and misinformation regarding the COVID-19 medication, etc. [12][6]. Elbedini et al. noticed the role of pharmacists in physical evaluation, blood pressure measurement, fever monitoring, and COVID-19 testing [77][33].
On the other hand, the pandemic intensified the activity of home-delivery for drugs PPE and disinfectants. This service focused on patients with COVID-19, the elderly, and patients with chronic diseases. In some countries (e.g., Germany, Finland, Latvia, UK), this service (drug home delivery and collect boxes) is remunerated [35][11]. The Australian Government introduced a new pharmaceutical service for home delivery of drugs and the Repatriation Pharmaceutical Benefits Scheme drugs. This service was subsidized for quarantined or vulnerable people [38][15]. The pharmacies could choose personal delivery (e.g., Portugal, UK), courier delivery (e.g., in Portugal based on the agreement between Associação Nacional das Farmácias and the Portuguese post office) or delivery by the Red Cross (e.g., Croatia, Italy, Spain). Thus, the Red Cross collaborated with local community pharmacies to ensure home delivery of medicines [48,84][29][34]. Over 22% of Croatian pharmacists and over one-third of Serbian pharmacists delivered drugs to patients’ homes [23][17]. The patients with chronic kidney disease were a special category because they need specific medications and dialysis consumables while being a category with a very high COVID-19 risk. Okoro presented the involvement of community pharmacists in the home delivery of these products for patients with chronic kidney disease [82][35].
In many countries, pharmacists have experience in conducting point-of-care tests for glycaemia, cholesterol, blood pressure or streptococcus A antigen, pharmacogenetic profile, etc. Hess et al. noticed that the involvement of pharmacies in COVID-19 testing played a vital public health role in mitigating the transmission of this infection [74][30]. On this basis, the authorities from 15 European countries (Austria, Belgium, the Czech Republic, France, Germany, Ireland, Italy, Malta, the Netherlands, Portugal, Romania, Spain, Sweden, Turkey, UK) involved pharmacists in COVID-19 testing, either by PCR (Polymerase chain reaction) or by rapid tests [22,35][13][11]. Moreover, in Germany pharmacists could perform rapid antigen tests for a fee and provide free FFP2 masks [60][36].
4. Specific Clinical and Hospital Pharmacy Services
Some studies present the clinical pharmacists’ roles during this period, such as: (a) therapeutic issues (identifying, prevention, treating); (b) therapeutic alternatives for out-of-stock drugs; (c) patients’ counseling including medication review (especially by phone, or by video conferences); (d) providing information about new therapies and about the safety, interactions or ADRs of the drugs (especially ibuprofen, glucocorticoids, ACEIs, BRAs, etc.); (e) contribution in clinical study teams; (g) elaboration of clinical guides together with doctors (dosage, precautions, interactions, ADRs, contraindication, special risk categories, etc.); (h) nurses and paramedic staff education on medication issue, reporting of ADRs, PPE usage, or manipulating the samples and residual materials from patients; (i) prevention of the COVID-19-related stigma through combating the negative behaviors or language and providing psychological counseling; (j) antimicrobial stewardship (collaboration with microbiology laboratories for assessment of COVID-19 tests, monitoring the patients’ compliance, drafting and implementing for the use of the antivirals or other drugs, management of the shortages, and the investigation of new drugs for the infection treatment) [12,13,42,81,93,94,95,96,97,98,99,100][6][7][20][37][38][39][40][41][42][43][44][45]. For example, Serbian pharmacists searched and evaluated the information about remdesivir [42][20]. Wang et al. showed that in a Chinese hospital with 2500 beds, 96% of the recommendations provided by the clinical pharmacist were accepted (e.g., discontinuation of treatment, choosing other medication, introducing a new drug in the therapeutic plan, dose adjustment, monitoring suspected ADRs or drug concentration, etc.). These are related to ADRs or treatment with antibiotics, antivirals, glucocorticoids, antifungal agents, mechanic ventilation, renal replacement therapy, artificial liver support [97][42]. Li et al. noticed the pharmacists’ assessment of the risk of stress-induced gastric mucosa damage, and the risk of venous thromboembolism [101][46]. The pharmacists from different institutions offered logistic and clinical support to obtain drugs for off-label use (e.g., remdesivir) through patient eligibility assessment, preparation of the file, and obtaining the authorization from the manufacturer and authorities to use these drugs [102][47].
The presence of pharmacists in healthcare teams is essential during a crisis, especially during the outbreak [29,61][48][49]. Since the beginning of the pandemic, pharmacists elaborated emergency drugs formularies, therapeutic plans, or documents with information on drugs used in COVID-19 in order to support the doctors’ activity; they participated in medical teams for treating COVID-19 patients with comorbidities. In addition, alongside doctors, they assessed the treatment. Another important part of their practice was to monitor drug ADRs and drug interactions, especially in critical patients or patients treated with narrow therapeutic index drugs [31,76,97,101,104][50][51][42][46][52]. Clinical pharmacists had to ensure that the off-label drug usage was appropriate. Thus, they had to monitor the treatment that could cause ADRs and adjust the doses depending on organ damage. The pharmacological meetings with the medical team were performed by alternative communication methods such as videoconferences or WhatsApp and all therapeutic adjustments were made with the involvement of pharmacists [104][52].
Among the collaborations of the clinical pharmacist with the medical teams for the treatment of COVID-19 patients, we can mention the proposal of the clinical pharmacist from the intensive care unit of the University Hospital of Amiens (France) for pulmonary administration of interferon-β-1b, after nebulization. Mary et al. showed that the 4 patients who did not respond to any other treatment had a favorable evolution after interferon administration [105][53].
A study conducted in 16 EU countries reported that pharmacists complained about limited recognition of their competence by the medical team or by the organizational management. The same study highlighted the lower acceptance rate of pharmacists’ interventions by the Swiss. Another problem noticed in Denmark referred to divergent information about experimental drugs received by the medical team staff at the beginning of the pandemic [42][20].
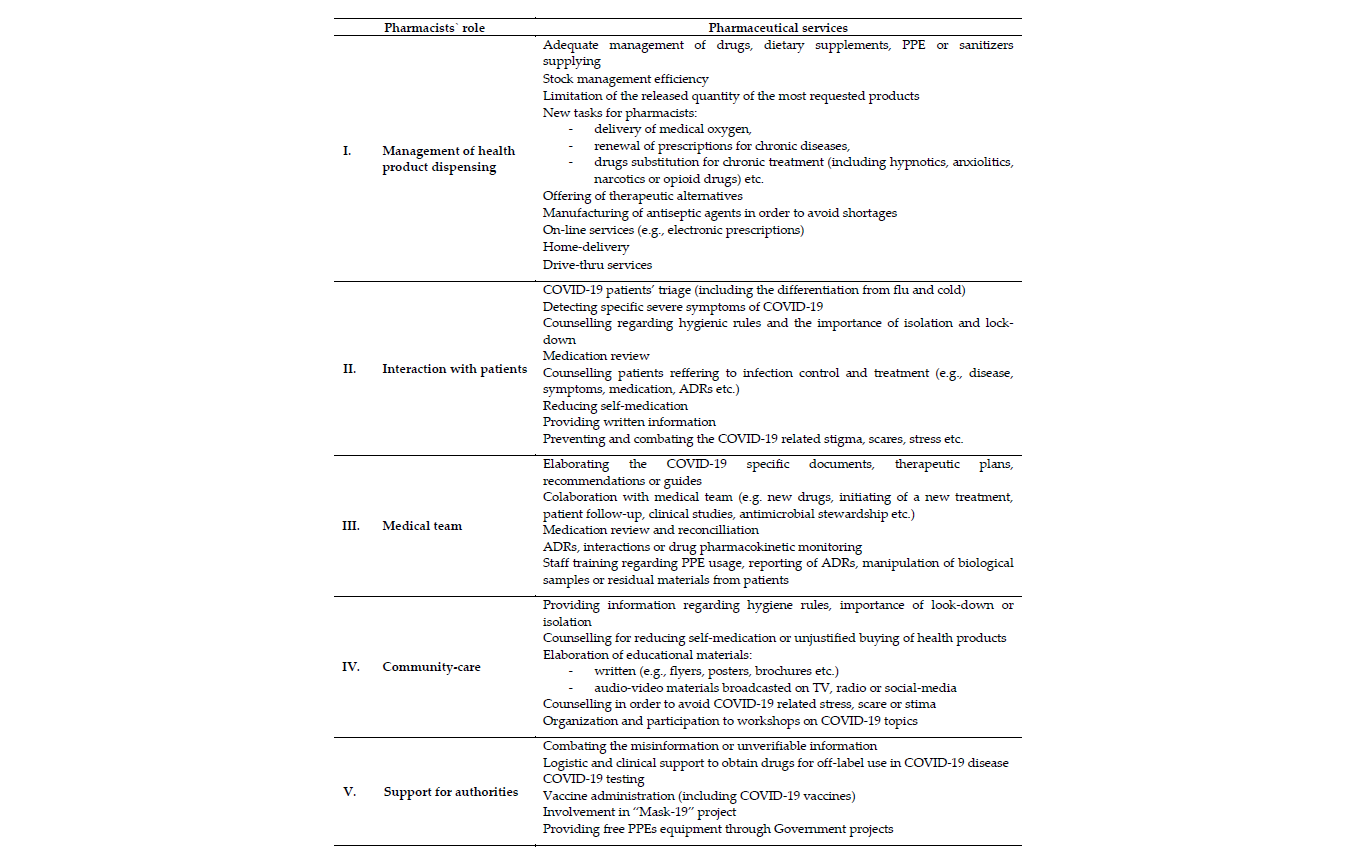
Since the beginning of the COVID-19 crisis, pharmacists were involved in different activities that lead to better management of the pandemic. They had a complex role in health product dispensing, patients’ counseling, medical teams, community-care, and as supporters for authorities’ activities (Table 1). So, the pharmaceutical services were diverse and essential for protecting the health status of the population, particularly to improve the life quality of COVID-19 patients. During this period, pharmacists achieved new skills and played an important role in healthcare and in crisis management.
Table 1. A brief overview of pharmaceutical care during the COVID-19 pandemic.
5. Conclusions
Together with this crisis, pharmaceutical care entered a new phase, demonstrating the ability of pharmacists to be competent and accessible providers of public health. The pharmaceutical care services are not standardized, differing from country to country, and dependent on the capacity and desire of each national authority to apply them as the main part of public health. It remains to be seen how much these services will evolve and what the official recognition of pharmacists’ activities by each authority or institution will be. Future studies should present a way to standardize pharmaceutical care activity. These could provide authorities a tool to monitor and even fund these services, which would help the healthcare system and improve the patients’ quality of life. Such tools would also be useful to react to a future health crisis.
References
- Hepler, C.D.; Strand, L.M. Opportunities and responsibilities in pharmaceutical care. Am. J. Hosp. Pharm. 1990, 47, 533–543.
- van Mil, J.W.F. Definitions of Pharmaceutical Care and Related Concepts. In The Pharmacist Guide to Implementing Pharmaceutical Care; Springer: Berlin/Heidelberg, Germany, 2019; pp. 3–10.
- Morgovan, C.; Cosma, S.; Burta, C.; Ghibu, S.; Polinicencu, C.; Vasilescu, D. Measures to reduce the effects of the economic and financial crisis in pharmaceutical companies. Farmacia 2010, 58, 400–407.
- Turcu-Stiolica, A.; Bogdan, M.; Subtirelu, M.-S.; Meca, A.-D.; Taerel, A.-E.; Iaru, I.; Kamusheva, M.; Petrova, G. Influence of COVID-19 on Health-Related Quality of Life and the Perception of Being Vaccinated to Prevent COVID-19: An Approach for Community Pharmacists from Romania and Bulgaria. J. Clin. Med. 2021, 10, 864.
- Ahmad, A.; Alkharfy, K.M.; Alrabiah, Z.; Alhossan, A. Saudi Arabia, pharmacists and COVID-19 pandemic. J. Pharm. Policy Pract. 2020, 13, 1–3.
- Bragazzi, N.; Mansour, M.; Bonsignore, A.; Ciliberti, R. The Role of Hospital and Community Pharmacists in the Management of COVID-19: Towards an Expanded Definition of the Roles, Responsibilities, and Duties of the Pharmacist. Pharmacy 2020, 8, 140.
- Al-Quteimat, O.M.; Amer, A.M. SARS-CoV-2 outbreak: How can pharmacists help? Res. Soc. Adm. Pharm. 2021, 17, 480–482.
- Erku, D.A.; Belachew, S.A.; Abrha, S.; Sinnollareddy, M.; Thomas, J.; Steadman, K.J.; Tesfaye, W.H. When fear and misinformation go viral: Pharmacists’ role in deterring medication misinformation during the “infodemic” surrounding COVID-19. Res. Soc. Adm. Pharm. 2021, 17, 1954–1963.
- Hayden, J.C.; Parkin, R. The challenges of COVID-19 for community pharmacists and opportunities for the future. Ir. J. Psychol. Med. 2020, 37, 198–203.
- Da Costa, F.A.; Lee, V.; Leite, S.N.; Murillo, M.D.; Menge, T.; Antoniou, S. Pharmacists reinventing their roles to effectively respond to COVID-19: A global report from the international pharmacists for anticoagulation care taskforce (iPACT). J. Pharm. Policy Pract. 2020, 13, 1–3.
- PGEU. Grup Position Paper on the Role of Community Pharmacists in COVID-19-Lessons Learned from the Pandemic. Available online: https://www.pgeu.eu/wp-content/uploads/2020/03/PGEU-Position-Paper-on-on-the-Lessons-Learned-from-COVID-19-ONLINE.pdf (accessed on 21 June 2021).
- Peckham, A.M.; Ball, J.; Colvard, M.D.; Dadiomov, D.; Hill, L.G.; Nichols, S.D.; Tallian, K.; Ventricelli, D.J.; Tran, T.H. Leveraging pharmacists to maintain and extend buprenorphine supply for opioid use disorder amid COVID-19 pandemic. Am. J. Health-Syst. Pharm. 2021, 78, 613–618.
- Baratta, F.; Visentin, G.M.; Enri, L.R.; Parente, M.; Pignata, I.; Venuti, F.; Di Perri, G.; Brusa, P. Community pharmacy practice in italy during the COVID-19 (SARS-CoV-2) pandemic: Regulatory changes and a cross-sectional analysis of seroprevalence. Int. J. Environ. Res. Public Health 2021, 18, 2302.
- Dinte, E.; Zehan, T.; Miclaus, G.F.; Sandor, N.I.; Vaida, D.; Vostinaru, S. Implication of pharmacists in Cluj County in managing the COVID-19 pandemic. Rom. J. Pharm. Pract. 2020, 13, 157–163.
- Health Portfolio Ministers. Ministers Department of Health Ensuring Continued Access to Medicines during the COVID-19 Pandemic. Available online: https://www.health.gov.au/ministers/the-hon-greg-hunt-mp/media/ensuring-continued-access-to-medicines-during-the-covid-19-pandemic (accessed on 21 June 2021).
- NHS. Standard Operating Procedure—Community Pharmacy. Available online: https://www.england.nhs.uk/coronavirus/publication/standard-operating-procedure-community-pharmacy/ (accessed on 21 June 2021).
- Novak, H.; Tadić, I.; Falamić, S.; Ortner Hadžiabdić, M. Pharmacists’ role, work practices, and safety measures against COVID-19: A comparative study. J. Am. Pharm. Assoc. 2021, 61, 398–407.
- Elbeddini, A.; Hooda, N.; Yang, L. Role of Canadian pharmacists in managing drug shortage concerns amid the COVID-19 pandemic. Can. Pharm. J. 2020, 153, 198–203.
- Elbeddini, A.; Yeats, A. Amid COVID-19 drug shortages: Proposed plan for reprocessing and reusing salbutamol pressurized metered dose inhalers (pMDIs) for shared use. Drugs Ther. Perspect. 2020, 36, 300–302.
- Paudyal, V.; Cadogan, C.; Fialová, D.; Henman, M.C.; Hazen, A.; Okuyan, B.; Lutters, M.; Stewart, D. Provision of clinical pharmacy services during the COVID-19 pandemic: Experiences of pharmacists from 16 European countries. Res. Soc. Adm. Pharm. 2020, 17, 1507–1517.
- PDA. COVID around the World—Snapshots of the COVID-19 Response as at 9th May 2020. Available online: https://www.the-pda.org/wp-content/uploads/COVID-19-around-the-world-final.pdf (accessed on 21 June 2021).
- Uwizeyimana, T.; Hashim, H.T.; Kabakambira, J.D.; Mujyarugamba, J.C.; Dushime, J.; Ntacyabukura, B.; Ndayizeye, R.; Adebisi, Y.A.; Lucero-Prisno, D.E. Drug supply situation in Rwanda during COVID-19: Issues, efforts and challenges. J. Pharm. Policy Pract. 2021, 14, 1–4.
- CNN. Women Are Using Code Words at Pharmacies to Escape Domestic Violence. Available online: https://edition.cnn.com/2020/04/02/europe/domestic-violence-coronavirus-lockdown-intl/index.html?utm_content=2020-04-02T14%3A24%3A06utm_source=twCNNutm_term=linkutm_medium=social (accessed on 21 June 2021).
- Ertan, D.; El-Hage, W.; Thierrée, S.; Javelot, H.; Hingray, C. COVID-19: Urgency for distancing from domestic violence. Eur. J. Psychotraumatol. 2020, 11, 1800245.
- United Nations. United Nations UN Supporting ‘Trapped’ Domestic Violence Victims during COVID-19 Pandemic. Available online: https://www.un.org/en/coronavirus/un-supporting-‘trapped’-domestic-violence-victims-during-covid-19-pandemic (accessed on 21 June 2021).
- Watson, K.E.; Schindel, T.J.; Barsoum, M.E.; Kung, J.Y. COVID the Catalyst for Evolving Professional Role Identity? A Scoping Review of Global Pharmacists’ Roles and Services as a Response to the COVID-19 Pandemic. Pharmacy 2021, 9, 99.
- HM. Government Guidance for Pharmacies Implementing the Ask for Ani Domestic Abuse Codeword Scheme #YouAreNotAlone. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/940379/Training_information_-_Ask_for_ANI.pdf (accessed on 21 June 2021).
- Robinson, J. Thousands of pharmacies participate in domestic abuse codeword scheme. Pharm. J. 2021, 306.
- Merks, P.; Jakubowska, M.; Drelich, E.; Świeczkowski, D.; Bogusz, J.; Bilmin, K.; Sola, K.F.; May, A.; Majchrowska, A.; Koziol, M.; et al. The legal extension of the role of pharmacists in light of the COVID-19 global pandemic. Res. Soc. Adm. Pharm. 2021, 17, 1807–1812.
- Hess, K.; Bach, A.; Won, K.; Seed, S.M. Community Pharmacists Roles during the COVID-19 Pandemic. J. Pharm. Pract. 2020, 897190020980626.
- Alshahrani, A. Readiness of community pharmacists to play a supportive and advocacy role in the fight against corona virus disease. Risk Manag. Healthc. Policy 2020, 13, 3121–3133.
- Zheng, S.Q.; Yang, L.; Zhou, P.X.; Li, H.B.; Liu, F.; Zhao, R.S. Recommendations and guidance for providing pharmaceutical care services during COVID-19 pandemic: A China perspective. Res. Soc. Adm. Pharm. 2021, 17, 1819–1824.
- Elbeddini, A.; Botross, A.; Gerochi, R.; Gazarin, M.; Elshahawi, A. Pharmacy response to COVID-19: Lessons learnt from Canada. J. Pharm. Policy Pract. 2020, 13, 1–8.
- Bukhari, N.; Rasheed, H.; Nayyer, B.; Babar, Z.U.D. Pharmacists at the frontline beating the COVID-19 pandemic. J. Pharm. Policy Pract. 2020, 13, 1–4.
- Okoro, R.N. COVID-19 pandemic: The role of community pharmacists in chronic kidney disease management supportive care. Res. Soc. Adm. Pharm. 2021, 17, 1925–1928.
- ABDA. Bundesvereinigung Deutscher Apothekerverbände Corona-Pandemie: Apotheker als Gefragte Arzneimittelexperten in Impfzentren. Available online: https://www.abda.de/aktuelles-und-presse/pressemitteilungen/detail/corona-pandemie-apotheker-als-gefragte-arzneimittelexperten-in-impfzentren/ (accessed on 22 June 2021).
- Mallhi, T.H.; Liaqat, A.; Abid, A.; Khan, Y.H.; Alotaibi, N.H.; Alzarea, A.I.; Tanveer, N.; Khan, T.M. Multilevel Engagements of Pharmacists During the COVID-19 Pandemic: The Way Forward. Front. Public Health 2020, 8, 561924.
- Claramunt-García, R.; Muñoz-Cid, C.L.; Sierra-Torres, M.I.; Merino-Almazán, M. Hospital pharmacy in COVID-19. Farm. Hosp. 2020, 44, 74.
- Goff, D.A.; Ashiru-Oredope, D.; Cairns, K.A.; Eljaaly, K.; Gauthier, T.P.; Langford, B.J.; Mahmoud, S.F.; Messina, A.P.; Michael, U.C.; Saad, T.; et al. Global contributions of pharmacists during the COVID-19 pandemic. J. Am. Coll. Clin. Pharm. 2020, 3, 1480–1492.
- Elbeddini, A.; Prabaharan, T.; Almasalkhi, S.; Tran, C. Pharmacists and COVID-19. J. Pharm. Policy Pract. 2020, 13, 1–4.
- Sridhar, S.B.; Rabbani, S.A. Pharmaceutical care services provided by pharmacists during COVID-19 pandemic: Perspectives from around the World. J. Pharm. Health Serv. Res. 2021.
- Wang, R.; Kong, L.; Xu, Q.; Yang, P.; Wang, X.; Chen, N.; Li, L.; Jiang, S.; Lu, X. On-ward participation of clinical pharmacists in a Chinese intensive care unit for patients with COVID-19: A retrospective, observational study. Res. Soc. Adm. Pharm. 2021, 17, 1853–1858.
- Stevens, M.P.; Patel, P.K.; Nori, P. Involving antimicrobial stewardship programs in COVID-19 response efforts: All hands on deck. Infect. Control Hosp. Epidemiol. 2020, 41, 744–745.
- Yiaslas, T.A.; Sood, A.; Ono, G.; Rogers-Soeder, T.S.; Kitazono, R.E.; Embree, J.; Spann, C.; Caputo, C.A.; Taylor, J.; Schaefer, S. The Design and Implementation of a Heart Disease Reversal Program in the Veterans Health Administration: Before and During the COVID-19 Pandemic. Fed. Pract. 2020, 37, 558–565.
- Liu, S.; Luo, P.; Tang, M.; Hu, Q.; Polidoro, J.P.; Sun, S.; Gong, Z. Providing pharmacy services during the coronavirus pandemic. Int. J. Clin. Pharm. 2020, 42, 299–304.
- Li, H.; Zheng, S.; Liu, F.; Liu, W.; Zhao, R. Fighting against COVID-19: Innovative strategies for clinical pharmacists. Res. Soc. Adm. Pharm. 2021, 17, 1813–1818.
- Gross, A.E.; MacDougall, C. Roles of the clinical pharmacist during the COVID-19 pandemic. J. Am. Coll. Clin. Pharm. 2020, 3, 564–566.
- Zeenny, R.M.; Ramia, E.; Akiki, Y.; Hallit, S.; Salameh, P. Assessing knowledge, attitude, practice, and preparedness of hospital pharmacists in Lebanon towards COVID-19 pandemic: A cross-sectional study. J. Pharm. Policy Pract. 2020, 13, 1–12.
- Tan, S.L.; Zhang, B.K.; Xu, P. Chinese pharmacists’ rapid response to the COVID-19 outbreak. Am. J. Health-Syst. Pharm. 2020, 77, 1096–1097.
- Aruru, M.; Truong, H.A.; Clark, S. Pharmacy Emergency Preparedness and Response (PEPR): A proposed framework for expanding pharmacy professionals’ roles and contributions to emergency preparedness and response during the COVID-19 pandemic and beyond. Res. Soc. Adm. Pharm. 2021, 17, 1967–1977.
- Visacri, M.B.; Figueiredo, I.V.; de Mendonça Lima, T. Role of pharmacist during the COVID-19 pandemic: A scoping review. Res. Soc. Adm. Pharm. 2021, 17, 1799–1806.
- Hussain, K.; Ambreen, G.; Muzammil, M.; Raza, S.S.; Ali, U. Pharmacy services during COVID-19 pandemic: Experience from a tertiary care teaching hospital in Pakistan. J. Pharm. Policy Pract. 2020, 13, 1–4.
- Mary, A.; Hénaut, L.; Macq, P.Y.; Badoux, L.; Cappe, A.; Porée, T.; Eckes, M.; Dupont, H.; Brazier, M. Rationale for COVID-19 Treatment by Nebulized Interferon-β-1b–Literature Review and Personal Preliminary Experience. Front. Pharmacol. 2020, 11, 1885.
