Milk contains approximately 3.5% by weight protein, which is a highly complex system. This milk protein is usually divided into two main fractions based on their solubility nature. Casein proteins are about 75% to 80% of the total protein in the milk and precipitate at pH 4.6 at 20 °C, while 20% of the protein remains in the serum.
- milk
- ultra-high temperature treatment
- casein
- whey protein
- immunoglobulin
1. Introduction
Milk is a complex biological fluid, produced in the mammary gland of female mammalian species. It is produced to feed the neonate from birth to weaning [1]. It is a highly nutritious and readily digested food, i.e., rich in minerals, proteins, and energy in an aqueous solution. This provides the neonate with essential amino acids and many other important compounds such as hormones, growth factors, and protective agents [2].
Furthermore, milk provides quality proteins (casein and whey proteins) that cannot be obtained from any other food material [3]. Milk proteins also exhibit a high range of positive health and therapeutic benefits, such as aiding muscle growth, promoting fat loss, and strengthening bones in both adults and children. Humans consume cow milk primarily; however, globally, people obtain milk from many other animals, including buffalo, yaks, goats, sheep, reindeer, and camels. Generally, cow’s milk contains 80% of casein and 20% of whey protein, and both of the proteins always provide health benefits and are sources of a wide range of peptides with potential bioactive properties [4][5][4,5]. The function of milk protein is to supply young mammals with the essential amino acids that are required for the growth and development of the muscular body [5]. For the past centuries, humans have used milk and dairy-derived products to supplement their diet, and these dairy products are still a major food source. Furthermore, the physicochemical properties and textural properties of various dairy products is dependent upon the functional properties of the milk proteins [6][7][6,7]. The properties of the milk proteins also influence the quality of milk and milk-based products during various processing and storage conditions. However, due to the perishable nature of milk, research efforts are focused on the investigation of different processes to improve the shelf-life of milk for a longer period. Therefore, severe heat treatments became more popular due to their potential to enhance the shelf-life of the milk without further requirement of refrigeration storage [4]. Ultra-high temperature (UHT) includes the heating of milk at 135–145 °C for 2–3 s, which kills the spore-forming pathogenic microorganism, resulting in a product with a shelf-life of several months when stored at room temperature [6]. Due to the extended shelf-life of UHT-treated milk, it could easily transport to remote areas without refrigeration. In comparison to pasteurized milk, due to excessive and severe heating, UHT milk has more cooked and sulphureous flavors. Additionally, during storage, changes in the physicochemical properties of milk can lead to off-flavors, undesirable browning, separation of fat, sediment formation, or gelation during the subsequent storage [7]. Several important factors such as processing parameters, time-temperature abuse (storage condition), and packaging type also influence the quality characteristics and consumer acceptance. As well, Psychrotrophs can grow in refrigerated milk, and the majority of these bacteria produce heat-stable extracellular proteases that remain active after UHT treatment. This gives the final UHT products a bitter flavor, and also leads to gelation [8]. The population of psychrotrophs in fresh raw milk obtained from healthy cows is less than 10 2 colony-forming unit (CFU) mL −1 in acceptable hygienic conditions, accounting for 10% of the total microbiota [9]. The ambient temperatures of processing and storage of UHT-processed milk can vary from 0 °C to greater than or equal to 50 °C in cold countries, tropical zones, and some storage facilities [10]. However, these variable conditions significantly influence the proteins of the milk products, which can undergo several changes before the product is consumed. To avoid a declining nutritional value and to ensure the stability of the product, these changes must be understood so that the damage to the product can be minimized [11]. During processing and storage, the changes in proteins are caused by enzymatic activity, physicochemical interactions, and microbiological contamination. Ultra-high temperature processed milk has a shelf life of 6–9 months; however, some companies are claiming a shelf life of 12 months for their products [6][12][6,12]. Moreover, there are many ways in which the product can be damaged during storage, even under proper conditions. The legal expiry date for UHT milk in some countries is <90 days [13]. In addition, milk proteins are the most important components of the milk, which are helpful in various biological functions in the human body; however, during storage of UHT milk, aggregates of milk proteins or protein particles of various sizes form that ultimately influence the overall quality of the milk [14]. Aggregation of milk proteins has been shown to increase with variation in storage temperature [15]. Moreover, the aggregation of milk protein is a three-dimensional network that occurs either through enzymatic or non-enzymatic (severe heat) processes. Besides, during storage, changes in the physicochemical properties of milk can lead to off-flavors, undesirable browning, separation of fat, sediment formation, or gelation during the subsequent storage. Several important factors such as processing parameters, storage condition, and packaging type also influence the quality characteristics and consumer acceptance of the milk; however, the influence of heat treatments on milk protein is inconstant. The major protein modifications that occur during UHT treatment are denaturation and aggregation of the protein, and chemical modifications of its amino acids. These UHT-induced protein alterations can change digestibility and the overall biological influence of the intake of these proteins [12][13][14][15][12,13,14,15]. Therefore, in this review, we discussed the structural chemistry of milk proteins and their fractions. The overall quality of UHT milk and the toxicological and physicochemical changes of UHT-treated milk proteins during processing and storage are elaborately discussed.
2. Milk Proteins and Their Fractions
Milk contains approximately 3.5% by weight protein, which is a highly complex system. This milk protein is usually divided into two main fractions based on their solubility nature. Casein proteins are about 75% to 80% of the total protein in the milk and precipitate at pH 4.6 at 20 °C, while 20% of the protein remains in the serum. In the serum, about 15% are whey proteins, which are soluble under the above-mentioned conditions. The rest of the proteins found in milk are trace fractions of glycoproteins [16]. Proteins are made up of a polypeptide chain of amino acid residues joined together by peptide bonds and cross-linked by disulfide bonds. An acid carboxyl group and a weak basic amino group are both joined by a hydrocarbon chain that is unique to each amino acid [17]. However, the casein micelle is spherical and made up of smaller units known as submicelles. Herein, the submicelles vary in composition, consisting of κ-casein and α s -casein, or α s -casein and β-casein. They are linked together by small calcium phosphate cluster bridges [11]. Moreover, quaternary, secondary, and tertiary structures are all part of the three-dimensional organization of proteins. The three-dimensional arrangement of amino acid residues that are close to each other in the linear sequence is referred to as a secondary structure. Secondary structures rising from regular and periodic steric linkages include the helix and pleated sheet. The tertiary structure refers to the spatial arrangement of amino acid residues that are wide apart in the linear sequence, allowing for more folding and coiling to occur [16]. A protein is considered a globular protein if it is tightly coiled and folded into a spherical shape, and a fibrous protein if it is made up of long peptide chains that are intermolecularly connected. When proteins with two or more polypeptide chain subunits are linked, they form a quaternary structure [12]. The milk proteins include α s1 -casein, α s2 -casein, β-casein, κ-casein, α-lactalbumin (αLA), and β-lactoglobulin (βLG), which are the main components of milk and whey products.
κ-casein is responsible for the stability of casein micelles, and it is very resistant to calcium precipitation. The Rennet cleavage at the Phe105-Met106 bond eliminates the stabilizing ability by leaving the hydrophobic portion, para-κ-casein, and a hydrophilic portion, called κ-casein glycomacropeptide [18][20].
Whey proteins, also known as serum proteins, are globular proteins that contribute 20% of the total protein in bovine milk and practically all of the protein in whey. They are complex structured proteins with a high level of secondary and tertiary protein structures. Moreover, whey proteins are further fractionated in various important fractions, such as β-lactoglobulin (55%), α-lactalbumin (20%), immunoglobulins (13%), and Bovine serum albumin (BSA) (7%), which are minor proteins (5%) [19][23]. β-lactoglobulin, which is the major whey protein in bovine milk, is highly structured with α-helices or β-sheets, forming a packed globular structure with strong internal disulfide (S-S) bridges. The physical properties of whey proteins are shown in Table 12 [20][24].
| Milk Proteins (Whey Proteins) |
% of Whey Protein | Isoelectric Point (pI) |
Concentration (g/L) |
Molecular Weight (kDa) |
References |
|---|---|---|---|---|---|
| β-lactoglobulin | 55–65 | 5.35–5.49 | 3.3 | 18.4 | [2][20] |
| α-lactalbumin | 15–25 | 4.2–4.5 | 1.2 | 14.2 | [2][20] |
| Bovine serum albumin (BSA) | 5–6 | 5.1 | 0.3 | 66.3 | [2][20] |
| Immunoglobulins | 10–15 | 5.5–8.3 | 0.5 | 80–900 | [2][20] |
| Proteose peptones | 10–20 | 5.1–6.0 | 0.2 | 4.1–80 | [2][20] |
Milk contains several minor proteins, including about 60 indigenous enzymes, such as oxidoreductase, xanthine, lactoperoxidase, phosphatases, proteinase, and lipoprotein lipase. These are scientifically important. Most of the minor proteins have biological functions and probably play very significant roles [21][29].
3. Types of UHT Processing Systems for Milk
Generally, UHT processing of food is a combination of heat treatment with aseptic packing, which provides a shelf-life to a food product with minimal chemical damages as compared to in-container sterilized food products. Despite the fact that UHT milk has a shelf-life of up to 12 months, pasteurized milk has a larger sector of the milk market, as it is utilized as a convenience product [22][30]. UHT milk may require long-term stability, but in the latter scenario, the desired shelf life may be shorter than three months. To obtain ‘commercial sterility’, i.e., unlikely to grow bacteria in the product under typical storage conditions, UHT treatment in the range of 135–150 °C is used in combination with proper holding times [23][31]. In practice, the products are checked for sterility by incubating at 55 °C for 7 days or at 30 °C for 15 days, and testing for bacterial growth. UHT processing mainly consists of two types, which are direct and indirect heat treatments. The saturated steam is directly mixed with milk, and with the help of a vacuum flash vessel, the steam (added water) is removed from the milk in the direct heating method [24][26]. On the other hand, the heating medium, namely hot water or steam, is used to heats the milk indirectly by convection or conduction through a barrier in the form of a heat exchanger in the indirect method, as shown in Figure 1 . The main difference between these two methods is that the effect on milk proteins can be differentiated in the temperature and time profiles. In the direct method of heating, the milk is heated and cooled very quickly from the sterilization temperature when compared with the indirect method of heating. Because of this reason, the direct method of heating would produce fewer chemical changes than the indirect method of heating. When the milk is processed by the direct method of UHT treatment, the formation of sediment occurs more as compared to the sedimentation that occurs in milk from indirect systems, and the rate of sedimentation is directly proportional to an increase in heat load and storage temperature [22][30]. Sterilization during aseptic packing of the milk is employed by the UHT process, and this operation enables UHT milk to be packaged without contamination, which provides a long shelf life to milk at an ambient temperature [23][31].
4. Effects of UHT Processing on Milk Proteins
There are many indications of milk proteins present in the UHT milk, and milk products are changed during processing and storage. Thermal treatments have a large range of impacts on milk components, depending on how intense they are, but they are usually associated with undesirable changes in product color, texture, and nutritional properties [25][32].
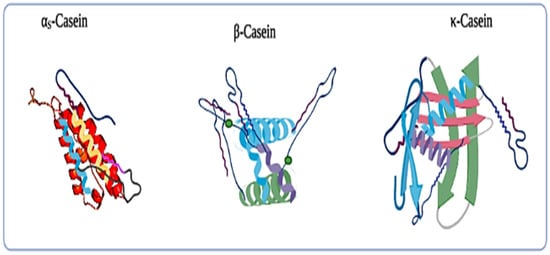
Most of the major whey proteins are highly ordered globular proteins, and thus they are very heat sensitive as they are liable to structural damage at the time of processing treatments in the dairy industries (e.g., high-pressure homogenization, and heat treatments). Generally, whey proteins tend to unfold from their original structure, revealing amino acids such as cysteine, which contains a free sulfhydryl (-SH) group when they are exposed to temperatures greater than 65 °C [26][39]. Moreover, the exposed free sulfhydryl group is now readily available and highly reactive, allowing other denatured whey proteins and proteins that contain the exposed sulfhydryl groups (e.g., κ-casein) to interact and aggregate to form a new (S-S) disulfide bond [27][35]. Whey protein fractions are composed of several proteins such as lactoferrin (LTF), α-lactalbumin (α-LA), β-lactoglobulin (β-LG) a small amount of lactoperoxidase (LP), bovine serum albumin (BSA), and immunoglobulin (IG). The structures of α-LA, β-LG, and BSA have been shown in Figure 23 . Moreover, β-LG has two disulfide bonds which maintain the structural integrity during the heat treatment and hydrolysis treatment, and one free cysteine group.
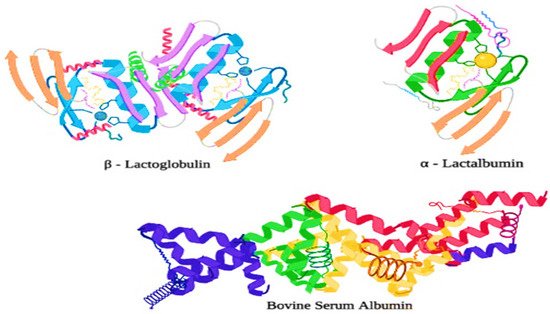
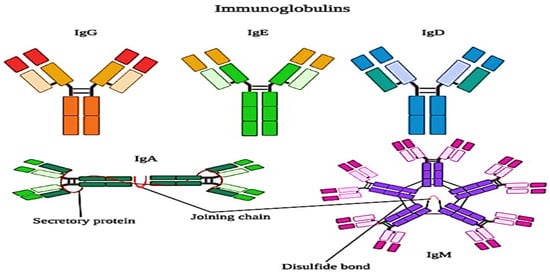
Whey proteins are more susceptible to heat denaturation, and their susceptibility depends upon the structural chemistry of the proteins. Immunoglobulins are the most heat-sensitive whey proteins, which are followed by bovine serum albumin (BSA), β-lactoglobulin (β-Lg), α-lactalbumin (α-La), and proteose peptones, though the last are not affected by heat. Mostly, the UHT treatments denature the immunoglobulins and most of the bovine serum albumin (BSA) because these proteins are present in lower concentrations than β-lactoglobulin and α-lactalbumin. The structure of immunoglobulins is explained in Figure 34 . The effects of denaturation and interaction with other proteins are minor as compared to β-lactoglobulin and α-lactalbumin, whereas, in the milk, the denaturation of whey proteins is dominated by the denaturation of β-lactoglobulin, which is about 50% of the whey proteins. However, α-lactalbumin is also important [26][39].
In addition, the denatured whey proteins can interact with casein fractions and form a composite of whey protein and κ-casein either in the serum phase or on the surface of the casein micelle [16]. This composite formation may prevent the dissociation of α s -casein and β-caseins with increasing temperature [28][41], and thus lowers their levels in the serum of UHT milk when compared with pasteurized milk. The dissociation of κ-casein from the casein micelle is an important factor related to the denaturation of β-lactoglobulin. During the UHT processing of skim milk (total solids 12–15%), the total percentage of κ-casein from the casein micelle at pH 6.6 was 30–60% [29][37]. Therefore, κ-casein interacts with denatured β-lactoglobulin in either part of the casein micelle or serum phase. The release of κ-casein from the casein micelle led to the denaturation of β-lactoglobulin [28][41]. Another important element is determining how much denatured whey protein is bound to κ-casein in the casein micelle and how much is associated with κ-casein in the serum phase [30][42]. When the milk is heated at a pH of 6.5, the denatured whey proteins are favorably attached to the casein micelle, whereas, at a greater pH > 6.8, most of the whey proteins or serum proteins are attached to the κ-casein, and they are found in the serum. The amount of attached whey proteins or serum proteins is highly sensitive to minor changes in the pH. The relative amounts of β-Lg and α-La that become attached to the micelle are also influenced by the heating process. Rapid heating, such as that used in indirect UHT processing, generates a large β-Lg: α-La ratio, but slower heating, such as that used in indirect UHT treatments, produces a lower ratio, indicating that more α-La and less β-Lg become attached [31][32][38,43].
