Nontuberculous mycobacteria (NTM) are the additional opportunistic pathogenic mycobacterial complex groups that consists of M. avium, M. marinum, M. hemophilum, M. kansasii, M. scrofulaceum, M. gordonae, M. abscessus, M. fortuitum and M. chelonae. They do not cause TB; however, they can produce pulmonary infections, lymphadenitis, skin disease, endometritis and disseminated disease. Thus, NTM are denoted by other names such as environmental mycobacteria or mycobacteria other than tuberculosis (MOTT) and atypical mycobacteria (ATM) [1–4].
- Nontuberculous mycobacteria
- Diagnostic challenges
- antimycobacterial agents
1. Introduction[1][2][3][4]
More than 200 different species of NTM have been identified in nature (https://www.bacterio.net/genus/mycobacterium), and among them; about 95% are environmental bacteria with maximum existence as saprophytes, opportunistic pathogens or nonpathogenic to humans and animals[5] [5]. NTM are generally found in the environment, mostly in wet soil, rivers, streams, estuaries, marshland and hospital settings. They are less pathogenic when compared to tuberculous mycobacteria, however they can cause illness to immunocompromised or pulmonary infected individuals[6] [6]. Among NTM pathogens, M. avium complex are the most significant and recurrent pathogenic organisms that causes pulmonary and extrapulmonary infections. In addition, M. xenopi, M. kansasii, M. malmoense are the most causative agents for pulmonary infections. Skin and cutaneous tissue infections are also caused by M. ulcerans and M. marinum[7] [7]. M. abscessus, M. fortuitum, M. chelonae, M. chimaera are the infectious agents accountable for most soft tissue infections[8] [8].
2. Classification
According to the Runyon classification (Figure 1), mycobacteria have broad categories based on phenotypic factors including pigmentation and the frequency of bacterial growth[9] [9]. They are classified as rapidly growing mycobacteria-RGM (visible colonies appear within seven days) and slow-growing mycobacteria-SGM (visible colonies appear after seven days). Most pathogenic mycobacteria are associated with the SGM, due to their virulence and growth rate. The members of the M. chelonae–M. abscessus complex and M. fortuitum complex are classified under the RGM family (Figure 1). The classification of mycobacteria remains greatly active and is continually developing, owing to the available technological progressions including sequencing of bacterial isolates. However, this improvement provides only taxonomy of the evolving novel mycobacteria, and still, their documentation remains uncertain and is obligatory to find the potential phenotypic and genetic polymorphisms of the M. abscessus complex.

Figure 1. Classification of nontuberculous mycobacteria.
RGM, M. chelonei, M. fortuitum and M. abscessus complex are well-renowned pathogens that often occur in cutaneous infections related to plastic surgery and cosmetic techniques. They appear widely in different pathologic conditions viz., cellulitis, superficial lymphadenitis, chronic nodular lesions, abscesses, nonhealing ulcers, verrucous lesions and commonly occur in the subcutaneous tissue and skin[10] [10].
M. abscessus is often misidentified as M. chelonae. It is documented that M. chelonae is seldom accountable for lung disease[11] [11]. In addition, M. chelonae fails to develop in the culture at 37 °C when compared to M. abscessus. M. chelonae is abundant in aquatic systems that can cause infection in immunocompromised hosts[12] [12]. Hence, this inappropriate identification of M. abscessus is highly possible in several pilot trials specifically in pulmonary contagions, consequently flouting the significance of this mycobacterium. Notably, the augmented occurrence of M. abscessus in the individual with cystic fibrosis directs that this pathogenic organism has developed progressively to become widespread in the past decade[13][14] [13,14]. The cultures of M. abscessus grow in less than seven days using agar medium (the combination of Bactec 12B and Middlebrook 7H10/ 7H11) and the strains of M. chelonae can be cultivated at 30 °C. Most of the NTM species can grow in the RGM culture medium at 30 °C, and M. xenopi can grow in the Lowenstein–Jensen (LJ) medium at 36 °C[15] [15].
The RGM organism M. abscessus possesses a high level of heterogeneity in the genotype and is capable of rapid evolution by phage mediated gene transfer[16][17] [16,17]. There are three subtypes in the complex of M. abscessus, namely, M. abscessus, M. bolletii and M. massiliense[5] [5]. M. abscessus possesses diverse structures in the cell wall due to the occurrence or absence of glycopeptidolipids (GPL)[18] [18]. Similarly, other NTM species have also shown structural variations. The colony morphology and GPL arrangements in M. abscessus are normally responsible for interactions with the host and regulating the environment of biofilm development and intracellular survival, which results in disease manifestations and clinical outcomes[19] [19]. The most common point of entry of NTM into the host occurs via direct invasion including trauma, iatrogenic acquisition or postsurgical infections[20] [20]. These bacteria can invade soft tissues and skin in immunodeficient patients during systemic dissemination[21][22] [21,22]. Shreds of evidence show that the possible human transmission of M. abscessus subsp. massiliense may occur among cystic fibrosis patients[23][24] [23,24].
3. Clinical Epidemiology of NTM
The diseases of NTM are often found in developed nations, where the peak occurrence rates was 10.6 cases per 100,000 individuals in 2000[25] [25]. Based on pulmonary research by various experts, the respiratory NTM are projected to be at least 15 times more common than TB with at least 200,000 cases per year in the U.S.A[25] [25]. In South Korea, the occurrence of NTM infections have been augmented to 39.6 cases/100,000 people in 2016 and yearly occurrence could be 19.0 cases/100,000 people. An investigation led in Germany described a growing incidence of NTM in 2009 from 2.3 cases/100,000 people to 3.3 cases/100,000 populace in 2014[26] [26]. Shreds of evidence associated with the occurrence of the disease of NTM and elevation levels are greater in Europe[26] [26], the United States[27][28][29] [27–29] and Japan[30] [30]. The higher rates of NTM infection have been reported in East Asian inhabitants particularly China, Vietnam, Hawaii, Philippines, Japan and Korea[27][28] [27,28]. The individuals with NTM in Japan and the Philippines were at higher risk for M. abscessus infection whereas Vietnam and Korean patients were often affected by M. fortuitum group infection[27] [27]. M. avium complex (MAC) and RGM including M. abscessus and M. chelonae have been attributed to 85% of pulmonary cases in the United States[31] [31]. Pulmonary diseases are strongly associated with advanced age and more often in women than men[10] [10].
The NTM diseases are generally caused by M. abscessus, M. fortuitum, MAC and M. chelonae. Among them, M. abscessus is often found with rising frequency and is most challenging to treat[32] [32]. The swiftly increasing NTMs are normally associated with catheter infections, post-cosmetic surgery of the soft tissue and skin and pulmonary infections[28] [28]. The clinical implications and location of infection of NTM are listed in Table 1. Several investigations have established that the incidence of NTM diseases are greatly escalating in numerous clinical conditions[21][33][34][35] [21,33–35]. The clinical range of the infections is highly connected based on the entry to the host and host susceptibility factors and these infections are multisystem and multigenic-based diseases[21][34] [21,34]. Disseminated NTM infections typically impact severely immunocompromised patients with primary immunodeficiencies, via inherited or acquired deficiency of the IL-12-IFN-γ pathway, HIV/AIDS, transplant-linked immunosuppression and anti-TNF-α receptor blockers treatment[34][36] [34,36].
Table 1.
Clinical significance and site of infection of nontuberculous mycobacteria (NTM).
|
List of NTM Species |
Clinical Relevance and Possible Site of Infection |
Reference |
|
M. abscessus |
Peripheral blood, peritoneal biopsy, pulmonary and permanent catheter tip. |
|
|
M. asiaticum |
Pulmonary |
|
|
M. avium |
Pulmonary |
|
|
M. celatum |
Pulmonary |
|
|
M. chelonae |
Breast abscesses, blood and peritoneal fluid, pleural fluid |
|
|
M. flavescens |
Pulmonary |
|
|
M. fortuitum |
Ascetic fluid, peritoneal dialysis fluid, pulmonary, lipoid pneumonia, mediastinal infection, a myocardial and abdominal abscess. |
|
|
M. gastri |
Pulmonary |
|
|
M. gordonae |
Urinary tract and rarely liver biopsies |
|
|
M. intracellulare |
Pulmonary and extrapulmonary |
|
|
M. kansasii |
Appendiceal abscess |
|
|
M. lentiflavum |
Extrapulmonary |
|
|
M. marinum |
Wound-elbow and nasal cavity |
|
|
M. riyadhense |
Pulmonary infection, sclerotic lesions, maxillary sinus, dural lesion |
|
|
M. scrofulaceum |
Extrapulmonary |
|
|
M. simiae |
Pulmonary |
|
|
M. smegmatis |
Pulmonary |
|
|
M. szulgai |
Joints/synovial aspiration |
|
|
M. terrae |
Pulmonary |
|
|
M. xenopi |
Pulmonary |
4. Challenges in Diagnosing and Treatment of NTM Diseases
RGM are usually isolated from blood, sputum or tissues for diagnosis and are often misidentified as diphtheroids. RGM species normally cultivate as routine culture in liquid broth blood culture medium or on solid agars that can grow quickly within seven days. These strains relatively stain with Gram stain not with Ziehl–Neelsen stain to demonstrate the acid-fast characteristics. A fresh young culture of RGM may not constantly show branching or beaded structures and exhibit weakly Gram-positive bacilli, thus misleading the diagnosis and often incorrectly concluded as diphtheroids[46] [46]. NTM in tissue specimens can also be identified based on the molecular method of determination, which includes, 16S rRNA gene sequencing, PCR analysis and HPLC. The diagnosis of NTM often fails to recognize the species and subspecies of the different samples from the affected individual. Most NTM microscopically appears similar to Mycobacterium tuberculosis (MTB), and the colony morphology varies in culture. The culture difference and microscopic appearance are shown in Figure 2. A total of 16S ribosomal RNA sequencing aids in individual NTM species identification[20] [20]. Diagnosis is generally completed by recurrent isolation accompanied by certain clinical and radiological features. There is no explicit treatment of NTM infections and therapy depends upon the particular species and its resistance to antibiotics[47] [47].
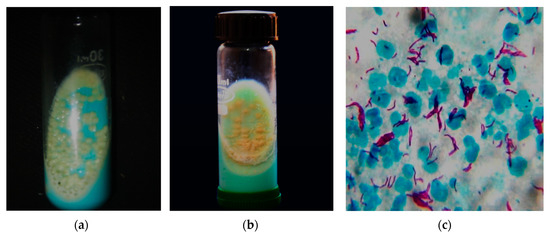
Figure 2.
NTM and
Mycobacterium tuberculosis (MTB) culture and microscopy. (
(MTB) culture and microscopy.
(
a
) NTM grown at 48 h of incubation in LJmedia with typical characteristics of moist, smooth glistening yellow colonies; (
b
) MTB grown at six weeks of incubation in LJ media with typical characteristics of rough, buff yellow-colored cauliflower-like colonies; (
c
) Long and slender pink-colored acid-fast tuberculous mycobacteria by Ziehl–Neelsen stain (100×). The above culture images differentiate the NTM and MTB with almost similar microscopical image
The diagnosis of NTM are difficult to confirm using acid-fast microscopy, which is the primary diagnostic tool for TB in numerous developing nations. As an outcome, most cases of NTM causing pulmonary infections are not recognized and eventually treated with traditional anti-TB medications. These treatments often fail because NTM are mostly resistant to anti-TB therapy[48] [48]. Hence, in developed nations, caseloads of 8.6/100,000 total population and 20.4/100,000 population over 50 years old are typical[49] [49]. In developing nations, the occurrence rate and diagnosis of NTM cannot be observed due to the lack of laboratory arrangement and identification of mycobacteria. Hence, the escalating rate of pathogenic NTM in developing nations has been greater particularly with the advent of HIV/AIDS patients. Normally, HIV/AIDS individuals with severe immunosuppression are at high risk of NTM infections, which often cause localized or disseminated infections[50] [50]. In addition, the failure of NTM treatment can frequently occur due to resistance to some of the available antibiotics (Table 2).
Table 2. Various treatment recommendations for NTM[51][52] [51,52].
|
Mycobacterium Species |
Established Regimens |
Additional or Suggested Agents |
|
M. avium complex |
rifampin, ethambutol, isoniazid, streptomycin or amikacin |
clarithromycin (azithromycin), ciprofloxacin, clofazimine |
|
M. scrofulaceum |
- |
clarithromycin (azithromycin), ciprofloxacin, clofazimine |
|
M. kansasii |
rifampin, ethambutol, isoniazid |
streptomycin, ciprofloxacin, clarithromycin |
|
M. marinum |
rifampin, ethambutol, doxycycline or trimethoprim-sulfamethoxazole |
streptomycin, ciprofloxacin |
|
M. xenopi |
rifampin, ethambutol, isoniazid |
streptomycin |
|
M. malmoense |
- |
clarithromycin (azithromycin), ciprofloxacin, clofazimine |
|
M. simiae |
- |
clarithromycin (azithromycin), ciprofloxacin, clofazimine |
|
M. szulgai |
- |
streptomycin, ciprofloxacin, clarithromycin |
|
M. hemophilum |
- |
rifampin, cefoxitin, doxycycline, trimethoprim-sulfamethoxazole |
|
M. fortuitum |
amikacin, ciprofloxacin, sulfonamides |
clofazimine, cefoxitin, imipenem, a cocktail of azithromycin or clarithromycin, doxycycline, fluoroquinolones, trimethoprim-sulfamethoxazole |
|
M. abscessus |
amikacin, streptomycin, cefoxitin |
clofazimine, clarithromycin, a cocktail of azithromycin, imipenem, clarithromycin, |
|
M. chelonae |
tobramycin, amikacin |
clofazimine, clarithromycin, doxycycline, a cocktail of azithromycin, imipenem, cefoxitin, clarithromycin, fluoroquinolones |
In addition, using these chemical agents produce various complications including, diarrhea, headache, renal failure and colitis. Mycobacteriosis is an acute/chronic, systemic, granulomatous disease caused by NTM, which is extremely challenging in selecting effective antimicrobial therapy based on the antimicrobial resistance[53] [53]. The RGM involves individualized treatment according to the outcomes found in vitro vulnerability tests for cefoxitin, amikacin, clarithromycin, sulfamethoxazole, ciprofloxacin, imipenem and doxycycline[54] [54]. The M. fortuitum and M. chelonae are members of M. abscessus complex and M. massiliense, M. abscessus and M. bolletii are subspecies, which are the chief NTM related to cutaneous tissue involvement[55] [55]. All these mycobacteria are regularly found with several skin lesions, however M. fortuitum is often found in a sole lesion[33] [33]. The susceptibility to antimicrobials generally depends upon the individual species. M. abscessus complex is likely to be vulnerable to the cocktail of amikacin, azithromycin, imipenem and cefoxitin, since, it is known that clarithromycin resistance due to the occurrence of the erm41 gene[56] [56].
In vivo study demonstrates that NTM isolates show resistance to azithromycin or clarithromycin[56][57] [56,57]. Azithromycin is normally the desired antibiotic for M. abscessus infections, while azithromycin or clarithromycin is highly efficient in the cases of M. massiliense[56][57] [56,57]. M. fortuitum, M. abscessus and M. chelonae are resistant to all of the existing anti-TB agents[10][56][57] [10,56,57]. M. fortuitum is highly susceptible to amikacin, trimethoprim-sulfamethoxazole, azithromycin or clarithromycin, fluoroquinolones and doxycycline. M. chelonae is also often susceptible to azithromycin or clarithromycin, tobramycin, fluoroquinolones and cefoxitin[55] [55]. The guideline of the therapy recommends performing susceptibility testing of NTM to enhance the option of a cocktail of the antimycobacterial drug relates clinically in vivo trials to antimicrobial treatment for various species of NTM. From the microbiologic perspective, heterogeneity of NTM needs sophisticated and rapid laboratory techniques. Since the present pharmacological treatment of NTM diseases are tricky, and often fails to scope the long-term removal of pathogens. Moreover, it is obligatory to hunt novel agents or treatment and dosage regimens for effective treatment of these NTM diseases, specifically serious in immunocompromised individuals.
Hence, it is necessary to find alternative remedial regimens. One of the alternative resources is traditional medicinal plants or their derivatives, which are well-known for their therapeutic properties. Most of the researchers have a positive approach toward natural products due to their natural origin and low noxious with fewer side effects[3][58][59][60][61][62][63][64][65][66] [3,58–66]. A trial of anti-Mycobacterial effects of these medicinal plants, particularly those that are conventionally used for pulmonary infections is significant.
Natural products as a source of medicine are potentially valuable due to their natural origin and low toxicity with lesser side effects. Medicinal herbs with the traditional practice of crude extracts or active principles have been widely used for treating and averting human illnesses for many centuries. These ethnopharmacological techniques have been reinforced to yield bioactive compounds that support to improve modern medicine as beneficial tools[67][68][69][70] [67–70]. Bioactive compounds often contribute a noteworthy function in drug finding by helping as a novel drug of interest and templates for synthetic agents[71][72][73] [71–73]. Copious investigations have established that natural bioactive compounds have possible antimycobacterial activities[2][60][74][75] [2,60,74,75]. The single-handed practice of bioactive compounds or cocktails with classical antibiotics signifies a greater alternative treatment. Additionally, the cocktails of those antimicrobial agents often require only a minor amount. Therefore, this smaller amount may provide less toxicity to the host, ensuring great lenience to the antibacterial drugs. Grounded on the existing information, there has been inadequate literature regarding antimycobacterial phytocompounds[76][77][78][79] [76–79].
5. Conclusions
NTM have developed into significant bacterial pathogens for both animals and humans. In particular, the concern is the high level of antimicrobial resistance displayed by these organisms, which complicates treatment and possible effective outcomes. The state of the existing antimycobacterial agents and their hitches is relatively serious. In developing nations, the incidence rate and diagnosis of NTM have often not been noticed as a deficiency of laboratory settings and mycobacteria identification. The escalating rate of pathogenic NTM in developing nations is significantly greater in HIV/AIDS patients, which leads to high levels of morbidity and mortality globally. Furthermore, there are restrictions evident by antimycobacterial drugs: the lower bactericidal ability, multidrug usage, high resistance and toxicity and organ damage. Hence, it is imperative to find new drugs as alternative therapies in which flavonoids are promising to be safe for usage, endowed with abundant pharmacological roles that are potentially active against NTM. Several flavonoids have been used in connotation with their antimycobacterial activities and can be potential and cost-effective. They have possible antimycobacterial effects at minor quantities by themselves or in synergistic combinations.
References
- Peyrani, P.; Ramirez, J.A. Nontuberculous mycobacterial pulmonary infections. In Pulmonary Complications of HIV; European Respiratory Society: Sheffield, UK, 2014; pp. 128–137.
- Suresh, M.; Rath, P.K.; Panneerselvam, A.; Dhanasekaran, D.; Thajuddin, N. Anti-mycobacterial effect of leaf extract of Centella asiatica. Res. J. Pharm. Technol. 2010, 3, 872–876.
- Lim, S.S.; Selvaraj, A.; Ng, Z.Y.; Palanisamy, M.; Mickmaray, S.; Cheong, P.C.H.; Lim, R.L.H. Isolation of actinomycetes with antibacterial activity against multi-drug resistant bacteria. Malays. J. Microbiol. 2018, 14, 293–305.
- Devi, C.A.; Dhanasekaran, D.; Suresh, M.; Thajuddin, N. Diagnostic value of real time PCR and associated bacterial and fungal infections in female genital tuberculosis. Biomed. Pharm. J. 2015, 3, 73–79.
- Tortoli, E.; Fedrizzi, T.; Meehan, C.J.; Trovato, A.; Grottola, A.; Giacobazzi, E.; Serpini, G.F.; Tagliazucchi, S.; Fabio, A.; Bettua, C.; et al. The new phylogeny of the genus mycobacterium: The old and the news. Infect. Genet. Evol. 2017, 56, 19–25.
- Catherinot, E.; Roux, A.-L.; Vibet, M.-A.; Bellis, G.; Ravilly, S.; Lemonnier, L.; Le Roux, E.; Bernède-Bauduin, C.; Le Bourgeois, M.; Herrmann, J.-L.; et al. Mycobacterium avium and mycobacterium abscessus complex target distinct cystic fibrosis patient subpopulations. J. Cyst. Fibros. 2013, 12, 74–80.
- Baldwin, S.L.; Larsen, S.E.; Ordway, D.; Cassell, G.; Coler, R.N. The complexities and challenges of preventing and treating nontuberculous mycobacterial diseases. PLoS Negl. Trop. Dis. 2019, 13, e0007083.
- Johansen, M.D.; Herrmann, J.-L.; Kremer, L. Non-tuberculous mycobacteria and the rise of mycobacterium abscessus. Nat. Rev. Microbiol. 2020, 18, 392–407.
- Turenne, C.Y. Nontuberculous mycobacteria: Insights on taxonomy and evolution. Infect. Genet. Evol. 2019, 72, 159–168.
- Franco-Paredes, C.; Marcos, L.A.; Henao-Martínez, A.F.; Rodríguez-Morales, A.J.; Villamil-Gómez, W.E.; Gotuzzo, E.; Bonifaz, A. Cutaneous mycobacterial infections. Clin. Microbiol. Rev. 2018, 32, e00069-18.
- Kim, B.-J.; Kim, B.-R.; Jeong, J.; Lim, J.-H.; Park, S.H.; Lee, S.-H.; Kim, C.K.; Kook, Y.-H.; Kim, B.-J. A description of mycobacterium chelonae subsp. gwanakae subsp. nov., a rapidly growing mycobacterium with a smooth colony phenotype due to glycopeptidolipids. Int. J. Syst. Evol. Microbiol. 2018, 68, 3772–3780.
- Jankovic, M.; Sabol, I.; Zmak, L.; Jankovic, V.K.; Jakopovic, M.; Obrovac, M.; Ticac, B.; Bulat, L.K.; Grle, S.P.; Marekovic, I.; et al. Microbiological criteria in non-tuberculous mycobacteria pulmonary disease: A tool for diagnosis and epidemiology. Int. J. Tuberc. Lung Dis. 2016, 20, 934–940.
- Olivier, K.N.; Weber, D.J.; Wallace, R.J.; Faiz, A.R.; Lee, J.-H.; Zhang, Y.; Brown-Elliot, B.A.; Handler, A.; Wilson, R.W.; Schechter, M.S.; et al. Nontuberculous Mycobacteria. Am. J. Respir. Crit. Care Med. 2003, 167, 828–834.
- Roux, A.L.; Catherinot, E.; Ripoll, F.; Soismier, N.; Macheras, E.; Ravilly, S.; Bellis, G.; Vibet, M.A.; Le Roux, E.; Lemonnier, L.; et al. Multicenter study of prevalence of nontuberculous Mycobacteria in patients with cystic fibrosis in France. J. Clin. Microbiol. 2009, 47, 4124–4128.
- Stephenson, D.; Perry, A.; Appleby, M.R.; Lee, D.; Davison, J.; Johnston, A.; Jones, A.L.; Nelson, A.; Bourke, S.J.; Thomas, M.F.; et al. An evaluation of methods for the isolation of nontuberculous mycobacteria from patients with cystic fibrosis, bronchiectasis and patients assessed for lung transplantation. BMC Pulm. Med. 2019, 19, 19.
- Choo, S.W.; Wee, W.Y.; Ngeow, Y.F.; Mitchell, W.; Tan, J.L.; Wong, G.J.; Zhao, Y.; Xiao, J. Genomic reconnaissance of clinical isolates of emerging human pathogen mycobacterium abscessus reveals high evolutionary potential. Sci. Rep. 2014, 4, 4061.
- Sapriel, G.; Konjek, J.; Orgeur, M.; Bouri, L.; Frézal, L.; Roux, A.-L.; Dumas, E.; Brosch, R.; Bouchier, C.; Brisse, S.; et al. Genome-wide mosaicism within mycobacterium abscessus: Evolutionary and epidemiological implications. BMC Genom. 2016, 17, 118.
- Viljoen, A.; Gutiérrez, A.V.; Dupont, C.; Ghigo, E.; Kremer, L. A simple and rapid gene disruption strategy in mycobacterium abscessus: On the design and application of glycopeptidolipid mutants. Front. Cell Infect. Microbiol. 2018, 8, 69.
- Gutiérrez, A.V.; Viljoen, A.; Ghigo, E.; Herrmann, J.-L.; Kremer, L. Glycopeptidolipids, a double-edged sword of the mycobacterium abscessus complex. Front. Microbiol. 2018, 9, 1145.
- Wallace, J.R.; Mangas, K.M.; Porter, J.L.; Marcsisin, R.; Pidot, S.J.; Howden, B.; Omansen, T.F.; Zeng, W.; Axford, J.K.; Johnson, P.D.R.; et al. Mycobacterium ulcerans low infectious dose and mechanical transmission support insect bites and puncturing injuries in the spread of Buruli ulcer. PLoS Negl. Trop. Dis. 2017, 11, e0005553.
- Wu, U.-I.; Holland, S.M. Host susceptibility to non-tuberculous mycobacterial infections. Lancet Infect. Dis. 2015, 15, 968–980.
- Zhang, M.; Feng, M.; He, J.-Q. Disseminated mycobacterium kansasii infection with cutaneous lesions in an immunocompetent patient. Int. J. Infect. Dis. 2017, 62, 59–63.
- Aitken, M.L.; Limaye, A.; Pottinger, P.; Whimbey, E.; Goss, C.H.; Tonelli, M.R.; Cangelosi, G.A.; Dirac, M.A.; Olivier, K.N.; Brown-Elliott, B.A.; et al. Respiratory outbreak of mycobacterium abscessus subspecies massiliense in a lung transplant and cystic fibrosis center. Am. J. Respir. Crit. Care Med. 2012, 185, 231–232.
- Bryant, J.M.; Grogono, D.M.; Greaves, D.; Foweraker, J.; Roddick, I.; Inns, T.; Reacher, M.; Haworth, C.S.; Curran, M.D.; Harris, S.R.; et al. Whole-genome sequencing to identify transmission of mycobacterium abscessus between patients with cystic fibrosis: A retrospective cohort study. Lancet 2013, 381, 1551–1560.
- Pedrero, S.; Tabernero, E.; Arana-Arri, E.; Urra, E.; Larrea, M.; Zalacain, R. Changing epidemiology of nontuberculous mycobacterial lung disease over the last two decades in a region of the Basque country. ERJ Open Res. 2019, 5, 00110–02018.
- Ringshausen, F.C.; Wagner, D.; de Roux, A.; Diel, R.; Hohmann, D.; Hickstein, L.; Welte, T.; Rademacher, J. Prevalence of nontuberculous Mycobacterial pulmonary disease, Germany, 2009–2014. Emerg. Infect. Dis. 2016, 70, 1102.
- Adjemian, J.; Olivier, K.N.; Seitz, A.E.; Holland, S.M.; Prevots, D.R. Prevalence of Nontuberculous Mycobacterial lung disease in U.S. medicare beneficiaries. Am. J. Respir. Crit. Care Med. 2012, 185, 881–886.
- Adjemian, J.; Olivier, K.N.; Seitz, A.E.; Falkinham, J.O.; Holland, S.M.; Prevots, D.R. Spatial clusters of Nontuberculous Mycobacterial lung disease in the United States. Am. J. Respir. Crit. Care Med. 2012, 186, 553–558.
- Henkle, E.; Hedberg, K.; Schafer, S.; Novosad, S.; Winthrop, K.L. Population-based incidence of pulmonary nontuberculous mycobacterial disease in Oregon 2007 to 2012. Ann. Am. Thorac. Soc. 2015, 12, 642–647.
- Morimoto, K.; Iwai, K.; Uchimura, K.; Okumura, M.; Yoshiyama, T.; Yoshimori, K.; Ogata, H.; Kurashima, A.; Gemma, A.; Kudoh, S. A steady increase in nontuberculous mycobacteriosis mortality and estimated prevalence in Japan. Ann. Am. Thorac. Soc. 2014, 11, 1–8.
- Cassidy, P.M.; Hedberg, K.; Saulson, A.; McNelly, E.; Winthrop Kevin, L. Nontuberculous mycobacterial disease prevalence and risk factors: A changing epidemiology. Clin. Infect. Dis. 2009, 49, e124–e129.
- Larsson, L.-O.; Polverino, E.; Hoefsloot, W.; Codecasa, L.R.; Diel, R.; Jenkins, S.G.; Loebinger, M.R. Pulmonary disease by non-tuberculous mycobacteria—Clinical management, unmet needs and future perspectives. Expert Rev. Respir. Med. 2017, 11, 977–989.
- Wang, S.-H.; Pancholi, P. Mycobacterial skin and soft tissue infection. Curr. Infect. Dis. Rep. 2014, 16, 438.
- Szymanski, E.P.; Leung, J.M.; Fowler, C.J.; Haney, C.; Hsu, A.P.; Chen, F.; Duggal, P.; Oler, A.J.; McCormack, R.; Podack, E.; et al. Pulmonary nontuberculous mycobacterial infection. A multisystem, multigenic disease. Am. J. Respir. Crit. Care Med. 2015, 192, 618–628.
- Ryu, Y.J.; Koh, W.-J.; Daley, C.L. Diagnosis and treatment of nontuberculous mycobacterial lung disease: Clinicians’ perspectives. Tuberc. Respir. Dis. 2016, 79, 74.
- Henkle, E.; Winthrop, K.L. Nontuberculous mycobacteria infections in immunosuppressed hosts. Clin. Chest Med. 2015, 36, 91–99.
- Mokaddas, E.; Ahmad, S. Species spectrum of nontuberculous mycobacteria isolated from clinical specimens in Kuwait. Curr. Microbiol. 2008, 56, 413–417.
- Al-Mahruqi, S.H.; van Ingen, J.; Al-Busaidy, S.; Boeree, M.J.; Al-Zadjali, S.; Patel, A.; Dekhuijzen, P.N.R.; van Soolingen, D. Clinical relevance of nontuberculous mycobacteria, Oman. Emerg. Infect. Dis. 2009, 15, 292–294.
- Varghese, B.; Memish, Z.; Abuljadayel, N.; Al-Hakeem, R.; Alrabiah, F.; Al-Hajoj, S.A. Emergence of clinically relevant non-tuberculous mycobacterial infections in Saudi Arabia. PLoS Negl. Trop. Dis. 2013, 7, e2234.
- Al-Harbi, A.; Al-Jahdali, H.; Al-Johani, S.; Baharoon, S.; Bin Salih, S.; Khan, M. Frequency and clinical significance of respiratory isolates of non-tuberculous mycobacteria in Riyadh, Saudi Arabia. Clin. Respir. J. 2014, 10, 198–203.
- Russell, C.D.; Claxton, P.; Doig, C.; Seagar, A.L.; Rayner, A.; Laurenson, I.F. Non-tuberculous mycobacteria: A retrospective review of Scottish isolates from 2000 to 2010. Thorax 2014, 69, 593–595.
- Jankovic, M.; Samarzija, M.; Sabol, I.; Jakopovic, M.; Katalinic Jankovic, V.; Zmak, L.; Ticac, B.; Marusic, A.; Obrovac, M.; van Ingen, J. Geographical distribution and clinical relevance of non-tuberculous mycobacteria in Croatia. Int. J. Tuberc. Lung Dis. 2013, 17, 836–841.
- Albayrak, N.; Simşek, H.; Sezen, F.; Arslantürk, A.; Tarhan, G.; Ceyhan, I. Evaluation of the distribution of non-tuberculous mycobacteria strains isolated in National Tuberculosis Reference Laboratory in 2009–2010, Turkey. Mikrobiyol. Bul. 2012, 46, 560–567.
- Simons, S.; van Ingen, J.; Hsueh, P.R.; Van Hung, N.; Dekhuijzen, P.N.; Boeree, M.J.; van Soolingen, D. Nontuberculous mycobacteria in respiratory tract infections, eastern Asia. Emerg. Infect. Dis. 2011, 17, 343–349.
- Lai, C.C.; Hsueh, P.R. Diseases caused by nontuberculous mycobacteria in Asia. Future Microbiol. 2014, 9, 93–106.
- Baron, E.J. Clinical Microbiology in Underresourced Settings. Clin. Lab. Med. 2019, 39, 359–369.
- Wu, M.-L.; Aziz, D.B.; Dartois, V.; Dick, T. NTM drug discovery: Status, gaps and the way forward. Drug Discov. Today 2018, 23, 1502–1519
- Fleshner, M.; Olivier, K.N.; Shaw, P.A.; Adjemian, J.; Strollo, S.; Claypool, R.J.; Folio, L.; Zelazny, A.; Holland, S.M.; Prevots, D.R. Mortality among patients with pulmonary non-tuberculous mycobacteria disease. Int. J. Tuberc. Lung Dis. 2016, 20, 582–587.
- Winthrop, K.L.; McNelley, E.; Kendall, B.; Marshall-Olson, A.; Morris, C.; Cassidy, M.; Saulson, A.; Hedberg, K. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features. Am. J. Respir. Crit. Care Med. 2010, 182, 977–982.
- Martínez González, S.; Cano Cortés, A.; Sota Yoldi, L.A.; García García, J.M.; Alba Álvarez, L.M.; Palacios Gutiérrez, J.J. Non-tuberculous mycobacteria. An emerging threat? Arch. Bronconeumol. 2017, 53, 554–560.
- Novosad, S.A.; Beekmann, S.E.; Polgreen, P.M.; Mackey, K.; Winthrop, K.L. Treatment of Mycobacterium abscessus Infection. Emerg. Infect. Dis. 2016, 22, 511–514.
- Wolinsky, E. Mycobacterial Diseases Other Than Tuberculosis. Clin. Infect. Dis. 1992, 15, 1–12.
- Redelman-Sidi, G.; Sepkowitz, K.A. Rapidly growing mycobacteria infection in patients with cancer. Clin. Infect. Dis. 2010, 51, 422–434.
- Esteban, J.; García-Coca, M. Mycobacterium biofilms. Front. Microbiol. 2017, 8, 2651.
- Forbes, B.A.; Hall, G.S.; Miller, M.B.; Novak, S.M.; Rowlinson, M.-C.; Salfinger, M.; Somoskövi, A.; Warshauer, D.M.; Wilson, M.L. Practice guidelines for clinical microbiology laboratories: Mycobacteria. Clin. Microbiol. Rev. 2018, 31.
- Mougari, F.; Guglielmetti, L.; Raskine, L.; Sermet-Gaudelus, I.; Veziris, N.; Cambau, E. Infections caused by mycobacterium abscessus: Epidemiology, diagnostic tools and treatment. Expert Rev. Anti-Infect. Ther. 2016, 14, 1139–1154.
- Marion, E.; Song, O.-R.; Christophe, T.; Babonneau, J.; Fenistein, D.; Eyer, J.; Letournel, F.; Henrion, D.; Clere, N.; Paille, V.; et al. Mycobacterial toxin induces analgesia in buruli ulcer by targeting the angiotensin pathways. Cell 2014, 157, 1565–1576.
- Suresh, M.; Rath, P.K.; Panneerselvam, A.; Dhanasekaran, D.; Thajuddin, N. Antifungal activity of selected Indian medicinal plant salts. J. Glob. Pharm. Technol. 2010, 2, 71–74.
- Prabakar, K.; Sivalingam, P.; Mohamed Rabeek, S.I.; Muthuselvam, M.; Devarajan, N.; Arjunan, A.; Karthick, R.; Suresh, M.M.; Wembonyama, J.P. Evaluation of antibacterial efficacy of phyto fabricated silver nanoparticles using Mukia scabrella (Musumusukkai) against drug resistance nosocomial gram negative bacterial pathogens. Colloids Surf. B Biointerfaces 2013, 104, 282–288.
- Mickymaray, S.; Al Aboody, M.S.; Rath, P.K.; Annamalai, P.; Nooruddin, T. Screening and antibacterial efficacy of selected Indian medicinal plants. Asian Pac. J. Trop. Biomed. 2016, 6, 185–191.
- Moorthy, K.; Punitha, T.; Vinodhini, R.; Mickymaray, S.; Shonga, A.; Tomass, Z.; Thajuddin, N. Efficacy of different solvent extracts of Aristolochia krisagathra and Thottea ponmudiana for potential antimicrobial activity. J. Pharm. Res. 2015, 9, 35–40.
- Mickymaray, S.; Alturaiki, W. Antifungal Efficacy of Marine Macroalgae against Fungal Isolates from Bronchial Asthmatic Cases. Molecules 2018, 23, 3032.
- Kannaiyan, M.; Manuel, V.N.; Raja, V.; Thambidurai, P.; Mickymaray, S.; Nooruddin, T. Antimicrobial activity of the ethanolic and aqueous extracts of Salacia chinensis Linn. against human pathogens. Asian Pac. J. Trop. Dis. 2012, 2, S416–S420.
- Mickymaray, S. Efficacy and mechanism of traditional medicinal plants and bioactive compounds against clinically important pathogens. Antibiotics 2019, 8, 257.
- Mickymaray, S.; Al Aboody, M.S. In vitro antioxidant and bactericidal efficacy of 15 common spices: Novel therapeutics for urinary tract infections? Medicina 2019, 55, 289.
- Suresh, M.; Alfonisan, M.; Alturaiki, W.; Aboody, M.S.A.; Alfaiz, F.A.; Premanathan, M.; Vijayakumar, R.; Umamagheswari, K.; Ghamdi, S.A.; Alsagaby, S.A. Investigations of bioactivity of Acalypha indica (L.), Centella asiatica (L.) and croton bonplandianus (Baill) against multidrug resistant bacteria and cancer cells. J. Herb. Med. 2020, 100359.
- Kumar, G.; Murugesan, A.G.; Rajasekara Pandian, M. Effect of Helicteres isora bark extract on blood glucose and hepatic enzymes in experimental diabetes. Pharmazie 2006, 61, 353–355.
- Ganesan, K.; Xu, B. Ethnobotanical studies on folkloric medicinal plants in Nainamalai, Namakkal District, Tamil Nadu, India. Trends Phytochem. Res. 2017, 1, 153–168.
- Kumar, G.; Banu, G.S.; Murugesan, A.G.; Pandian, M.R. Hypoglycaemic effect of Helicteres isora bark extract in rats. J. Ethnopharmacol. 2006, 107, 304–307.
- Sinaga, M.; Ganesan, K.; Kumar Nair, S.K.P.; Gani, S.B. Preliminary phytochemical analysis and in vitro antibacterial activity of bark and seeds of Ethiopian neem (Azadirachta indica A. Juss). World J Pharm. Pharma. Sci. 2016, 5, 1714–1723.
- Zhang, T.; Jayachandran, M.; Ganesan, K.; Xu, B. Black truffle aqueous extract attenuates oxidative stress and inflammation in STZ-induced hyperglycemic rats via Nrf2 and NF-κB pathways. Front. Pharmacol. 2018, 9, 1257.
- Jayachandran, M.; Wu, Z.; Ganesan, K.; Khalid, S.; Chung, S.M.; Xu, B. Isoquercetin upregulates antioxidant genes, suppresses inflammatory cytokines and regulates AMPK pathway in streptozotocin-induced diabetic rats. Chem. Biol. Interact. 2019, 303, 62–69.
- Sukalingam, K.; Ganesan, K.; Xu, B. Trianthema portulacastrum L. (giant pigweed): Phytochemistry and pharmacological properties. Phytochem. Rev. 2017, 16, 461–478.
- Vijayakumar, R.; Sandle, T.; Al-Aboody, M.S.; AlFonaisan, M.K.; Alturaiki, W.; Mickymaray, S.; Premanathan, M.; Alsagaby, S.A. Distribution of biocide resistant genes and biocides susceptibility in multidrug-resistant Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii—A first report from the Kingdom of Saudi Arabia. J. Infect. Public Health 2018, 11, 812–816.
- Vinodhini, R.; Moorthy, K.; Suresh, M. Incidence and virulence traits of Candida dubliniensis isolated from clinically suspected patients. Asian J. Pharm. Clin. Res. 2016, 9, 77.
- Chandran, R.P.; Kumar, S.N.; Manju, S.; Kader, S.A.; Dileep Kumar, B.S. In vitro α-glucosidase inhibition, antioxidant, anticancer, and antimycobacterial properties of ethyl acetate extract of Aegle tamilnadensis Abdul Kader (Rutaceae) leaf. Appl. Biochem. Biotechnol. 2015, 175, 1247–1261.
- Vianna, J.S.; Machado, D.; Ramis, I.B.; Silva, F.P.; Bierhals, D.V.; Abril, M.A.; von Groll, A.; Ramos, D.F.; Lourenço, M.C.S.; Viveiros, M.; et al. The Contribution of Efflux Pumps in Mycobacterium abscessus Complex Resistance to Clarithromycin. Antibiotics 2019, 8, 153.
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272.
- Talevi, A. Multi-target pharmacology: Possibilities and limitations of the “skeleton key approach” from a medicinal chemist perspective. Front. Pharmacol. 2015, 6, 205.
