A wide variety of bioactive ceramic scaffolds, which encompass CaP, doped HA and CS, bioactive glasses and ceramics, and their respective composites, have been used as the constructs for replacing cancellous bone (restoration of the bone loss), shown in. Synthetic HA, which is similar to the CaP minerals within bones and teeth, is successfully applied as a bone implant with suitable mechanical strength and osteoconductivity. Bioactive scaffolds processed through PBSLP with the various modes of functionalization through the incorporation of drugs, stem cells, and growth factors facilitate bone regeneration and ameliorate critical-sized bone defects based on the fracture site length for personalized medicine. The keyhole pores, apart from the designed porosity from the CAD design, usually affect the mechanical strength of the scaffolds when fabricated through powder bed selective laser processing techniques.
- additive manufacturing
- bone tissue engineering
- ceramics
- calcium phosphate
- calcium silicate
- critical-sized defects
- drugs
- laser powder bed fusion
- scaffolds
- selective laser melting
- selective laser sintering
1. Introduction
The worldwide incidence of bone diseases and disorders requiring arthroplasty and/or other surgical intervention is persistently increasing. A particular challenge in ongoing developments for the treatments of orthopedic critical injuries originates from the need to balance design criteria and material biocompatibility with the mechanical and geometric constraints regulated by the physiological environment of the bone defect. Appropriate material designs for bone tissue engineering (BTE) require a deep understanding of the architecture, hierarchy, and composition of native bone tissue and the ability to mimic its physiochemical properties.
The bone is a natural composite of collagen type I (COL-1) and nanosized calcium-deficient carbonated hydroxyapatite crystals deposited in parallel with the collagen fibers. The hard, bony tissue at the nanolevel is a combination of strength and ductility [1], which enables energy to be absorbed prior to bone fracture. At the microscale level, an osteon is a basic unit for cortical bone. The dense and trabecular bone both comprise collagen fibrils [2], which are reinforced with hydroxyapatite (HA) particles. At the macrolevel, the bone can be contemplated as a composite of osteons and body fluids. Mimicking the features of natural tissue is a nontrivial task requiring special fabrication methods and techniques, as well as mechanically reliable and biophysically compatible materials. Fulfilling the intricate design of bioinspired scaffolds is hardly possible with traditional manufacturing methods due to the complex architecture of bone. The conventional techniques (e.g., freeze-drying, solvent casting, electrospinning, sol−gel, gas foaming, etc.) have several drawbacks in the forming and fabricating of complex shapes, such as the low mechanical properties of produced coupons, the use of toxic organic solvents, large energy utilization, the evolution of additional phases in synthetic matrices, etc. Recent progress in additive manufacturing (AM) has provided a platform to produce biomimetic components with geometric freedom [3] and the possibility of in situ development of functional structures.
The specific target of scaffold functionality in adapting peculiar designs and utilizing novel ceramic materials is diminishing orthopedic ailments, such as donor-site morbidity, bone tumor, non-unions, and clinical constraints. Numerous AM methods have recently been utilized to produce the tailored structures, i.e., L-PBF, material extrusion (ME), stereolithography (SLA), directed energy deposition (DED), binder jetting (3DP), vat polymerization, etc. [4][7].
Laser additive manufacturing (LAM) through the powder bed or L-PBF involves two techniques, i.e., (SLS and SLM) or accumulatively called PBSLP. Nowadays, PBSLP has been used as an attractive technique enabling different modes of functionalization of biomaterials through novel engineering to develop drug-delivery systems (DDS) and personalized medicine [5]. PBSLP has the unique potential to directly incorporate active biomolecules, such as drugs and other moieties, directly into the powder bed even though they are temperature sensitive or can be incorporated after the scaffold fabrication [6] and their release can be tailored by tampering with the SLM or SLS parameters [7]. PBSLP has the significant potential to print biomaterials (printlets) with several tailored engineering properties such as immediate, control, multilayered, multireservoir, and visually impaired printlets for targeting specific patient groups for personalized medicine [5].
This review’s specific topic is the LAM of bioactive ceramic scaffolds processed only through direct PBSLP using SLM or SLS. The second section provides detailed information on the PBSLP and its advantages over the other AM techniques used for the fabrication of bioactive ceramics. The third section will brief about the biological, architectural, and mechanical requirements of the bioactive ceramic scaffolds. The fourth section presents bioactive ceramic scaffolds based on CaP and CS and their respective composites processed through a direct L-PBF approach (SLS/SLM). In the last section, an effort has also been made to convey the practical aspects of PBSLP for the bioactive scaffolds (CaP and CS) as a potential technique for the various modes of functionalization through the incorporation of drugs, stem cells, and growth factors to ameliorate critical-sized bone defects based on the fracture site length for a specific clinical target. The potential developments for fabrication through PBSLP and the design of bioactive ceramics are also discussed.
2. Bioactive Scaffold Parameters for Bone Tissue Growth
Usually, porous bioactive ceramic scaffolds are considered as a potential replacement for the trabecular or cancellous region of the bone. Only a few works have been reported on the fabrication of single-phase bioactive ceramic scaffolds for long bone or load-bearing defects, which simultaneously promote bone regeneration [8][9][10][11][12][73,74,75,76,77].
In the case of BTE scaffold, the Kokubo solution has become a common necessity to study the bioactivity and biomineralization process by the formation of biomimetic HA on the surface of novel structures [13][100]. However, bioactivity and biocompatibility are reflected not only by the formation of HA on the surfaces but also by inducing molecular signaling pathways. The bioactive ceramic scaffolds possess the potential to trigger the signaling molecules. At the molecular level (involving bioactive ions released from bioceramic scaffold, growth factors, and integrins) without having any adverse effect or cytotoxicity on the host tissue [14][101].
All these aforementioned parameters are an essential prerequisite for the fabrication of a bioactive scaffold to be considered as a potential bone graft substitute. PBSLP provides freedom to fine tune the mechanical properties, biodegradability, porosity, and design of the bioactive scaffolds by carefully controlling the exposure of the laser to the bioactive ceramic powder bed.
3. Bioactive Ceramic Scaffolds Processed by PBSLP
Functionally graded CaP bioactive ceramics were studied by Salimi, E [15][132]. The fabrication of the gradient implant for the osteochondral defect is one of the most important issues among researchers. Therefore, understanding the different phases of the graded CaP bioactive ceramics with the interaction of the laser at the bone−cartilage interphase is considered very important for the tissue-specific interactions [16][133]. For that matter, the biomechanical functionality aspect co-relating with the assorted phases of the CaP bioactive ceramics is better anticipated for the subchondral bone and calcified cartilage (osteochondral interphase) [17][134].
The potential of the PBSLP of CaP bioactive ceramics lies in modulating the energy density according to the authors. The varying energy density will escort the formation of the different phases on the CaP powder feedstock to fabricate bioactive smart implants with varying bioactivity and degradability, which can open new avenues for the personalized implants specifically for the osteochondral defects in a single powder feedstock. However, there is still a gap and scope for research to fabricate CaP scaffolds with improved mechanical properties for load-bearing applications, which can essentially be fulfilled by designing triple periodic minimal surface (TPMS) bioactive ceramic scaffolds.
The pure CaSiO 3 scaffolds have been fabricated through the direct SLS method without involving any binder [18][144] and have demonstrated that with the increasing laser energy, the low temperature polymorph (β-CaSiO 3) is transformed to a higher temperature polymorph (α-CaSiO 3) at 1125 °C. Graphene was also incorporated in the direct powder bed fusion with CaSiO 3 to form graphene-CaSiO 3 composite scaffolds with enhanced mechanical properties of the bioactive scaffolds [19][145]. In this study too, the conversion of β-CaSiO 3 to α-CaSiO 3 with the impact of increasing laser energy is observed . Furthermore, the bioactivity of CS bioceramic was enhanced by incorporating Poly(3-hydroxybutyrate- co -3-hydroxyvalerate) (PHBV) into the powder bed by direct SLS process. The PHBV/CS composite scaffolds proved to accelerate the proliferation and osteogenic differentiation of stem cells by mimicking Collagen Type 1 (COL-1) when compared to the pure CaSiO 3 scaffolds, as shown in [20][146].
Diopside-based composite scaffolds reinforced with graphene nanoplatelets (GNP) with a few layers of graphene were also studied through PBSLP. The composite scaffolds exhibited enhanced bioactivity, compression strength, and fracture toughness (toughening mechanism of GNP) when studied against pure diopside scaffolds [21][147]. Similarly, akermanite-reinforced PHBV composite scaffolds were also studied [22][148]. They comprehensively studied the effect of akermanite particles (micro/nano) affecting mechanical properties, water up-taking ability on the composite akermanite/PHBV scaffolds.
4. Synthetic Bioactive Ceramic Scaffolds for Critical Size Bone Defects by PBSLP
Bone defects can be characterized into different classifications according to the position of the fracture: long bones, assorted regions of the spinal cord, maxillofacial and craniofacial. The most frequent bone fracture sites are the femur, shoulder, hip, wrist (radius, ulna), tibia, ankle (distal tibia/fibula fractures), vertebral, maxilla-, and craniofacial (jawbone, calvaria) fractures [23][156]. The fractures also depend on the size and the length at the damage sites, as shown in Figure 1 .
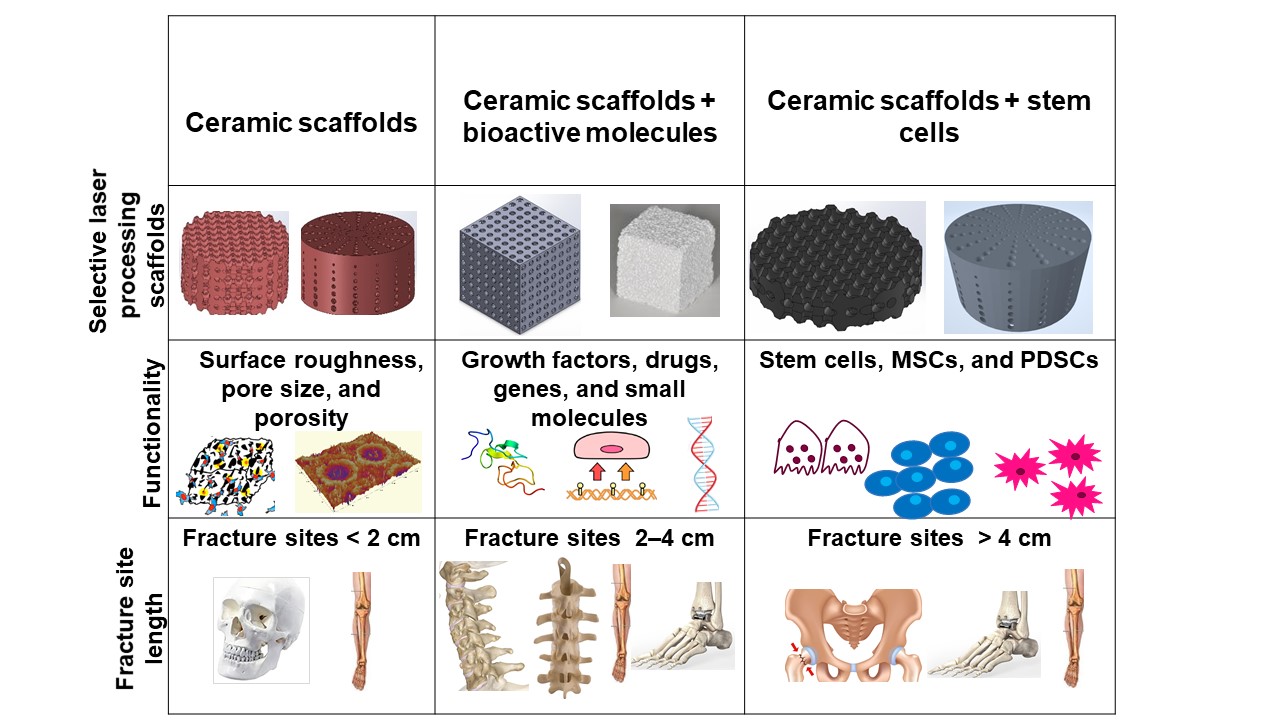
Critical-sized bone defects usually range between 1 and 4.5 cm [24][157]. A permanent, direct, and sustainable solution is a requisite to overcome the imperfections of natural bone scaffolds to heal and repair critical-sized bone defects. Synthetic bioactive ceramic scaffolds mask an indispensable role in BTE, which therefore can mimic the natural bone characteristics such as providing support similar to ECM by facilitating and providing a 3D network for regenerating the defected bone. The critical-sized defects of bone can be refurbished by bioactive scaffolds through the assorted added functionalities of 3D printing, such as surface roughness, pore design, porosity, and infusion of cell-based targeted functionality through the scaffold. Scaffolds are engineered to heal bone depending on the severity of the trauma. The three main divisions or strategies for bone repair using ceramic elements involving PBSLP in Figure 1 encompass: (i) 3D synthetic bioactive scaffolds, (ii) synthetic bioactive scaffolds combined with active moieties; and (iii) synthetic bioactive scaffolds in combination with cell-based products.
In BTE, osteoconductive synthetic bioactive ceramic scaffolds alone sometimes cannot cause rapid healing and rejuvenation of the lost bone tissue due to the paucity of active biomolecules, which cannot promote cell differentiation and proliferation. Growth factors, genes, and stem cell therapy are vital quintessence for instantaneous bone healing, morphogenesis, and tissue rejuvenation since they are osteoinductive. Bioactive scaffolds manufactured by PBSLP can also be integrated with active biomolecules to achieve active biomolecule delivery with the required loading and efficacy to the defective bone (clinical target), as shown in column II and III of Figure 1.
The fabrication of [25][169] customized apatite-wollastonite glass-ceramic scaffolds by SLS process by recruiting stem cells taken from the femoral head has been reported for its osteosupportive capacity. The scaffolds comprising the polymer and bioactive ceramic component can further enhance osteogenesis since scaffolds mimic both the ceramic and organic components of the bone. The ASCs were incorporated [26][170] onto the polycarpolactone-TCP-collagen scaffolds with interporous connections by the SLS process. These bioactive composite scaffolds have been reported to enhance the proliferation and osteogenic differentiation of ASCs cells, both in vitro and in vivo environments. Additionally, cells seeded onto the scaffolds (platform) can be genetically engineered ex vivo for the continuous and/or increased secretion of the growth factors by a variety of DDS [27][28][171,172]. Thus, the unification of bone tissue engineering and synthetic bioactive ceramic bone scaffolds using PBSLP has become a synergistic approach, nowadays, for repairing critical-sized bone defects which depend on the loading of active biomolecules. Furthermore, 3D-customized bioactive ceramic scaffolds are a current trend, as they are biocompatible, osteoconductive, and can precisely fit into a defect in a patient’s cranial, maxillofacial, femur, and tibia bones for critical-sized defects.
