The scope of the present entry was to highlight possible sources of bias that could be encountered when evaluating HRQOL (Health-Related Quality of Life) in patients treated for oral cancer. The second aim was to lay the foundation of a standardized protocol for cohort selection, data collection, and stratification that could enhance knowledge in the field.
- quality of life
- HRQOL
- head
- neck
- oral cancer
- oncology
1. Introduction
Patient-reported outcomes (PROs) provide precious information about troubles in everyday life and the perception of psychological and physical wellness from the patient’s perspective. Over recent decades, PROs have gained more relevance in treatment decision making, so much so that the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) consider them — including the quality of life — as a relevant end point to approve new therapies [1,2,3][1][2][3]. To approach such a complex topic as the quality of life in oncological patients, they commonly refer to health-related quality of life (HRQOL). A distinction between these concepts has been made to exclude influences from domains that are not related to the patient’s health status [4], at least theoretically.
The concept of “quality of life” was firstly introduced by Heckscher [5], and in 1977 was adopted as a “keyword” by the United States National Library of Medicine [6]. Since then, several definitions have been proposed [7,8][7][8]. The WHO defined quality of life as “individuals perceptions of their position in context of the culture and value systems in which they live and in relation to their goal, expectations, standards, and concerns” [9].
Head and neck tumors and their treatment may negatively affect patients’ HRQOL, which is considered an essential secondary outcome of treatment nowadays [10,11][10][11]. For this reason, having reliable evaluation tests is mandatory to better understand how and why specific medical interventions should be chosen and adapted according to individual needs. The quest towards the perfect quality of life evaluation test led researchers to understand some key points to be focused on: a test should be reproducible, sensitive, and easy to understand [12]. Questionnaires developed by the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Group are widely used in current literature to address these needs. A core questionnaire (EORTC QLQ-C30) is associated with site-specific validated modules (EORTC QLQ-H&N35/43), consisting of single- and multi-item scales that measure several head and neck symptoms [13,14][13][14].
HRQOL is a complex topic and needs to be analyzed taking into account every potential influencing factor. Various sociodemographic, disease-specific, and treatment-specific aspects have been recognized as affecting HRQOL [12,15,16,17,18,19,20][12][15][16][17][18][19][20]. Several researchers have investigated its intrinsic multidimensionality, concluding that HRQOL plays a role in treatment decision making, but none have verified what the relevant items are and how this feature is assessed. The scope of the present review was to highlight possible sources of bias that could be encountered when evaluating HRQOL in patients treated for oral cancer. The second aim was to lay the foundation of a standardized protocol for cohort selection, data collection, and stratification that could enhance knowledge in the field.
2. Disease- and Treatment-Specific Variables
2.1. Cancer Site
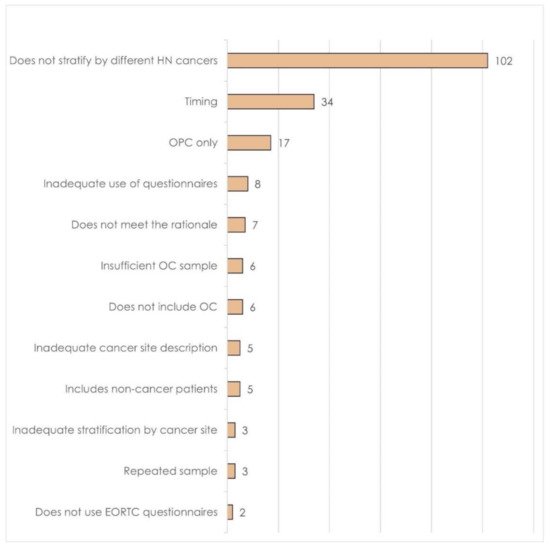
2.2. Cancer Stage
2.3. Mandibular Resection
2.4. Extent of Resection
2.5. Surgical Approach
2.6. Neck Dissection
2.7. Reconstruction
2.8. Radiotherapy and Chemotherapy
2.9. Synchronous Lesions, Recurrences, and Metachronous Lesions
2.10. Major Surgical Complications and Secondary Surgery
3. Conclusions and Recommendations for Future Studies
References
- European Medicines Agency. Pre-Authorisation Evaluation of Medicines for Human Use. Doc. Ref. EMEA/CHMP/EWP/139391/2004. London. 2005. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-regulatory-guidance-use-healthrelated-quality-life-hrql-measures-evaluation_en.pdf (accessed on 28 August 2021).
- Apolone, G.; De Carli, G.; Brunetti, M.; Garattini, S. Health-related quality of life (HR-QOL) and regulatory issues. An assessment of the European Agency for the Evaluation of Medicinal Products (EMEA) recommendations on the use of HR-QOL measures in drug approval. PharmacoEconomics 2001, 19, 187–195.
- Marquis, P.; Caron, M.; Emery, M.-P.; Scott, J.A.; Arnould, B.; Acquadro, C. The Role of Health-Related Quality of Life Data in the Drug Approval Processes in the US and Europe. Pharm. Med. 2011, 25, 147–160.
- Ferrans, C.E.P. Definitions and Conceptual Models of Quality of Life. Outcomes Assessment in Cancer: Measures, Methods, and Applications; Cambridge University Press (CUP): New York, NY, USA, 2005; pp. 14–30.
- Heckscher, A.L. The quality of American culture. In Goals for Americans: (The Report of the Presidential Commission on National Goals and Chapters Submitted for the Consideration of the Commission of the American Assembly); Prentice-Hall Washington: Washington, DC, USA, 1960; pp. 127–146.
- Morton, R.P. Evolution of quality of life assessment in head and neck cancer. J. Laryngol. Otol. 1995, 109, 1029–1035.
- Dietz, A.; Meyer, A.; Singer, S. Lebensqualitätsmessungen bei Patienten mit Kopf-Hals-Malignomen. HNO 2009, 57, 857–865.
- Shumaker, S.A.; Ellis, S.; Naughton, M. Assessing health-related quality of life in HIV disease: Key measurement issues. Qual. Life Res. 1997, 6, 475–480.
- Khandelwal, A.; Neeli, A.; Gadiyar, A.; Khandelwal, A. Assessment of quality of life of patients 1–5 years after treatment for oral cancer. Indian J. Dent. Res. 2017, 28, 538.
- Crombie, A.K.; Farah, C.S.; Batstone, M.D. Health-related quality of life of patients treated with primary chemoradiotherapy for oral cavity squamous cell carcinoma: A comparison with surgery. Br. J. Oral Maxillofac. Surg. 2014, 52, 111–117.
- Smith, S.C.; Lamping, D.L.; Banerjee, S.; Harwood, R.H.; Foley, B.; Smith, P.; Cook, J.C.; Murray, J.; Prince, M.; Levin, E.; et al. Development of a new measure of health-related quality of life for people with dementia: DEMQOL. Psychol. Med. 2006, 37, 737–746.
- Torres-Carranza, E.; Infante-Cossío, P.; Hernández-Guisado, J.M.; Hens-Aumente, E.; Gutierrez-Pérez, J.L. Assessment of quality of life in oral cancer. Med. Oral Patol. Oral Cir. Bucal 2008, 13, E735–E741.
- Bjordal, K.; de Graeff, A.; Fayers, P.; Hammerlid, E.; van Pottelsberghe, C.; Curran, D.; Ahlner-Elmqvist, M.; Maher, E.; Meyza, J.; Brédart, A.; et al. A 12 country field study of the EORTC QLQ-C30 (version 3.0) and the head and neck cancer specific module (EORTC QLQ-H&N35) in head and neck patients. Eur. J. Cancer 2000, 36, 1796–1807.
- Singer, S.; Amdal, C.D.; Hammerlid, E.; Tomaszewska, I.M.; Silva, J.C.; Mehanna, H.; Santos, M.; Inhestern, J.; Brannan, C.; Yarom, N.; et al. International validation of the revised European Organisation for Research and Treatment of Cancer Head and Neck Cancer Module, the EORTC QLQ-HN43: Phase IV. Head Neck 2019, 41, 1725–1737.
- Quinten, C.; Coens, C.; Ghislain, I.; Zikos, E.; Sprangers, M.A.; Ringash, J.; Martinelli, F.; Ediebah, D.E.; Maringwa, J.; Reeve, B.B.; et al. The effects of age on health-related quality of life in cancer populations: A pooled analysis of randomized controlled trials using the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 involving 6024 cancer patients. Eur. J. Cancer 2015, 51, 2808–2819.
- Chandu, A.; Smith, A.C.; Rogers, S.N. Health-Related Quality of Life in Oral Cancer: A Review. J. Oral Maxillofac. Surg. 2006, 64, 495–502.
- Murphy, B.A.; Ridner, S.; Wells, N.; Dietrich, M. Quality of life research in head and neck cancer: A review of the current state of the science. Crit. Rev. Oncol. 2007, 62, 251–267.
- Samuel, S.R.; Maiya, A.G.; Fernandes, D.J.; Guddattu, V.; Saxena, P.P.; Kurian, J.R.; Lin, P.-J.; Mustian, K.M. Effectiveness of exercise-based rehabilitation on functional capacity and quality of life in head and neck cancer patients receiving chemo-radiotherapy. Support. Care Cancer 2019, 27, 3913–3920.
- So, W.; Chan, R.; Chan, D.; Hughes, B.; Chair, S.; Choi, K.; Chan, C. Quality-of-life among head and neck cancer survivors at one year after treatment—A systematic review. Eur. J. Cancer 2012, 48, 2391–2408.
- Talmi, Y.P. Quality of life issues in cancer of the oral cavity. J. Laryngol. Otol. 2002, 116, 785–790.
- Infante-Cossio, P.; Torres-Carranza, E.; Cayuela, A.; Hens-Aumente, E.; Pastor-Gaitan, P.; Gutiérrez-Pérez, J. Impact of treatment on quality of life for oral and oropharyngeal carcinoma. Int. J. Oral Maxillofac. Surg. 2009, 38, 1052–1058.
- Kovács, A.; Stefenelli, U.; Thorn, G. Long-term quality of life after intensified multi-modality treatment of oral cancer including intra-arterial induction chemotherapy and adjuvant chemoradiation. Ann. Maxillofac. Surg. 2015, 5, 26–31.
- Oates, J.; Clark, J.R.; Read, J.; Reeves, N.; Gao, K.; O’Brien, C.J. Integration of prospective quality of life and nutritional assessment as routine components of multidisciplinary care of patients with head and neck cancer. ANZ J. Surg. 2008, 78, 34–41.
- Pierre, C.S.; Dassonville, O.; Chamorey, E.; Poissonnet, G.; Ettaiche, M.; Santini, J.; Peyrade, F.; Benezery, K.; Sudaka, A.; Bozec, A. Long-term quality of life and its predictive factors after oncologic surgery and microvascular reconstruction in patients with oral or oropharyngeal cancer. Eur. Arch. Oto-Rhino-Laryngol. 2013, 271, 801–807.
- Bozec, A.; Poissonnet, G.; Chamorey, E.; Casanova, C.; Vallicioni, J.; Demard, F.; Mahdyoun, P.; Peyrade, F.; Follana, P.; Bensadoun, R.-J.; et al. Free-Flap Head and Neck Reconstruction and Quality of Life: A 2-Year Prospective Study. Laryngoscope 2008, 118, 874–880.
- Leeuw, I.M.V.-D.; Buffart, L.M.; Heymans, M.; Rietveld, D.H.; Doornaert, P.; de Bree, R.; Buter, J.; Aaronson, N.K.; Slotman, B.J.; Leemans, C.R.; et al. The course of health-related quality of life in head and neck cancer patients treated with chemoradiation: A prospective cohort study. Radiother. Oncol. 2014, 110, 422–428.
- van Gemert, J.; Holtslag, I.; van der Bilt, A.; Merkx, M.A.; Koole, R.; Van Cann, E. Health-related quality of life after segmental resection of the lateral mandible: Free fibula flap versus plate reconstruction. J. Cranio-Maxillofac. Surg. 2015, 43, 658–662.
- Canis, M.; Weiss, B.G.; Ihler, F.; Hummers-Pradier, E.; Matthias, C.; Wolff, H.A. Quality of life in patients after resection of pT3 lateral tongue carcinoma: Microvascular reconstruction versus primary closure. Head Neck 2014, 38, 89–94.
- Ferri, A.; Perlangeli, G.; Montalto, N.; Lizarazo, J.L.C.; Bianchi, B.; Ferrari, S.; Nicolai, P.; Sesenna, E.; Grammatica, A. Transoral resection with buccinator flap reconstruction vs. pull-through resection and free flap reconstruction for the management of T1/T2 cancer of the tongue and floor of the mouth. J. Cranio-Maxillofac. Surg. 2020, 48, 514–520.
- Lin, N.-C.; Lin, S.-L.; Tsai, K.-Y. Barrel-shaped design of the forearm free flap for lower lip reconstruction: A pilot case-control study. BMC Surg. 2020, 20, 132.
- Mair, M.D.; Nair, S.; Nikam, S.; Nair, D.; Agarwal, J.P.; Chaturvedi, P. Longitudinal and cross-sectional assessment of quality of life in surgically treated advanced (T4) cancer of the buccal mucosa. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 124, 529–536.
- Petruson, K.; Mercke, C.; Lundberg, L.-M.; Silander, E.; Hammerlid, E. Longitudinal evaluation of patients with cancer in the oral tongue, tonsils, or base of tongue—Does interstitial radiation dose affect quality of life? Brachytherapy 2005, 4, 271–277.
- Beck-Broichsitter, B.E.; Huck, J.; Küchler, T.; Hauke, D.; Hedderich, J.; Wiltfang, J.; Becker, S.T. Self-perception versus professional assessment of functional outcome after ablative surgery in patients with oral cancer. J. Cancer Res. Clin. Oncol. 2016, 143, 305–311.
- Becker, S.T.; Menzebach, M.; Küchler, T.; Hertrampf, K.; Wenz, H.-J.; Wiltfang, J. Quality of life in oral cancer patients—Effects of mandible resection and socio-cultural aspects. J. Cranio-Maxillofac. Surg. 2012, 40, 24–27.
- Borggreven, P.A.; Aaronson, N.K.; Leeuw, I.M.V.-D.; Muller, M.J.; Heiligers, M.L.; de Bree, R.; Langendijk, J.A.; Leemans, C.R. Quality of life after surgical treatment for oral and oropharyngeal cancer: A prospective longitudinal assessment of patients reconstructed by a microvascular flap. Oral Oncol. 2007, 43, 1034–1042.
- Bozec, A.; Majoufre, C.; De Boutray, M.; Gal, J.; Chamorey, E.; Roussel, L.-M.; Philouze, P.; Testelin, S.; Coninckx, M.; Bach, C.; et al. Oral and oropharyngeal cancer surgery with free-flap reconstruction in the elderly: Factors associated with long-term quality of life, patient needs and concerns. A GETTEC cross-sectional study. Surg. Oncol. 2020, 35, 81–88.
- Klug, C.; Neuburg, J.; Gläser, C.; Schwarz, B.; Kermer, C.; Millesi, W. Quality of life 2–10 years after combined treatment for advanced oral and oropharyngeal cancer. Int. J. Oral Maxillofac. Surg. 2002, 31, 664–669.
- Yoshimura, R.-I.; Shibuya, H.; Miura, M.; Watanabe, H.; Ayukawa, F.; Hayashi, K.; Toda, K. Quality of Life of Oral Cancer Patients After Low-Dose-Rate Interstitial Brachytherapy. Int. J. Radiat. Oncol. 2009, 73, 772–778.
- Huang, T.-L.; Tsai, W.-L.; Chien, C.-Y.; Lee, T.-F.; Fang, F.-M. Quality of life for head and neck cancer patients treated by combined modality therapy: The therapeutic benefit of technological advances in radiotherapy. Qual. Life Res. 2010, 19, 1243–1254.
- Schliephake, H.; Neukam, F.W.; Schmelzeisen, R.; Varoga, B.; Schneller, H. Long-term quality of life after ablative intraoral tumour surgery. J. Cranio-Maxillofac. Surg. 1995, 23, 243–249.
- Hartl, D.M.; Dauchy, S.; Escande, C.; Bretagne, E.; Janot, F.; Kolb, F. Quality of life after free-flap tongue reconstruction. J. Laryngol. Otol. 2008, 123, 550–554.
- Zhang, P.-P.; Meng, L.; Shen, J.; Liu, H.; Zhang, J.; Xiang, X.; Yan, Y.-B. Free radial forearm flap and anterolateral thigh flap for reconstruction of hemiglossectomy defects: A comparison of quality of life. J. Cranio-Maxillofac. Surg. 2018, 46, 2157–2163.
- Goh, H.K.C.; Ng, Y.H.; Teo, D.T.W. Minimally invasive surgery for head and neck cancer. Lancet Oncol. 2010, 11, 281–286.
- Hartl, D.M.; Ferlito, A.; Silver, C.E.; Takes, R.P.; Stoeckli, S.J.; Suárez, C.; Rodrigo, J.P.; Sesterhenn, A.M.; Snyderman, C.H.; Terris, D.J.; et al. Minimally invasive techniques for head and neck malignancies: Current indications, outcomes and future directions. Eur. Arch. Oto-Rhino-Laryngol. 2011, 268, 1249–1257.
- Rauso, R.; Colella, G.; Franco, R.; Ronchi, A.; Chirico, F. Ossified Carcinoma Ex Pleomorphic Adenoma in accessory lobe of parotid gland: Complexity in clinical, imaging and histologic diagnosis and minimally invasive surgery. Oral Oncol. 2019, 92, 95–98.
- Hsu, D.; Sayan, A.; Ramchandani, P.; Ilankovan, V. Minimally-invasive neck dissection and free flap reconstruction in patients with cancer of the head and neck. Br. J. Oral Maxillofac. Surg. 2016, 55, 46–49.
- Madhura, M.G.; Rao, R.; Patil, S.; Alhazmi, Y.A.; Jafer, M.; Habib, S.R.; Awan, K.H. Minimally invasive procedures for the recognition and diagnosis of oral precancer and cancer. Disease-a-Month 2020, 66, 101033.
- Gilbert, R.W. Reconstruction of the oral cavity; past, present and future. Oral Oncol. 2020, 108, 104683.
- Lam, L.; Samman, N. Speech and swallowing following tongue cancer surgery and free flap reconstruction—A systematic review. Oral Oncol. 2013, 49, 507–524.
- Saint-Cyr, M.; Wong, C.; Schaverien, M.; Mojallal, A.; Rohrich, R.J. The Perforasome Theory: Vascular Anatomy and Clinical Implications. Plast. Reconstr. Surg. 2009, 124, 1529–1544.
- Colella, G.; Rauso, R.; De Cicco, D.; Boschetti, C.E.; Iorio, B.; Spuntarelli, C.; Franco, R.; Tartaro, G. Clinical management of squamous cell carcinoma of the tongue: Patients not eligible for free flaps, a systematic review of the literature. Expert Rev. Anticancer. Ther. 2021, 21, 9–22.
- Rauso, R.; Chirico, F.; Federico, F.; Nicoletti, G.F.; Colella, G.; Fragola, R.; Pafundi, P.C.; Tartaro, G. Maxillo-facial reconstruction following cancer ablation during COVID-19 pandemic in southern Italy. Oral Oncol. 2021, 115, 105114.
- Joseph, S.; Naveen, B.S.; Mohan, M.T.; Tharayil, J. Comparison of islanded facial artery myomucosal flap with fasciocutaneous free flaps in the reconstruction of lateral oral tongue defects. Int. J. Oral Maxillofac. Surg. 2020, 49, 1000–1006.
- Gao, R.W.; Nuyen, B.A.; Divi, V.; Sirjani, D.; Rosenthal, E.L. Outcomes in Head and Neck Resections That Require Multiple-Flap Reconstructions. JAMA Otolaryngol. Neck Surg. 2018, 144, 746–752.
- Sukato, D.C.; Timashpolsky, A.; Ferzli, G.; Rosenfeld, R.M.; Gordin, E.A. Systematic Review of Supraclavicular Artery Island Flap vs Free Flap in Head and Neck Reconstruction. Otolaryngol. Neck Surg. 2018, 160, 215–222.
- Rauso, R.; Tartaro, G.; Califano, L.; Rugge, L.; Chirico, F.; Colella, G. Pedicled palatal flap for surgical repair of oro-nasal fistula. J. Biol. Regul. Homeost. Agents 2018, 32, 1565–1567.
- Halama, D.; Dreilich, R.; Lethaus, B.; Bartella, A.; Pausch, N.C. Donor-site morbidity after harvesting of radial forearm free flaps—Comparison of vacuum-assisted closure with conventional wound care: A randomized controlled trial. J. Cranio-Maxillofac. Surg. 2019, 47, 1980–1985.
- Hu, S.; Fan, C.; Bs, B.P.; Rosenberg, J.D. Submental island flap vs free tissue transfer in oral cavity reconstruction: Systematic review and meta-analysis. Head Neck 2020, 42, 2155–2164.
- Ling, X.F.; Peng, X. What Is the Price to Pay for a Free Fibula Flap? A Systematic Review of Donor-Site Morbidity following Free Fibula Flap Surgery. Plast. Reconstr. Surg. 2012, 129, 657–674.
- Lakhiani, C.; DeFazio, M.V.; Han, K.; Falola, R.; Evans, K. Donor-Site Morbidity Following Free Tissue Harvest from the Thigh: A Systematic Review and Pooled Analysis of Complications. J. Reconstr. Microsurg. 2016, 32, 342–357.
- Ettyreddy, A.R.; Chen, C.L.; Zenga, J.; Simon, L.; Pipkorn, P. Complications and Outcomes of Chimeric Free Flaps: A Systematic Review. Otolaryngol. Neck Surg. 2019, 161, 568–575.
- Rauso, R.; Nicoletti, G.F.; Sesenna, E.; Faro, C.L.; Chirico, F.; Fragola, R.; Giudice, G.L.; Tartaro, G. Superficial Temporal Artery Perforator Flap: Indications, Surgical Outcomes, and Donor Site Morbidity. Dent. J. 2020, 8, 117.
- Chambers, M.S.; Garden, A.S.; Kies, M.S.; Martin, J.W. Radiation-induced Xerostomia in patients with head and neck cancer: Pathogenesis, impact on quality of life, and management. Head Neck 2004, 26, 796–807.
- De Felice, F.; Pranno, N.; Papi, P.; Brugnoletti, O.; Tombolini, V.; Polimeni, A. Xerostomia and Clinical Outcomes in Definitive Intensity Modulated Radiotherapy (IMRT) Versus Three-dimensional Conformal Radiotherapy (3D-CRT) for Head and Neck Squamous Cell Carcinoma: A Meta-analysis. In Vivo 2020, 34, 623–629.
- Jensen, S.B.; Pedersen, A.M.L.; Vissink, A.; Andersen, E.; Brown, C.G.; Davies, A.N.; Dutilh, J.; Fulton, J.S.; Jankovic, L.; Lopes, N.N.F.; et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: Management strategies and economic impact. Support. Care Cancer 2010, 18, 1061–1079.
- Marta, G.N.; Silva, V.; De Andrade Carvalho, H.; de Arruda, F.F.; Hanna, S.A.; Gadia, R.; da Silva, J.L.F.; Correa, S.F.M.; Vita Abreu, C.E.C.; Riera, R. Intensity-modulated radiation therapy for head and neck cancer: Systematic review and meta-analysis. Radiother. Oncol. 2014, 110, 9–15.
- O’Sullivan, B.; Rumble, R.; Warde, P. Intensity-modulated Radiotherapy in the Treatment of Head and Neck Cancer. Clin. Oncol. 2012, 24, 474–487.
- Rademaker, A.W.; Vonesh, E.F.; Logemann, J.A.; Pauloski, B.R.; Liu, D.; Lazarus, C.L.; Newman, L.A.; Ma, A.H.M.; Ms, E.M.; Gaziano, J.; et al. Eating ability in head and neck cancer patients after treatment with chemoradiation: A 12-month follow-up study accounting for dropout. Head Neck 2003, 25, 1034–1041.
- Kotz, T.; Costello, R.; Li, Y.; Posner, M.R. Swallowing dysfunction after chemoradiation for advanced squamous cell carcinoma of the head and neck. Head Neck 2004, 26, 365–372.
- Klein, J.; Livergant, J.; Ringash, J. Health related quality of life in head and neck cancer treated with radiation therapy with or without chemotherapy: A systematic review. Oral Oncol. 2014, 50, 254–262.
- Bian, X.; Song, T.; Wu, S. Outcomes of xerostomia-related quality of life for nasopharyngeal carcinoma treated by IMRT: Based on the EORTC QLQ-C30 and H&N35 questionnaires. Expert Rev. Anticancer. Ther. 2014, 15, 109–119.
- Arpino, L.; Iavarone, A.; Parlato, C.; Moraci, A. Prognostic role of depression after lumbar disc surgery. Neurol. Sci. 2004, 25, 145–147.
- Mathews, A.; Ridgeway, V. Personality and surgical recovery: A review. Br. J. Clin. Psychol. 1981, 20, 243–260.
- Powell, R.; Scott, N.; Manyande, A.; Bruce, J.; Vögele, C.; Byrne-Davis, L.M.T.; Unsworth, M.; Osmer, C.; Johnston, M. Psychological preparation and postoperative outcomes for adults undergoing surgery under general anaesthesia. Cochrane Database Syst. Rev. 2016, 5, CD008646.
- Bozec, A.; Poissonnet, G.; Chamorey, E.; Casanova, C.; Vallicioni, J.; Demard, F.; Peyrade, F.; Follana, P.; Bensadoun, R.J.; Benezery, K.; et al. Quality of life after oral and oropharyngeal recon-struction with a radial forearm free flap: Prospective study. J. Otolaryngol. Head Neck Surg. = Le J. D’oto-Rhino-Laryngol. Chir. Cervico-Faciale 2009, 38, 401–408.
- Kessler, P.A.; Bloch-Birkholz, A.; Leher, A.; Neukam, F.W.; Wiltfang, J. Evaluation of quality of life of patients with oral squamous cell carcinoma. Comparison of two treatment protocols in a prospective study. Radiother. Oncol. 2004, 70, 275–282.
- Girod, D.A.; Sykes, K.; Jorgensen, J.; Tawfik, O.; Tsue, T. Acellular dermis compared to skin grafts in oral cavity reconstruction. Laryngoscope 2009, 119, 2141–2149.
- Tribius, S.; Meyer, M.S.; Pflug, C.; Hanken, H.; Busch, C.-J.; Krüll, A.; Petersen, C.; Bergelt, C. Socioeconomic status and quality of life in patients with locally advanced head and neck cancer. Strahlenther. Onkol. 2018, 194, 737–749.
- Winkler, J.; Stolzenberg, H. Social class index in the Federal Health Survey. Gesundh. (Bundesverb. Arzte Offentlichen Gesundh. (Ger.)) 1999, 61, S178–S183.
- Peisker, A.; Raschke, G.F.; Guentsch, A.; Roshanghias, K.; Eichmann, F.; Schultze-Mosgau, S. Longterm quality of life after oncologic surgery and microvascular free flap reconstruction in patients with oral squamous cell carcinoma. Medicina Oral Patologia Oral y Cirugia Bucal 2016, 21, e420–e424.
