MicroRNAs (miRNAs) are a class of small non-coding RNAs that post-transcriptionally regulate gene expression by translational repression or messenger RNA (mRNA) degradation. MiRNAs can, either directly or via other effectors, modulate the remodeling process after MI. The balance between pro-fibrotic miRNAs and anti-fibrotic miRNAs seems to be the key to repairing the injured myocardium.
- miRNA
- myocardial infarction
- heart failure
- left ventricular remodeling
- biomarker
- prognostic
1. Introduction
Coronary artery disease (CAD) is the leading cause of death for both women and men in developed as well as in developing countries, acute myocardial infarction (MI) being responsible for most of the CAD mortality [1]. Over the past two decades, MI mortality and morbidity have significantly decreased due to extensive prevention programs and effective revascularization procedures. Furthermore, novel drug therapies and standard of care protocols have been implemented to avoid further complications such as life-threatening arrhythmias and heart failure [2].
Post-MI heart failure is the result of left ventricular remodeling (LVR). LVR refers to the ventricular shape, size, and function changes consequent to cardiomyocyte apoptosis and hypertrophy [3]. Ultimately, fibrosis leads to impairment of the left ventricle’s systolic and diastolic functions [4], underlining the need for LVR early predictive biomarkers and preventive therapies.
Currently, several circulating biomarkers could predict with decent accuracy post-MI cardiovascular events, of which, N-terminal pro-brain natriuretic peptide (NT-pro-BNP) and cardiac troponin (cTn) are the most promising [5]. However, their use is limited, primarily due to their fluctuating levels in the blood and the influence of other comorbidities, such as renal or hepatic failure [6].
MicroRNAs (miRNAs) are a class of small non-coding RNAs that post-transcriptionally regulate gene expression by translational repression or messenger RNA (mRNA) degradation [7]. MiRNAs can, either directly or via other effectors, modulate the remodeling process after MI. The balance between pro-fibrotic miRNAs and anti-fibrotic miRNAs seems to be the key to repairing the injured myocardium. In this review, we highlight the most relevant miRNAs involved in post-MI LVR.
2. miRNAs and Post-MI Remodeling
LVR is initiated in the acute phase of MI when macrophages release factors that stimulate fibroblasts. Most frequently, this factor is transforming growth factor-beta (TGF-β), secreted as a latent complex, unable to associate with its receptors. TGF-β activation involves cleavage of the C-terminal and N-terminal pro-domains by extracellular proteases, such as furin, plasmin, or Matrix Metalloproteinases (MMP)-2 and MMP-9 [8][9]. Other cellular pro-fibrotic mediators include connective tissue growth factor (CTGF), platelet-derived growth factor (PDGF), tumor necrosis factor alfa (TNF-α), interleukin 10 (IL-10,) and Angiotensin II ( AngII ) [9][10].
Besides the Smad canonical pathway, TGF-β activates many non-canonical signaling pathways, such as ERK (extracellular signal-regulated kinase), MAPK (mitogen-activated protein kinase), AKT (protein kinase B), and NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) pathways. Ultimately, this leads to proliferation, differentiation, and the activation of fibroblasts, which deposit ECM components in the surrounding tissue [10][11][11,12].
MicroRNA modulation of collagen production through canonical/Smad and non-canonical TGF-β pathway is instrumental for understanding post-MI fibrosis. Some miRNAs (miR-7, miR-29, miR-34a, miR-92, miR-125b, miR-133a, miR-208, miR-210, miR-370, and miR-1254) target only the canonical/Smad pathway, while others (miR-19a/b, miR-150, miR-199b, miR-223, let-7) target non-Smad pathways at multiple levels ( Figure 1 and Figure 2 ).
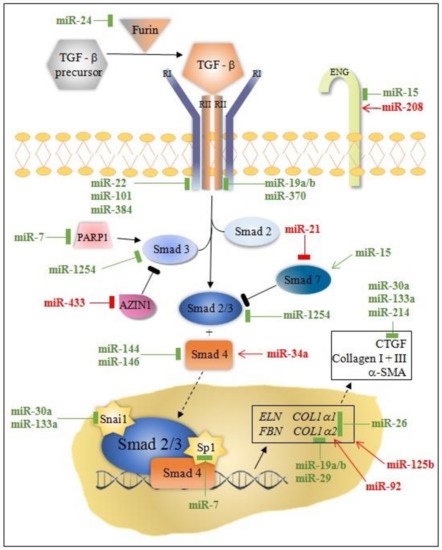
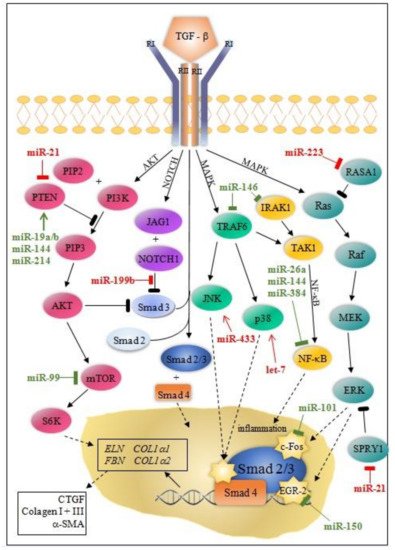
MiRNAs can either directly or indirectly modulate collagen synthesis and other molecules involved in the progression of cardiac fibrosis. Most of the miRNAs involved in post-MI LVR modulate the TGFβ signaling pathway through fibroblast activation, while others (e.g., miR-7, miR-146, miR-199, miR-214) are linked to fibrosis through cardiomyocytes’ apoptosis. Interestingly, the activity of these miRNAs is cell-specific: miR-15, miR-133a, miR-146, miR-210, miR-370 in cardiomyocytes and fibroblasts; miR-21, miR-26a in endothelial cells, miR-155 in macrophages; and, exceptionally, miR-19a/19b in cardiomyocytes, fibroblasts, and macrophages.
3. miRNAs as Prognostic Biomarkers of Post-MI LVR
Gao and colleagues found a positive association between miR-21 serum levels and the neutrophil-lymphocyte ratio in 184 patients with MI [12][92]. The 30-day survival rate of patients with high miR-21 serum levels and the elevated neutrophil-lymphocyte ratio was significantly lower than patients with low miR-21 expression and low neutrophil-lymphocyte ratio, suggesting that miR-21 can be used as an independent predictor for survival post-MI.
In 2014, Pin et al. assessed the prognostic significance of miR-34a and miR-208b levels in blood sampled in admission on a large cohort (n = 359) of MI patients [13][95]. Echocardiography was performed after admission (day 1–4) and at the 6-month follow-up. LVR parameters were measured, and an increase of more than 10% in LVEDV defined the cardiac remodeling group ( n = 116). Both miRNAs showed higher blood levels in the LVR group compared with the non-LVR group. The primary endpoint consisting of cardiovascular death or heart failure (clinical symptoms, LVEF < 40% and high NT-proBNP levels) was noted in 83 patients and associated with significantly increased miR-208b and miR-34a levels. Furthermore, the combination of these two plasma miRNAs outperformed NT-proBNP in both LVR and endpoint prediction after MI.
Later that year, the same authors published data evaluating a signature combination of four miRNAs (miR-16/27a/101/150) to predict post-MI LVR [14][90]. One hundred fifty patients with MI were enrolled in the study, blood samples were obtained at discharge (to determine NT-proBNP plasma levels and the four miRNAs), but this time the wall motion index score as an indicator for LV systolic dysfunction was applied. They built two multi-variable models to assess LV contractility: one model included clinical variables, including NT-proBNP, and the second model included all the parameters of model 1 and the four miRNAs. Patients with anterior MI or a history of MI and elevated NT-proBNP were at high risk of impaired LV contractility. Patients with low levels of miR-150/101 or elevated levels of miR-16/27a were also at increased risk of poor LV contractility. Statistical analysis confirmed that the panel of four miRNAs improved the prognostic value of the multi-variable clinical model, including NT-proBNP.
In 2017, Liu and colleagues proved that miR-208 could serve as a predictor for post-MI LVR [15][98]. The study cohort consisted of 100 MI patients (of which 59 underwent PCI), 80 patients with unstable angina, and 80 healthy controls. Venous blood samples were collected on admission and after PCI. Echocardiographic assessment of cardiac function (LVEDV, LVESV, and LVEF) was completed on admission and at the 6-month follow-up. The LVR group was defined as at least a 10% increase in LVEDV from baseline ( n = 14). Major clinical cardiovascular events (MACE) were evaluated in all MI patients who underwent PCI ( n = 20). Circulating miR-208b levels were significantly higher in MI patients than in unstable angina patients and healthy controls. Additionally, miR-208b level was increased in three-vessel coronary artery disease (CAD) patients compared to single- and two-vessel CAD. Last but not least, miR-208b blood levels were higher in the LVR and MACE groups. Interestingly, the plasma level of miR-208b was significantly decreased after PCI compared to levels before PCI.
4. miRNA as Therapeutic Targets
Chen et al. injected intramyocardially miR-133-conditioned mesenchymal stem cells and reported a reduction of apoptosis and the infarct size and a significant increase in cardiac function in a rat model of acute myocardial infarction an effect presumably mediated by the local release of miR-133 [16][106].
The administration of antimiR-21 by intracoronary perfusion in pigs subjected to transient percutaneous occlusion of the left coronary artery suppressed pathological post-MI fibrosis and the inflammatory response in macrophages [17][107]. Interestingly, the use of anti-miR-21-coated stents leads to an efficient reduction of in-stent restenosis, with no significant off-target side effects [18][19][108,109]. Bellera and colleagues showed that a single, early (at the reperfusion time) intracoronary administration of encapsulated in polylactidecoglycolide anti-miR-92 stimulates angiogenesis and prevents LVR in an adult pig model of AMI, without evidence of systemic or local adverse effects [20][42].
Song et al. injected miRNA-21-enriched exosomes (isolated from conditioned HEK293T cells) directly into the infarcted area and documented a rapid (four hours) uptake of miR-21 in both cardiomyocytes and endotheliocytes, followed by a significant attenuation of apoptosis and improvement of cardiac function [21][111]. Ma et al. used mesenchymal cells-derived exosomes injected intramyocardially to deliver miR-132 in infarcted mouse hearts and described an improved vascularization in the peri-infarcted area, with significant enhancement of cardiac function [22][112]. Early intravenous administration of exosomes derived from miR-146a-conditioned adipose-derived stem cells significantly reduced the cardiac damage and improved cardiac function in a rat model of acute myocardial infarction [23][113]. Zhao et al. injected intramyocardially exosomes derived from mesenchymal stromal cells and showed that exosomal miR-182 reduced cardiac inflammation and infarct size in a mouse model of myocardial infarction [24][114]. Charles et al. reported a significant reduction in the infarcted size and improved cardiac function after IV administration of MSC-derived exosomes as early as day seven post-infarction [25][115].
However, the therapeutic use of exosomes is still hampered by its low and variable recovery yield from cell cultures, their widely heterogeneous size, and the cumbersome purification procedures [26][27][116,117]. Alternatives to the exosome, such as targeted modified nanoparticles and low molecular weight heparin (LMWH) nanocarriers, have been recently proposed. Hyaluronan-sulfate miR-21-carrying anionic targeted at macrophages in the infarcted area turned them in reparative mode and significantly reduced fibrosis and apoptosis, with enhanced cardiac function [28][118]. Even more exciting, LMWH nanocarriers loaded with anti-miR-1 overcame micro-thrombotic obstruction and reduced cardiomyocyte apoptosis, cardiac fibrosis, and promoted tissular repair [29][119].
