Paper-based sensors are getting increasing attention for reliable indoor/outdoor onsite detection with non-expert operation due to low cost, portability, easy disposal, and high accuracy, as well as bulky reduced laboratory equipment. They have become powerful analysis tools in trace detection with ultra-low detection limits and extremely high accuracy, resulting in their great popularity in biological detection, environmental inspection, and other applications. However, the current paper-based sensors still encounter insufficiencies such as harsh storage, short shelf time, singleplex analyte detection, disability of holographic strain detection, and low reproducibility for direct detection of the actual sample without pretreatment. Efforts should be made to paper-based sensors with those concerns before their broad commercial application.
- paper-based sensor
- lateral flow test strips
- microfluidics
- bioassay trace
Note: The entry will be online only after author check and submit it.
1. Introduction
| Output Signal |
Principle | Analyte | Limit | Shelf Time | Year | Ref | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| current | Swelling of cellulose network after absorbing water causes the separation of the conductive carbon nanotubes attached to cellulose network, increasing sensor’s resistance, then the sensor’s current changed by the humidity. | humidity | - | Three months | 2020 | [18] | |||||
| resistance | Applied pressure reduces the gap between the conductive paper layers carrying the reduced graphene oxide material, then the sensor’s resistance decreases. | pressure | - | - | 2017 | [19] | |||||
| resistance | Conductive molybdenum carbide-graphene layers with porous and coral-shaped microstructure on the paper matrix allow micro-cracks under external compressive or tensile pressures, which eventually causes a resistance increase of the sensor. | pressure | - | - | 2020 | [20] | |||||
| current | Low pressure reduces the gap between the PVP layer containing conductive carbon nanotubes and the interdigital electrodes, resulting in a resistance decrease. While high pressure reduces the gap between the conductive carbon nanotubes inside the PVP layer to reduce the sensor’s resistance, leading to an increased current. | pressure | 8 Pa | - | 2019 | [21] | |||||
| resistance | Vibration caused deformation of the sensor, which increased/decreased the distance between the conductive grooves’ two walls and subsequently increase/decrease the resistance. | vibration | - | - | 2021 | [22] | |||||
| capacitance | External pressure caused an increment of the contact area among the ionic and conductive cellulose fibers, leading to a capacitance rise of the ISP sensor. | pressure | 6.25 Pa | - | 2019 | [23] | |||||
| current | After capturing the target miRNA, PtCuMOFs/ DNA3, and PtCuMOFs/DNA4 reporter probes are hybridized to the captured miRNA on the electrode surface and generates faradaic current with conjugated methylene blue and ferrocene. |
miR−141, miR−21 |
0.1 fM/L | - | 2019 | [24] | |||||
| current | The hairpin structure of H1 is opened after the miRNA−155 binding. Then the hairpin structure H2 competes with miRNA−155 from H1 through a stronger combination with H1, then continuously going through this process. Then the amplified H1-H2 duplexes combine with S1-AuNPs@Cu-MOFs to oxidize glucose to generate an electrical signal. | miRNA−155 | 0.35 fM/L | - | 2017 | [25] | |||||
| current | After the mutation-related DNA is captured on the sensor, it then hybridizes with another DNA strand labeled with HRP and catalyzes hydrogen peroxide to generate electrical signals. | EGFR mutation DNA | 0.167 nM/L | Four weeks | 2017 | [12] | |||||
| fluorescence | NH | 3 | -triggered Structure change of NH | 2 | -MIL-125(Ti) on the sensor is utilized for the visible fluorescence immunoassay of target CEA by coupling with a sandwich-type detection mode in the microplates. |
CEA | 0.041 ng/mL | - | 2018 | [26] | |
| chemiluminescence | Lactate oxidase on the sensor oxidizes lactate to generate hydrogen peroxide. Subsequently, the HRP fixed on the sensor catalyzes the oxidation of TMB by hydrogen peroxide to realize the lactate-concentration-dependent color delivery. | L-lactate | 0.1 mM/L | - | 2017 | [27] | |||||
| electrochemical potential | The glucose oxidase on the sensor oxidizes glucose to generate hydrogen peroxide, which changes the redox potential of the solution directly. | glucose | 0.02 mM/L | One month | 2018 | [28] | |||||
| resistance | Adsorption of PSA through the specific reaction between PSA and PSA antibody increases the distance between the multi-wall carbon nanotubes on the sensor, forcing the resistance increment. | PSA | 1.18 ng/mL | Two hours | 2018 | [15] | |||||
| fluorescence | In the presence of the analytes, a layer-by-layer self-assembly reaction is allowed due to the recognition between the β-cyclodextrin-coated gold nanoparticles and 1-adamantane acetic acid or tetrakis (4-carboxyphenyl) porphyrin. And then, the accumulation of Au nanoparticles will give an increasing fluorescence. | carcinoembryonic antigen, p24 antigen | dozens of molecules per strip | - | 2019 | [29] | |||||
| fluorescence | Reduced Ag | + | in the silver nanoparticles to Ag atoms will aggregate into oligomeric clusters, distributing to the UV–vis absorption band. | AA | 82.8 µM/L | Three weeks | 2015 | [8] | |||
| current | Adsorption of norepinephrine or serotonin gives an electrode fouling, leading to the decrease of oxidation current and the shift of the positive potential peak. | NE, 5-HT |
2.5 µM/L, 0.5 µM/L |
- | 2017 | [30] | |||||
| chemiluminescence | Doxycycline relieves the expression of the reporter of the yeast bacteria, causing a noticeable color change in the sensor. | doxycycline | - | One year | 2015 | [16] | |||||
| current | Ethanol oxidase on the senor oxides ethanol to generate hydrogen peroxide. Then the hydrogen peroxide reacted with the Prussian blue/carbon black nanoparticles to trigger an electric current on the sensor. | ethanol | 0.52 mM/L | Three weeks | 2017 | [31] | |||||
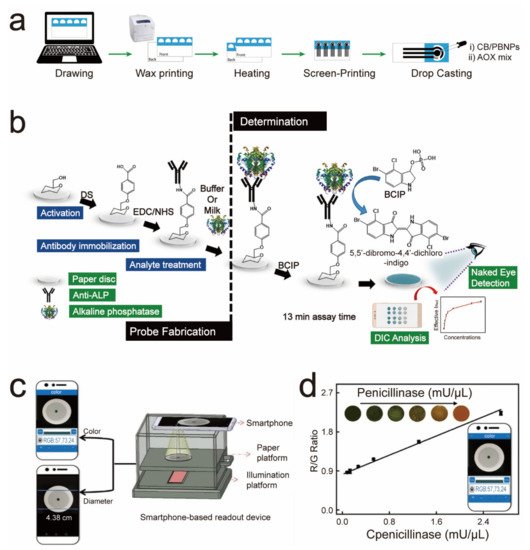
| After the capture of the ALP by the ALP antibody on the sensor, it will catalyze the color-developing substrate to deliver an ALP-concentration-dependent color change. | ALP | 0.87 U/mL | Four weeks | 2019 | [32] | ||||||
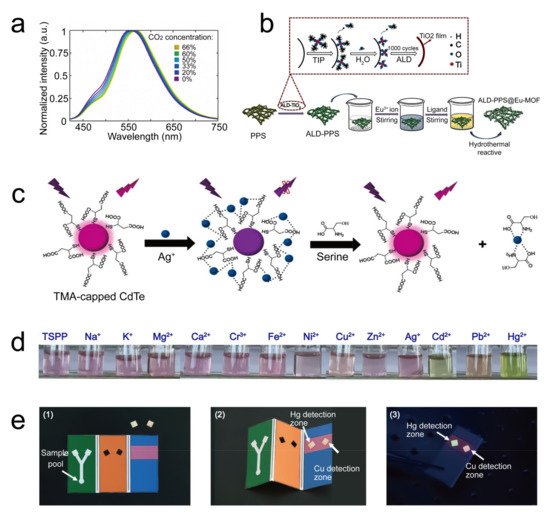
| fluorescence | In the presence of CO | 2 | , the fluorescent spectra peak of the P4VB on the sensor shows a CO | 2 | -concentration-dependent redshift. | CO | 2 | 5.7 ppm | - | 2020 | [33] |
| chemiluminescence | After the oxidation of phenol substrates by the polyphenol oxidase, the oxidization product will react with the benzothiazolinone hydrazine to present a color change of the sensor paper. | catechol, phenol, p-cresol, 4-methyl catechol |
0.5 µM/L | One month | 2020 | [34] | |||||
| fluorescence | Ag | + | binding quenches the fluorescence of the CdTe quantum dots. | Ag | + | 13.16 nM/L | - | 2020 | [35] | ||
| fluorescence | In the presence of Cu | 2+ | , the fluorescence of the fluorophore P2017 decreased while that of B001 was maintained well, thus giving a ratio-based fluorescence detection technology. | Cu | 2+ | 2.4 nM/L | - | 2019 | [36] |
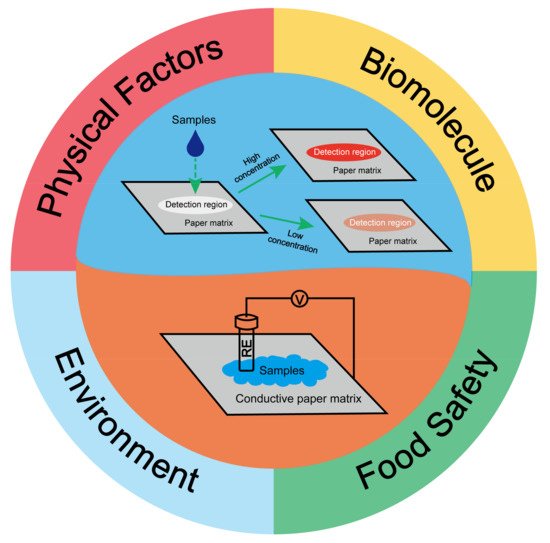
2. Paper-Based Sensors for Physical Factors Sensing
2.1. Humidity Sensing
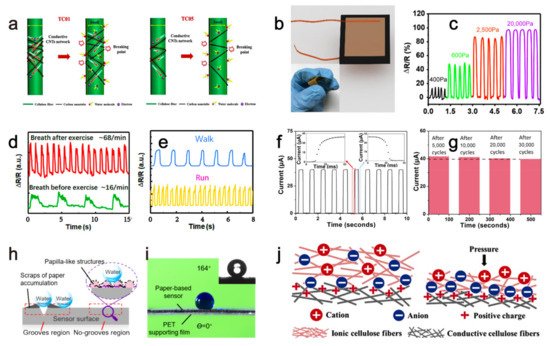
2.2. Mechanical Sensing
3. Paper-Based Sensor for Biomolecule Sensing
3.1. Nucleic Acid Sensing
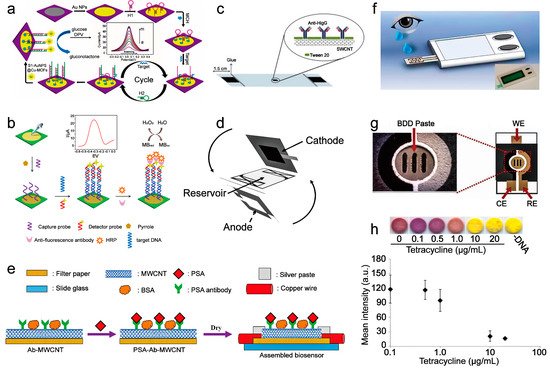
3.2. Protein Sensing
3.3. Sugar Sensing
3.4. Antigen/Antibody Sensing
3.5. Vitamin Sensing
3.6. Neurotransmitter Sensing
3.7. Antibiotic Sensing
4. Paper-Based Sensors for Food Safety Testing
5. Paper-Based Sensors for Environmental Quality Inspection
trochemical sensors for analysis of methamphetamine: A review. Chemosphere 2021, 278, 130393. [CrossRef]
2. Meena, K.V.; Sankar, A.R. Biomedical Catheters with Integrated Miniature Piezoresistive Pressure Sensors: A Review. IEEE Sens.
J. 2021, 21, 10241–10290. [CrossRef]
3. Fuh, Y.K.; Wang, B.S. Near field sequentially electrospun three-dimensional piezoelectric fibers arrays for self-powered sensors of
human gesture recognition. Nano Energy 2016, 30, 677–683. [CrossRef]
4. Singh, A.T.; Lantigua, D.; Meka, A.; Taing, S.; Pandher, M.; Camci-Unal, G. Paper-Based Sensors: Emerging Themes and
Ap-plications. Sensors 2018, 18, 2838. [CrossRef]
5. White, D.; Keramane, M.; Capretta, A.; Brennan, J.D. A paper-based biosensor for visual detection of glucose-6-phosphate
dehydrogenase from whole blood. Analyst 2020, 145, 1817–1824. [CrossRef]
6. Choi, J.R.; Hu, J.; Tang, R.; Gong, Y.; Feng, S.; Ren, H.; Wen, T.; Li, X.; Abas, W.A.B.W.; Pingguan-Murphy, B.; et al. An inte-grated
paper-based sample-to-answer biosensor for nucleic acid testing at the point of care. Lab Chip 2016, 16, 611–621. [CrossRef]
7. Alkasir, R.S.J.; Rossner, A.; Andreescu, S. Portable Colorimetric Paper-Based Biosensing Device for the Assessment of Bisphenol
A in Indoor Dust. Environ. Sci. Technol. 2015, 49, 9889–9897. [CrossRef]
8. Ferreira, D.C.M.; Giordano, G.F.; Soares, C.C.S.P.; de Oliveira, J.F.A.; Mendes, R.K.; Piazzetta, M.H.; Gobbi, A.L.; Cardoso, M.B.
Optical paper-based sensor for ascorbic acid quantification using silver nanoparticles. Talanta 2015, 141, 188–194. [CrossRef]
9. Ming, T.; Luo, J.; Liu, J.; Sun, S.; Xing, Y.; Wang, H.; Xiao, G.; Deng, Y.; Cheng, Y.; Yang, Z.; et al. Paper-based microfluidic
aptasensors. Biosens. Bioelectron. 2020, 170, 112649. [CrossRef]
10. Tai, H.; Duan, Z.; Wang, Y.; Wang, S.; Jiang, Y. Paper-Based Sensors for Gas, Humidity and Strain Detections: A Review. ACS
Appl. Mater. Interfaces 2020, 12, 31037–31053. [CrossRef]
11. Wang, T.; Reid, R.C.; Minteer, S.D. A Paper-based Mitochondrial Electrochemical Biosensor for Pesticide Detection. Electroa-nalysis
2016, 28, 854–859. [CrossRef]
12. Tian, T.; Liu, H.; Li, L.; Yu, J.; Ge, S.; Song, X.; Yan, M. Paper-based biosensor for noninvasive detection of epidermal growth
factor receptor mutations in non-small cell lung cancer patients. Sens. Actuators B 2017, 251, 440–445. [CrossRef]
13. Fakhri, N.; Abarghoei, S.; Dadmehr, M.; Hosseini, M.; Sabahi, H.; Ganjali, M.R. Paper based colorimetric detection of miR-NA-21
using Ag/Pt nanoclusters. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227, 117529. [CrossRef]
14. Kumar, S.; Kumar, S.; Srivastava, S.; Yadav, B.K.; Lee, S.H.; Sharma, J.G.; Doval, D.C.; Malhotra, B.D. Reduced graphene oxide
modified smart conducting paper for cancer biosensor. Biosens. Bioelectron. 2015, 73, 114–122. [CrossRef] [PubMed]
15. Ji, S.; Lee, M.; Kim, D. Detection of early stage prostate cancer by using a simple carbon nanotube@paper biosensor. Biosens.
Bioelectron. 2018, 102, 345–350. [CrossRef] [PubMed]
16. Weaver, A.A.; Halweg, S.; Joyce, M.; Lieberman, M.; Goodson, H.V. Incorporating yeast biosensors into paper-based analytical
tools for pharmaceutical analysis. Anal. Bioanal. Chem. 2015, 407, 615–619. [CrossRef]
17. Chaiyo, S.; Mehmeti, E.; Siangproh, W.; Hoang, T.L.; Nguyen, H.P.; Chailapakul, O.; Kalcher, K. Non-enzymatic electro-chemical
detection of glucose with a disposable paper-based sensor using a cobalt phthalocyanine–ionic liquid–graphene composite.
Biosens. Bioelectron. 2018, 102, 113–120. [CrossRef]
18. Zhu, P.; Kuang, Y.; Wei, Y.; Li, F.; Ou, H.; Jiang, F.; Chen, G. Electrostatic self-assembly enabled flexible paper-based humidity
sensor with high sensitivity and superior durability. Chem. Eng. J. 2020, 404, 127105. [CrossRef]
19. Tao, L.; Zhang, K.; Tian, H.; Liu, Y.; Wang, D.; Chen, Y.; Yang, Y.; Ren, T. Graphene-Paper Pressure Sensor for Detecting Human
Motions. ACS Nano 2017, 11, 8790–8795. [CrossRef]
20. Long, Y.; He, P.; Xu, R.; Hayasaka, T.; Shao, Z.; Zhong, J.; Lin, L. Molybdenum-carbide-graphene composites for paper-based
strain and acoustic pressure sensors. Carbon 2020, 157, e594–e601. [CrossRef]
21. Wang, C.; Hou, X.; Cui, M.; Yu, J.; Fan, X.; Qian, J.; He, J.; Geng, W.; Mu, J.; Chou, X. An ultra-sensitive and wide measuring range
pressure sensor with paper-based CNT film/interdigitated structure. Sci. China Mater. 2019, 63, 403–412. [CrossRef]
22. Liu, L.; Jiao, Z.; Zhang, J.; Wang, Y.; Zhang, C.; Meng, X.; Jiang, X.; Niu, S.; Han, Z.; Ren, L. Bioinspired, Superhydrophobic,
and Paper-Based Strain Sensors for Wearable and Underwater Applications. ACS Appl. Mater. Interfaces 2021 , 13, 1967–1978.
[CrossRef] [PubMed]
23. Li, S.; Pan, N.; Zhu, Z.; Li, R.; Li, B.; Chu, J.; Li, G.; Chang, Y.; Pan, T. All-in-One Iontronic Sensing Paper. Adv. Funct. Mater. 2019 ,
29, 1807343. [CrossRef]
24. Tian, R.; Li, Y.; Bai, J. Hierarchical assembled nanomaterial paper based analytical devices for simultaneously electrochemical
detection of microRNAs. Anal. Chim. Acta 2019, 1058, e89–e96. [CrossRef] [PubMed]
25. Wang, H.; Jian, Y.; Kong, Q.; Liu, H.; Lan, F.; Liang, L.; Ge, S.; Yu, J. Ultrasensitive electrochemical paper-based biosensor for
microRNA via strand displacement reaction and metal-organic frameworks. Sens. Actuators B 2018, 257, 561–569. [CrossRef]
26. Lv, S.; Tang, Y.; Zhang, K.; Tang, D. Wet NH 3 -Triggered NH 2 -MIL-125(Ti) structural switch for visible fluorescence immunoassay
impregnated on paper. Anal. Chem. 2018, 90, 14121–14125. [CrossRef] [PubMed]
27. Calabria, D.; Caliceti, C.; Zangheri, M.; Mirasoli, M.; Simoni, P.; Roda, A. Smartphone–based enzymatic biosensor for oral fluid
L-lactate detection in one minute using confined multilayer paper reflectometry. Biosens. Bioelectron. 2017 , 94, 124–130. [CrossRef]
[PubMed]
Sensors 2021, 21, 5998 20 of 22
28. Leonor, G.; Novell, M.; Blondeau, P.; Andrade, F.J. A disposable, simple, fast and low-cost paper-based biosensor and its
ap-plication to the determination of glucose in commercial orange juices. Food Chem. 2018, 265, 64–69. [CrossRef]
29. Huang, X.; Zhou, Y.; Ding, L.; Yu, G.; Leng, Y.; Lai, W.; Xiong, Y.; Chen, X. Supramolecular Recognition-Mediated Lay-er-by-Layer
Self-Assembled Gold Nanoparticles for Customized Sensitivity in Paper-Based Strip Nanobiosensors. Small 2019 , 15, 1903861.
[CrossRef]
30. Nantaphol, S.; Channon, R.B.; Kondo, T.; Siangproh, W.; Chailapakul, O.; Henry, C.S. Boron Doped Diamond Paste Electrodes for
Microfluidic Paper-Based Analytical Devices. Anal. Chem. 2017, 89, 4100–4107. [CrossRef]
31. Cinti, S.; Basso, M.; Moscone, D.; Arduini, F. A paper-based nanomodified electrochemical biosensor for ethanol detection in
beers. Anal. Chim. Acta 2017, 960, 123–130. [CrossRef]
32. Mahato, K.; Chandra, P. Paper-based miniaturized immunosensor for naked eye ALP detection based on digital image col-
orimetry integrated with smartphone. Biosens. Bioelectron. 2019, 128, 9–16. [CrossRef]
33. Wang, H.; Vagin, S.I.; Rieger, B.; Meldrum, A. An Ultrasensitive Fluorescent Paper-Based CO 2 Sensor. ACS Appl. Mater. In-terfaces
2020, 12, 20507–20513. [CrossRef] [PubMed]
34. Noori, R.; Perwez, M.; Mazumder, J.A.; Sardar, M. Development of low-cost paper-based biosensor of polyphenol oxidase for
detection of phenolic contaminants in water and clinical samples. Environ. Sci. Pollut. Res. 2020 , 27, 30081–30092. [CrossRef]
[PubMed]
35. Chen, H.; Hu, O.; Fu, H.; Fan, Y.; Xu, L.; Meng, Q.; Zhang, L.; Lan, W.; Wu, C.; Tang, S.; et al. Paper-based sensor for visual
detection of Ag + based on a “turn-off-on” fluorescent design. Microchem. J. 2020, 157, 104887. [CrossRef]
36. Zhou, J.Q.; Wu, X.; Chen, X.; Qin, G.; Zhang, M.; Wu, H.; Fang, Y.; Lu, H.; Yu, L.; Li, L.; et al. Two-component ratiometric sensor
for Cu 2+ detection on paper-based device. Anal. Bioanal. Chem. 2019, 411, 6165–6172. [CrossRef] [PubMed]
37. Duan, Z.; Jiang, Y.; Yan, M.; Wang, S.; Yuan, Z.; Zhao, Q.; Sun, P.; Xie, G.; Du, X.; Tai, H. Facile, Flexible, Cost-Saving, and
Environment-Friendly Paper-Based Humidity Sensor for Multifunctional Applications. ACS Appl. Mater. Interfaces 2019 , 11,
21840–21849. [CrossRef]
38. Castro, C.; Rosillo, C.; Tsutsui, H. Characterizing effects of humidity and channel size on imbibition in paper-based microfluidic
channels. Microfluid Nanofluid 2017, 21, 21. [CrossRef]
39. Li, Z.; Wang, J.; Xu, Y.; Shen, M.; Duan, C.; Dai, L.; Ni, Y. Green and sustainable cellulose-derived humidity sensors: A review.
Carbohydr. Polym. 2021, 270, 118385. [CrossRef]
40. Shen, L.-L.; Zhang, G.-R.; Etzold, B.J.M. Paper-Based Microfluidics for Electrochemical Applications. ChemElectroChem 2020 , 7,
10–30. [CrossRef]
41. Nishat, S.; Jafry, A.T.; Martinez, A.W.; Awan, F.R. Paper-based microfluidics: Simplified fabrication and assay methods. Sens.
Actuators B Chem. 2021, 336, 129681. [CrossRef]
42. Xu, Y.; Fei, Q.; Page, M.; Zhao, G.; Ling, Y.; Stoll, S.B.; Yan, Z. Paper-based wearable electronics. iScience 2021 , 24, 102736.
[CrossRef]
43. Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, V.S.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect
in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [CrossRef]
44. Wang, Y.; Zhou, W.; Cao, K.; Hu, X.; Gao, L.; Lu, Y. Architectured graphene and its composites: Manufacturing and structural
applications. Compos. Part A Appl. Sci. Manuf. 2021, 140, 106177. [CrossRef]
45. Yang, T.; Mativetsky, J.M. Paper-Based Mechanical Sensors Enabled by Folding and Stacking. ACS Appl. Mater. Interfaces 2019 , 11,
26339–26345. [CrossRef] [PubMed]
46. Li, Q.; Liu, H.; Zhang, S.; Zhang, D.; Liu, X.; He, Y.; Mi, L.; Zhang, J.; Liu, C.; Shen, C.; et al. Superhydrophobic Electrically
Conductive Paper for Ultrasensitive Strain Sensor with Excellent Anticorrosion and Self-Cleaning Property. ACS Appl. Mater.
Interfaces 2019, 11, 21904–21914. [CrossRef]
47. Mao, K.; Min, X.; Zhang, H.; Zhang, K.; Cao, H.; Guo, Y.; Yang, Z. Paper-based microfluidics for rapid diagnostics and drug
delivery. J. Control. Release 2020, 322, 187–199. [CrossRef] [PubMed]
48. Caspersson, T.; Schultz, J. Nucleic Acid Metabolism of the Chromosomes in Relation to Gene Reproduction. Nature 1938 , 142,
294–295. [CrossRef]
49. Hisae, T.-K.; Naoki, S. Roles of non-canonical structures of nucleic acids in cancer and neurodegenerative diseases. Nucleic Acids
Res. 2021, 49, 7839–7855. [CrossRef]
50. Gilboa, T.; Garden, P.M.; Cohen, L. Single-molecule analysis of nucleic acid biomarkers—A review. Anal. Chim. Acta 2020 , 1115,
61–85. [CrossRef]
51. Zhao, Y.; Zuo, X.; Li, Q.; Chen, F.; Chen, Y.-R.; Deng, J.; Han, D.; Hao, C.; Huang, F.; Huang, Y.; et al. Nucleic acids analysis. Sci.
China Chem. 2021, 64, 171–203. [CrossRef] [PubMed]
52. Liu, X.; Wang, P.; Zhang, C.; Ma, Z. Epidermal growth factor receptor (EGFR): A rising star in the era of precision medicine of
lung cancer. Oncotarget 2017, 8, 50209–50220. [CrossRef] [PubMed]
53. Lin, H.; Wang, X.; Wu, J.; Li, H.; Li, F. Equipment-Free and Visualized Biosensor for Transcription Factor Rapid Assay Based on
Dopamine-Functionalized Cellulose Paper. J. Mater. Chem. B 2019, 7, 5461–5464. [CrossRef] [PubMed]
54. Pozuelo, M.; Blondeau, P.; Novell, M.; Andrade, F.J.; Rius, F.X.; Riu, J. Paper-based chemiresistor for detection of ultralow
concentrations of protein. Biosens. Bioelectron. 2013, 49, 462–465. [CrossRef]
Sensors 2021, 21, 5998 21 of 22
55. Fischer, C.; Fraiwan, A.; Choi, S. A 3D paper-based enzymatic fuel cell for self-powered, low-cost glucose monitoring. Biosens.
Bioelectron. 2016, 79, 193–197. [CrossRef]
56. Gartia, M.R.; Misra, S.K.; Ye, M.; Schwartz-Duval, A.; Plucinski, L.; Zhou, X.; Kellner, D.; Labriola, L.T.; Pan, D. Point-of-service,
quantitative analysis of ascorbic acid in aqueous humor for evaluating anterior globe integrity. Sci. Rep. 2015 , 5, 16011. [CrossRef]
57. Duyen, T.T.M.; Matsuura, H.; Ujiie, K.; Muraoka, M.; Harada, K.; Hirata, K. Paper-based colorimetric biosensor for antibiotics
inhibiting bacterial protein synthesis. J. Biosci. Bioeng. 2017, 123, 96–100. [CrossRef]
58. Wagner, D.R.; Heyward, V.H. Measures of body composition in blacks and whites: A comparative review. Am. J. Clin. Nutr. 2000 ,
71, 1392–1402. [CrossRef] [PubMed]
59. Verhelst, X.; Dias, A.M.; Colombel, J.-F.; Vermeire, S.; Vlierberghe, H.V.; Callewaert, N.; Pinho, S.S. Protein Glycosylation as
a Diagnostic and Prognostic Marker of Chronic Inflammatory Gastrointestinal and Liver Diseases. Gastroenterology 2020 , 158,
95–110. [CrossRef]
60. Zhang, Y.; Deng, Y.; Yang, X.; Xue, H.; Lang, Y. The Relationship between Protein S-Nitrosylation and Human Diseases: A Review.
Neurochem. Res. 2020, 45, 2815–2827. [CrossRef]
61. Ki, H.; Jang, H.; Oh, J.; Han, G.; Lee, H.; Kim, S.; Kim, M. Simultaneous Detection of Serum Glucose and Glycated Albumin on a
Paper-Based Sensor for Acute Hyperglycemia and Diabetes Mellitus. Anal. Chem. 2020, 92, 11530–11534. [CrossRef]
62. Parrilla, M.; Cánovas, R.; Andrade, F.J. Paper-based enzymatic electrode with enhanced potentiometric response for moni-toring
glucose in biological fluids. Biosens. Bioelectron. 2017, 90, 110–116. [CrossRef]
63. Huang, P.; Hong, C.P.; Zhu, J.F.; Chen, T.; Chan, C.; Ko, Y.; Lin, T.; Pan, Z.; Sun, N.; Wang, Y.; et al. Ag@Au nanoprisms-metal
organic frameworks-based paper for extending glucose sensing range in human serum and urine. Dalton Trans. 2017 , 46,
6985–6993. [CrossRef] [PubMed]
64. Nunez-Bajo, E.; Fernández-Abedul, M.T. Paper-based platforms with coulometric readout for ascorbic acid determination in fruit
juices. Analyst 2020, 145, 3431–3439. [CrossRef] [PubMed]
65. Sarada, B.V.; Rao, T.N.; Tryk, D.A.; Fujishima, A. Electrochemical Oxidation of Histamine and Serotonin at Highly Bo-ron-Doped
Diamond Electrodes. Anal. Chem. 2000, 72, 1632–1638. [CrossRef] [PubMed]
66. Anh, H.Q.; Le, T.P.Q.; Le, N.D.; Lu, X.X.; Duong, T.T.; Garnier, J.; Rochelle-Newall, E.; Zhang, S.; Oh, N.-H.; Oeurng, C.; et al.
Antibiotics in surface water of East and Southeast Asian countries: A focused review on contamination status, pollution sources,
potential risks, and future perspectives. Sci. Total Environ. 2021, 764, 142865. [CrossRef] [PubMed]
67. Magureanu, M.; Bilea, F.; Bradu, C.; Hong, D. A review on non-thermal plasma treatment of water contaminated with anti-biotics.
J. Hazard. Mater. 2021, 417, 125481. [CrossRef] [PubMed]
68. Mentana, A.; Palermo, C.; Nardiello, D.; Quinto, M.; Centonze, D. Simultaneous and accurate real time monitoring of glucose and
ethanol in alcoholic drinks, must, and biomass by a dual amperometric biosensor. J. Agric. Food Chem. 2013 , 61, 61–68. [CrossRef]
69. Xu, J.; Chen, X.; Khan, H.; Yang, L. A dual-readout paper-based sensor for onsite detection of penicillinase with a smartphone.
Sens. Actuators B Chem. 2021, 335, 129707. [CrossRef]
70. Hidayat, M.A.; Maharani, D.A.; Purwanto, D.A.; Kuswandi, B.; Yuwono, M. Simple and Sensitive Paper-based Colorimetric
Biosensor for Determining Total Polyphenol Content of the Green Tea Beverages. Biotechnol. Bioprocess Eng. 2020 , 25, 255–263.
[CrossRef]
71. Zhang, D.; Li, C.; Ji, D.; Wang, Y. Paper-Based Microfluidic Sensors for Onsite Environmental Detection: A Critical Review. Crit.
Rev. Anal. Chem. 2021, 1886900. [CrossRef]
72. Lin, Y.; Gritsenko, D.; Feng, S.; Teh, Y.C.; Lu, X.; Xu, J. Detection of heavy metal by paper-based microfluidics. Biosens. Bioe-lectron.
2016, 83, 256–266. [CrossRef] [PubMed]
73. Dabhade, A.; Jayaraman, S.; Paramasivan, B. Colorimetric paper bioassay by horseradish peroxidase for the detection of cat-echol
and resorcinol in aqueous samples. Prep. Biochem. Biotechnol. 2020, 50, 849–856. [CrossRef] [PubMed]
74. Dungchai, W.; Sameenoi, Y.; Chailapakul, O.; Volckens, J.; Henry, C.S. Determination of aerosol oxidative activity using silver
nanoparticle aggregation on paper-based analytical devices. Analyst 2013, 138, 6766–6773. [CrossRef] [PubMed]
75. Li, Y.; Liang, F. Progress in Paper-based Colorimetric Sensor Array. Chin. J. Anal. Chem. 2020, 48, 1448–1457. [CrossRef]
76. Wang, X.; Sun, J.; Tong, J.; Guan, X.; Bian, C.; Xia, S. Paper-Based Sensor Chip for Heavy Metal Ion Detection by SWSV.
Mi-cromachines 2018, 9, 150. [CrossRef]
77. Cinti, S.; Talarico, D.; Palleschi, G.; Moscone, D.; Arduini, F. Novel reagentless paper-based screen-printed electrochemical sensor
to detect phosphate. Anal. Chim. Acta 2016, 919, 78–84. [CrossRef]
78. Yakoh, A.; Rattanarat, P.; Siangproh, W.; Chailapakul, O. Simple and selective paper-based colorimetric sensor for determi-nation
of chloride ion in environmental samples using label-free silver nanoprisms. Talanta 2018, 178, 134–140. [CrossRef] [PubMed]
79. Yen, Y.; Lee, K.; Lin, C.; Zhang, S.; Wang, C.; Liu, T. Portable Nanohybrid Paper-Based Chemiresistive Sensor for Free Chlorine
Detection. ACS Omega 2020, 5, 25209–25215. [CrossRef] [PubMed]
80. Maruthupandi, M.; Thiruppathi, D.; Vasimalai, N. One minute synthesis of green fluorescent copper nanocluster: The prep-aration
of smartphone aided paper-based kit for onsite monitoring of nanomolar level mercury and sulfide ions in environ-mental
samples. J. Hazard. Mater. 2020, 392, 122294. [CrossRef]
81. Liu, Y.; Hsu, C.; Lu, B.; Lin, P.; Ho, M. Determination of nitrite ions in environment analysis with a paper-based microfluidic
device. Dalton Trans. 2018, 47, 14799–14807. [CrossRef] [PubMed]
References
- Khorablou, Z.; Shahdost-fard, F.; Razmi, H.; Yola, M.L.; Karimi-Maleh, H. Recent advances in developing optical and elec-trochemical sensors for analysis of methamphetamine: A review. Chemosphere 2021, 278, 130393.
- Meena, K.V.; Sankar, A.R. Biomedical Catheters with Integrated Miniature Piezoresistive Pressure Sensors: A Review. IEEE Sens. J. 2021, 21, 10241–10290.
- Fuh, Y.K.; Wang, B.S. Near field sequentially electrospun three-dimensional piezoelectric fibers arrays for self-powered sensors of human gesture recognition. Nano Energy 2016, 30, 677–683.
- Singh, A.T.; Lantigua, D.; Meka, A.; Taing, S.; Pandher, M.; Camci-Unal, G. Paper-Based Sensors: Emerging Themes and Ap-plications. Sensors 2018, 18, 2838.
- White, D.; Keramane, M.; Capretta, A.; Brennan, J.D. A paper-based biosensor for visual detection of glucose-6-phosphate dehydrogenase from whole blood. Analyst 2020, 145, 1817–1824.
- Choi, J.R.; Hu, J.; Tang, R.; Gong, Y.; Feng, S.; Ren, H.; Wen, T.; Li, X.; Abas, W.A.B.W.; Pingguan-Murphy, B.; et al. An inte-grated paper-based sample-to-answer biosensor for nucleic acid testing at the point of care. Lab Chip 2016, 16, 611–621.
- Alkasir, R.S.J.; Rossner, A.; Andreescu, S. Portable Colorimetric Paper-Based Biosensing Device for the Assessment of Bisphenol A in Indoor Dust. Environ. Sci. Technol. 2015, 49, 9889–9897.
- Ferreira, D.C.M.; Giordano, G.F.; Soares, C.C.S.P.; de Oliveira, J.F.A.; Mendes, R.K.; Piazzetta, M.H.; Gobbi, A.L.; Cardoso, M.B. Optical paper-based sensor for ascorbic acid quantification using silver nanoparticles. Talanta 2015, 141, 188–194.
- Ming, T.; Luo, J.; Liu, J.; Sun, S.; Xing, Y.; Wang, H.; Xiao, G.; Deng, Y.; Cheng, Y.; Yang, Z.; et al. Paper-based microfluidic aptasensors. Biosens. Bioelectron. 2020, 170, 112649.
- Tai, H.; Duan, Z.; Wang, Y.; Wang, S.; Jiang, Y. Paper-Based Sensors for Gas, Humidity and Strain Detections: A Review. ACS Appl. Mater. Interfaces 2020, 12, 31037–31053.
- Wang, T.; Reid, R.C.; Minteer, S.D. A Paper-based Mitochondrial Electrochemical Biosensor for Pesticide Detection. Electroa-nalysis 2016, 28, 854–859.
- Tian, T.; Liu, H.; Li, L.; Yu, J.; Ge, S.; Song, X.; Yan, M. Paper-based biosensor for noninvasive detection of epidermal growth factor receptor mutations in non-small cell lung cancer patients. Sens. Actuators B 2017, 251, 440–445.
- Fakhri, N.; Abarghoei, S.; Dadmehr, M.; Hosseini, M.; Sabahi, H.; Ganjali, M.R. Paper based colorimetric detection of miR-NA-21 using Ag/Pt nanoclusters. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227, 117529.
- Kumar, S.; Kumar, S.; Srivastava, S.; Yadav, B.K.; Lee, S.H.; Sharma, J.G.; Doval, D.C.; Malhotra, B.D. Reduced graphene oxide modified smart conducting paper for cancer biosensor. Biosens. Bioelectron. 2015, 73, 114–122.
- Ji, S.; Lee, M.; Kim, D. Detection of early stage prostate cancer by using a simple carbon [email protected] biosensor. Biosens. Bioelectron. 2018, 102, 345–350.
- Weaver, A.A.; Halweg, S.; Joyce, M.; Lieberman, M.; Goodson, H.V. Incorporating yeast biosensors into paper-based analytical tools for pharmaceutical analysis. Anal. Bioanal. Chem. 2015, 407, 615–619.
- Chaiyo, S.; Mehmeti, E.; Siangproh, W.; Hoang, T.L.; Nguyen, H.P.; Chailapakul, O.; Kalcher, K. Non-enzymatic electro-chemical detection of glucose with a disposable paper-based sensor using a cobalt phthalocyanine–ionic liquid–graphene composite. Biosens. Bioelectron. 2018, 102, 113–120.
- Zhu, P.; Kuang, Y.; Wei, Y.; Li, F.; Ou, H.; Jiang, F.; Chen, G. Electrostatic self-assembly enabled flexible paper-based humidity sensor with high sensitivity and superior durability. Chem. Eng. J. 2020, 404, 127105.
- Tao, L.; Zhang, K.; Tian, H.; Liu, Y.; Wang, D.; Chen, Y.; Yang, Y.; Ren, T. Graphene-Paper Pressure Sensor for Detecting Human Motions. ACS Nano 2017, 11, 8790–8795.
- Long, Y.; He, P.; Xu, R.; Hayasaka, T.; Shao, Z.; Zhong, J.; Lin, L. Molybdenum-carbide-graphene composites for paper-based strain and acoustic pressure sensors. Carbon 2020, 157, e594–e601.
- Wang, C.; Hou, X.; Cui, M.; Yu, J.; Fan, X.; Qian, J.; He, J.; Geng, W.; Mu, J.; Chou, X. An ultra-sensitive and wide measuring range pressure sensor with paper-based CNT film/interdigitated structure. Sci. China Mater. 2019, 63, 403–412.
- Liu, L.; Jiao, Z.; Zhang, J.; Wang, Y.; Zhang, C.; Meng, X.; Jiang, X.; Niu, S.; Han, Z.; Ren, L. Bioinspired, Superhydrophobic, and Paper-Based Strain Sensors for Wearable and Underwater Applications. ACS Appl. Mater. Interfaces 2021, 13, 1967–1978.
- Li, S.; Pan, N.; Zhu, Z.; Li, R.; Li, B.; Chu, J.; Li, G.; Chang, Y.; Pan, T. All-in-One Iontronic Sensing Paper. Adv. Funct. Mater. 2019, 29, 1807343.
- Tian, R.; Li, Y.; Bai, J. Hierarchical assembled nanomaterial paper based analytical devices for simultaneously electrochemical detection of microRNAs. Anal. Chim. Acta 2019, 1058, e89–e96.
- Wang, H.; Jian, Y.; Kong, Q.; Liu, H.; Lan, F.; Liang, L.; Ge, S.; Yu, J. Ultrasensitive electrochemical paper-based biosensor for microRNA via strand displacement reaction and metal-organic frameworks. Sens. Actuators B 2018, 257, 561–569.
- Lv, S.; Tang, Y.; Zhang, K.; Tang, D. Wet NH3-Triggered NH2-MIL-125(Ti) structural switch for visible fluorescence immunoassay impregnated on paper. Anal. Chem. 2018, 90, 14121–14125.
- Calabria, D.; Caliceti, C.; Zangheri, M.; Mirasoli, M.; Simoni, P.; Roda, A. Smartphone–based enzymatic biosensor for oral fluid L-lactate detection in one minute using confined multilayer paper reflectometry. Biosens. Bioelectron. 2017, 94, 124–130.
- Leonor, G.; Novell, M.; Blondeau, P.; Andrade, F.J. A disposable, simple, fast and low-cost paper-based biosensor and its ap-plication to the determination of glucose in commercial orange juices. Food Chem. 2018, 265, 64–69.
- Huang, X.; Zhou, Y.; Ding, L.; Yu, G.; Leng, Y.; Lai, W.; Xiong, Y.; Chen, X. Supramolecular Recognition-Mediated Lay-er-by-Layer Self-Assembled Gold Nanoparticles for Customized Sensitivity in Paper-Based Strip Nanobiosensors. Small 2019, 15, 1903861.
- Nantaphol, S.; Channon, R.B.; Kondo, T.; Siangproh, W.; Chailapakul, O.; Henry, C.S. Boron Doped Diamond Paste Electrodes for Microfluidic Paper-Based Analytical Devices. Anal. Chem. 2017, 89, 4100–4107.
- Cinti, S.; Basso, M.; Moscone, D.; Arduini, F. A paper-based nanomodified electrochemical biosensor for ethanol detection in beers. Anal. Chim. Acta 2017, 960, 123–130.
- Mahato, K.; Chandra, P. Paper-based miniaturized immunosensor for naked eye ALP detection based on digital image col-orimetry integrated with smartphone. Biosens. Bioelectron. 2019, 128, 9–16.
- Wang, H.; Vagin, S.I.; Rieger, B.; Meldrum, A. An Ultrasensitive Fluorescent Paper-Based CO2 Sensor. ACS Appl. Mater. In-terfaces 2020, 12, 20507–20513.
- Noori, R.; Perwez, M.; Mazumder, J.A.; Sardar, M. Development of low-cost paper-based biosensor of polyphenol oxidase for detection of phenolic contaminants in water and clinical samples. Environ. Sci. Pollut. Res. 2020, 27, 30081–30092.
- Chen, H.; Hu, O.; Fu, H.; Fan, Y.; Xu, L.; Meng, Q.; Zhang, L.; Lan, W.; Wu, C.; Tang, S.; et al. Paper-based sensor for visual detection of Ag+ based on a “turn-off-on” fluorescent design. Microchem. J. 2020, 157, 104887.
- Zhou, J.Q.; Wu, X.; Chen, X.; Qin, G.; Zhang, M.; Wu, H.; Fang, Y.; Lu, H.; Yu, L.; Li, L.; et al. Two-component ratiometric sensor for Cu2+ detection on paper-based device. Anal. Bioanal. Chem. 2019, 411, 6165–6172.
- Duan, Z.; Jiang, Y.; Yan, M.; Wang, S.; Yuan, Z.; Zhao, Q.; Sun, P.; Xie, G.; Du, X.; Tai, H. Facile, Flexible, Cost-Saving, and Environment-Friendly Paper-Based Humidity Sensor for Multifunctional Applications. ACS Appl. Mater. Interfaces 2019, 11, 21840–21849.
- Castro, C.; Rosillo, C.; Tsutsui, H. Characterizing effects of humidity and channel size on imbibition in paper-based microfluidic channels. Microfluid Nanofluid 2017, 21, 21.
- Li, Z.; Wang, J.; Xu, Y.; Shen, M.; Duan, C.; Dai, L.; Ni, Y. Green and sustainable cellulose-derived humidity sensors: A review. Carbohydr. Polym. 2021, 270, 118385.
- Shen, L.-L.; Zhang, G.-R.; Etzold, B.J.M. Paper-Based Microfluidics for Electrochemical Applications. ChemElectroChem 2020, 7, 10–30.
- Yang, T.; Mativetsky, J.M. Paper-Based Mechanical Sensors Enabled by Folding and Stacking. ACS Appl. Mater. Interfaces 2019, 11, 26339–26345.
- Nishat, S.; Jafry, A.T.; Martinez, A.W.; Awan, F.R. Paper-based microfluidics: Simplified fabrication and assay methods. Sens. Actuators B Chem. 2021, 336, 129681.
- Xu, Y.; Fei, Q.; Page, M.; Zhao, G.; Ling, Y.; Stoll, S.B.; Yan, Z. Paper-based wearable electronics. iScience 2021, 24, 102736.
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, V.S.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669.
- Wang, Y.; Zhou, W.; Cao, K.; Hu, X.; Gao, L.; Lu, Y. Architectured graphene and its composites: Manufacturing and structural applications. Compos. Part A Appl. Sci. Manuf. 2021, 140, 106177.
- Li, Q.; Liu, H.; Zhang, S.; Zhang, D.; Liu, X.; He, Y.; Mi, L.; Zhang, J.; Liu, C.; Shen, C.; et al. Superhydrophobic Electrically Conductive Paper for Ultrasensitive Strain Sensor with Excellent Anticorrosion and Self-Cleaning Property. ACS Appl. Mater. Interfaces 2019, 11, 21904–21914.
- Mao, K.; Min, X.; Zhang, H.; Zhang, K.; Cao, H.; Guo, Y.; Yang, Z. Paper-based microfluidics for rapid diagnostics and drug delivery. J. Control. Release 2020, 322, 187–199.
- Caspersson, T.; Schultz, J. Nucleic Acid Metabolism of the Chromosomes in Relation to Gene Reproduction. Nature 1938, 142, 294–295.
- Hisae, T.-K.; Naoki, S. Roles of non-canonical structures of nucleic acids in cancer and neurodegenerative diseases. Nucleic Acids Res. 2021, 49, 7839–7855.
- Gilboa, T.; Garden, P.M.; Cohen, L. Single-molecule analysis of nucleic acid biomarkers—A review. Anal. Chim. Acta 2020, 1115, 61–85.
- Zhao, Y.; Zuo, X.; Li, Q.; Chen, F.; Chen, Y.-R.; Deng, J.; Han, D.; Hao, C.; Huang, F.; Huang, Y.; et al. Nucleic acids analysis. Sci. China Chem. 2021, 64, 171–203.
- Pozuelo, M.; Blondeau, P.; Novell, M.; Andrade, F.J.; Rius, F.X.; Riu, J. Paper-based chemiresistor for detection of ultralow concentrations of protein. Biosens. Bioelectron. 2013, 49, 462–465.
- Fischer, C.; Fraiwan, A.; Choi, S. A 3D paper-based enzymatic fuel cell for self-powered, low-cost glucose monitoring. Biosens. Bioelectron. 2016, 79, 193–197.
- Gartia, M.R.; Misra, S.K.; Ye, M.; Schwartz-Duval, A.; Plucinski, L.; Zhou, X.; Kellner, D.; Labriola, L.T.; Pan, D. Point-of-service, quantitative analysis of ascorbic acid in aqueous humor for evaluating anterior globe integrity. Sci. Rep. 2015, 5, 16011.
- Duyen, T.T.M.; Matsuura, H.; Ujiie, K.; Muraoka, M.; Harada, K.; Hirata, K. Paper-based colorimetric biosensor for antibiotics inhibiting bacterial protein synthesis. J. Biosci. Bioeng. 2017, 123, 96–100.
- Liu, X.; Wang, P.; Zhang, C.; Ma, Z. Epidermal growth factor receptor (EGFR): A rising star in the era of precision medicine of lung cancer. Oncotarget 2017, 8, 50209–50220.
- Lin, H.; Wang, X.; Wu, J.; Li, H.; Li, F. Equipment-Free and Visualized Biosensor for Transcription Factor Rapid Assay Based on Dopamine-Functionalized Cellulose Paper. J. Mater. Chem. B 2019, 7, 5461–5464.
- Wagner, D.R.; Heyward, V.H. Measures of body composition in blacks and whites: A comparative review. Am. J. Clin. Nutr. 2000, 71, 1392–1402.
- Verhelst, X.; Dias, A.M.; Colombel, J.-F.; Vermeire, S.; Vlierberghe, H.V.; Callewaert, N.; Pinho, S.S. Protein Glycosylation as a Diagnostic and Prognostic Marker of Chronic Inflammatory Gastrointestinal and Liver Diseases. Gastroenterology 2020, 158, 95–110.
- Zhang, Y.; Deng, Y.; Yang, X.; Xue, H.; Lang, Y. The Relationship between Protein S-Nitrosylation and Human Diseases: A Review. Neurochem. Res. 2020, 45, 2815–2827.
- Ki, H.; Jang, H.; Oh, J.; Han, G.; Lee, H.; Kim, S.; Kim, M. Simultaneous Detection of Serum Glucose and Glycated Albumin on a Paper-Based Sensor for Acute Hyperglycemia and Diabetes Mellitus. Anal. Chem. 2020, 92, 11530–11534.
- Parrilla, M.; Cánovas, R.; Andrade, F.J. Paper-based enzymatic electrode with enhanced potentiometric response for moni-toring glucose in biological fluids. Biosens. Bioelectron. 2017, 90, 110–116.
- Huang, P.; Hong, C.P.; Zhu, J.F.; Chen, T.; Chan, C.; Ko, Y.; Lin, T.; Pan, Z.; Sun, N.; Wang, Y.; et al. [email protected] nanoprisms-metal organic frameworks-based paper for extending glucose sensing range in human serum and urine. Dalton Trans. 2017, 46, 6985–6993.
- Nunez-Bajo, E.; Fernández-Abedul, M.T. Paper-based platforms with coulometric readout for ascorbic acid determination in fruit juices. Analyst 2020, 145, 3431–3439.
- Sarada, B.V.; Rao, T.N.; Tryk, D.A.; Fujishima, A. Electrochemical Oxidation of Histamine and Serotonin at Highly Bo-ron-Doped Diamond Electrodes. Anal. Chem. 2000, 72, 1632–1638.
- Anh, H.Q.; Le, T.P.Q.; Le, N.D.; Lu, X.X.; Duong, T.T.; Garnier, J.; Rochelle-Newall, E.; Zhang, S.; Oh, N.-H.; Oeurng, C.; et al. Antibiotics in surface water of East and Southeast Asian countries: A focused review on contamination status, pollution sources, potential risks, and future perspectives. Sci. Total Environ. 2021, 764, 142865.
- Magureanu, M.; Bilea, F.; Bradu, C.; Hong, D. A review on non-thermal plasma treatment of water contaminated with anti-biotics. J. Hazard. Mater. 2021, 417, 125481.
- Mentana, A.; Palermo, C.; Nardiello, D.; Quinto, M.; Centonze, D. Simultaneous and accurate real time monitoring of glucose and ethanol in alcoholic drinks, must, and biomass by a dual amperometric biosensor. J. Agric. Food Chem. 2013, 61, 61–68.
- Xu, J.; Chen, X.; Khan, H.; Yang, L. A dual-readout paper-based sensor for onsite detection of penicillinase with a smartphone. Sens. Actuators B Chem. 2021, 335, 129707.
- Hidayat, M.A.; Maharani, D.A.; Purwanto, D.A.; Kuswandi, B.; Yuwono, M. Simple and Sensitive Paper-based Colorimetric Biosensor for Determining Total Polyphenol Content of the Green Tea Beverages. Biotechnol. Bioprocess Eng. 2020, 25, 255–263.
- Zhang, D.; Li, C.; Ji, D.; Wang, Y. Paper-Based Microfluidic Sensors for Onsite Environmental Detection: A Critical Review. Crit. Rev. Anal. Chem. 2021, 1886900.
- Lin, Y.; Gritsenko, D.; Feng, S.; Teh, Y.C.; Lu, X.; Xu, J. Detection of heavy metal by paper-based microfluidics. Biosens. Bioe-lectron. 2016, 83, 256–266.
- Dabhade, A.; Jayaraman, S.; Paramasivan, B. Colorimetric paper bioassay by horseradish peroxidase for the detection of cat-echol and resorcinol in aqueous samples. Prep. Biochem. Biotechnol. 2020, 50, 849–856.
- Dungchai, W.; Sameenoi, Y.; Chailapakul, O.; Volckens, J.; Henry, C.S. Determination of aerosol oxidative activity using silver nanoparticle aggregation on paper-based analytical devices. Analyst 2013, 138, 6766–6773.
- Li, Y.; Liang, F. Progress in Paper-based Colorimetric Sensor Array. Chin. J. Anal. Chem. 2020, 48, 1448–1457.
- Wang, X.; Sun, J.; Tong, J.; Guan, X.; Bian, C.; Xia, S. Paper-Based Sensor Chip for Heavy Metal Ion Detection by SWSV. Mi-cromachines 2018, 9, 150.
- Cinti, S.; Talarico, D.; Palleschi, G.; Moscone, D.; Arduini, F. Novel reagentless paper-based screen-printed electrochemical sensor to detect phosphate. Anal. Chim. Acta 2016, 919, 78–84.
- Yakoh, A.; Rattanarat, P.; Siangproh, W.; Chailapakul, O. Simple and selective paper-based colorimetric sensor for determi-nation of chloride ion in environmental samples using label-free silver nanoprisms. Talanta 2018, 178, 134–140.
- Yen, Y.; Lee, K.; Lin, C.; Zhang, S.; Wang, C.; Liu, T. Portable Nanohybrid Paper-Based Chemiresistive Sensor for Free Chlorine Detection. ACS Omega 2020, 5, 25209–25215.
- Maruthupandi, M.; Thiruppathi, D.; Vasimalai, N. One minute synthesis of green fluorescent copper nanocluster: The prep-aration of smartphone aided paper-based kit for onsite monitoring of nanomolar level mercury and sulfide ions in environ-mental samples. J. Hazard. Mater. 2020, 392, 122294.
- Liu, Y.; Hsu, C.; Lu, B.; Lin, P.; Ho, M. Determination of nitrite ions in environment analysis with a paper-based microfluidic device. Dalton Trans. 2018, 47, 14799–14807.
- Amr, A.E.E.; Kamel, A.H.; Almehizia, A.A.; Sayed, A.Y.A.; Elsayed, E.A.; Abd-Rabboh, H.S.M. Paper-Based Potentiometric Sensors for Nicotine Determination in Smokers’ Sweat. ACS Omega 2021, 6, 11340–11347.
- Yu, Y.; Pan, D.; Qiu, S.; Ren, L.; Huang, S.; Liu, R.; Wang, L.; Wang, H. Polyphenylene sulfide paper-based sensor modified by Eu-MOF for efficient detection of Fe3+. React. Funct. Polym. 2021, 165, 104954.
- Prabpal, J.; Vilaivan, T.; Praneenararat, T. Paper-Based Heavy Metal Sensors from the Concise Synthesis of an Anionic Por-phyrin: A Practical Application of Organic Synthesis to Environmental Chemistry. J. Chem. Educ. 2017, 94, 1137–1142.
- Qi, J.; Li, B.; Wang, X.; Zhang, Z.; Wang, Z.; Han, J.; Chen, L. Three-dimensional paper-based microfluidic chip device for multiplexed fluorescence detection of Cu2+ and Hg2+ ions based on ion imprinting technology. Sens. Actuators B Chem. 2017, 251, 224–233.
- Ding, R.; Cheong, Y.H.; Ahamed, A.; Lisak, G. Heavy Metals Detection with Paper-Based Electrochemical Sensors. Anal. Chem. 2021, 93, 1880–1888.
