Probiotics are defined as those microorganisms that, when administered in sufficient quantities, confer a health benefit. Some pathologies in which dysbiosis is present and the therapeutic role of probiotics has been explored are atopic dermatitis, psoriasis and acne.
- probiotic
- skin microbiome
- gut dysbiosis
- atopic dermatitis
- acne
- psoriasis
- microbiota
1. Introduction
Probiotics are defined as those microorganisms that, when administered in sufficient quantities, confer a health benefit [1]. Some pathologies in which dysbiosis is present and the therapeutic role of probiotics has been explored are atopic dermatitis, psoriasis and acne [2]. The pathophysiology in each of them, the hypotheses about the role of gut and skin dysbiosis and possible mechanisms of action of some different strains of probiotics will be reviewed in this article. To find the information and references included in this systematic review, all authors searched electronic literature databases (mainly https://pubmed.ncbi.nlm.nih.gov and https://europepmc.org/ , accessed on 4 July 2021) and proposed a total of 210 articles. All these articles were revised by one author, who contacted some experts for more information on the topic and finally made the decision about the references to be included in this entry.
The skin is the organ with the largest surface area in the human body. It serves to separate and protect us from the environment and one of its main functions is to serve as a physical barrier against external agents. Its ecosystem is made up of diverse habitats that harbor a large number of saprophytic microorganisms, including bacteria, fungi, and viruses, as well as some mites. Many of them are harmless or may even perform beneficial functions for the individual. For example, they help protect us against the invasion of pathogenic organisms through their settlement in different epithelial niches and also have an important role in the maduration of skin T cells [3][4].
The skin microbiota is made up of four main bacterial phyla: Actinobacteria, Firmicutes, Proteobacteria, and Bacteroidetes, and of more than 40 identified bacterial genera. Depending on the body area and the individual themself, the proportions of these vary. In sebaceous areas, the genus Propionibacterium predominates, while Staphylococcus and Corynebacterium are more abundant in areas with moist skin. Gram-negative bacterial genera represent the majority in dry skin [5].
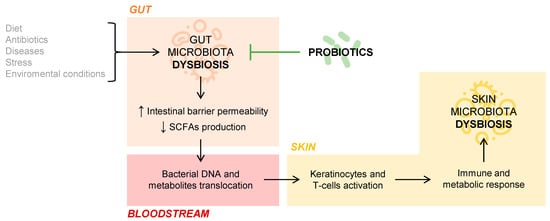
But what is the role of the gut microbiota in cutaneous homeostasis? Several studies document the immunological and metabolic impact of the intestinal microbiota on other organs of the body, including the skin, through the mechanisms of action of commensal bacteria and their metabolites [6]. If an intestinal dysbiosis occurs, that is, a loss of balance in the individual’s habitual microbial composition, the intestinal barrier may be affected so that it increases its permeability and, thus, a bacterial and intestinal metabolite translocation into the bloodstream is possible [7]. This fact has been observed in patients with psoriasis, in whom intestinal bacterial DNA has been isolated in blood samples when they present disease activity [8]. The SCFAs propionate, acetate, and butyrate, coming from the intestinal fermentation of dietary fiber, are decisive in the fact that the phenomenon of bacterial translocation appears. Those patients who have an intestinal microbiome rich in bacteria that produce these SCFAs have a lower tendency to suffer bacterial translocation phenomena. This phenomenon may be partly responsible for the interconnection between the intestinal and skin microbiota, conditioning the composition of the skin’s own microbiota, as this DNA and bacterial metabolites of intestinal origin present in the blood act on keratinocytes and skin T cells. Ultimately, this activation would provoke an immune and metabolic response of the skin, which would affect the microbial composition of this organ itself [7][9]. The connection between gut and skin microbiota is represented in Figure 1 .
2. Atopic Dermatitis
3. Psoriasis
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514.
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut-Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021, 9, 353.
- Ellis, S.R.; Nguyen, M.; Vaughn, A.R.; Notay, M.; Burney, W.A.; Sandhu, S.; Sivamani, R.K. The Skin and Gut Microbiome and Its Role in Common Dermatologic Conditions. Microorganisms 2019, 7, 550.
- Salem, I.; Ramser, A.; Isham, N.; Ghannoum, M.A. The Gut Microbiome as a Major Regulator of the Gut-Skin Axis. Front. Microbiol. 2018, 9, 1459.
- Sebastian Domingo, J.J.; Sanchez Sanchez, C. From the intestinal flora to the microbiome. Rev. Esp. Enferm. Dig. 2018, 110, 51–56.
- Polkowska-Pruszynska, B.; Gerkowicz, A.; Krasowska, D. The gut microbiome alterations in allergic and inflammatory skin diseases-an update. J. Eur. Acad. Derm. Venereol. 2020, 34, 455–464.
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587.
- Ramirez-Bosca, A.; Navarro-Lopez, V.; Martinez-Andres, A.; Such, J.; Frances, R.; Horga de la Parte, J.; Asin-Llorca, M. Identification of Bacterial DNA in the Peripheral Blood of Patients with Active Psoriasis. JAMA Dermatol. 2015, 151, 670–671.
- Ning, L.; Lifang, P.; Huixin, H. Prediction Correction Topic Evolution Research for Metabolic Pathways of the Gut Microbiota. Front. Mol. Biosci. 2020, 7, 600720.
- Ahn, C.; Huang, W. Clinical Presentation of Atopic Dermatitis. Adv. Exp. Med. Biol. 2017, 1027, 39–46.
- Cheok, S.; Yee, F.; Song Ma, J.Y.; Leow, R.; Ho, M.S.L.; Yew, Y.W.; Tay, Y.K.; Rebello, S.A.; Luo, N.; Koh, M.J.A. Prevalence and descriptive epidemiology of atopic dermatitis and its impact on quality of life in Singapore. Br. J. Dermatol. 2018, 178, 276–277.
- Bin, L.; Leung, D.Y. Genetic and epigenetic studies of atopic dermatitis. Allergy Asthma Clin. Immunol. 2016, 12, 52.
- Justiz Vaillant, A.A.; Modi, P.; Jan, A. Atopy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK542187/ (accessed on 5 June 2021).
- David Boothe, W.; Tarbox, J.A.; Tarbox, M.B. Atopic Dermatitis: Pathophysiology. Adv. Exp. Med. Biol. 2017, 1027, 21–37.
- Liu, F.T.; Goodarzi, H.; Chen, H.Y. IgE, mast cells, and eosinophils in atopic dermatitis. Clin. Rev. Allergy Immunol. 2011, 41, 298–310.
- Hanifin, J.M.; Rajka, G. Diagnostic Features of Atopic-Dermatitis. Acta Derm. Venereol. 1980, 92, 44–47.
- Silverberg, J.I.; Vakharia, P.P.; Chopra, R.; Sacotte, R.; Patel, N.; Immaneni, S.; White, T.; Kantor, R.; Hsu, D.Y. Phenotypical Differences of Childhood- and Adult-Onset Atopic Dermatitis. J. Allergy Clin. Immunol. Pract. 2018, 6, 1306–1312.
- Stalder, J.F.; Taïeb, A.; Atherton, D.J.; Bieber, P.; Bonifazi, E.; Broberg, A.; Calza, A.; Coleman, Y.; De Prost, J.F.; Stalder, C.; et al. Severity scoring of atopic dermatitis: The SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology 1993, 186, 23–31.
- Chopra, R.; Vakharia, P.P.; Sacotte, R.; Patel, N.; Immaneni, S.; White, T.; Kantor, R.; Hsu, D.Y.; Silverberg, J.I. Severity strata for Eczema Area and Severity Index (EASI), modified EASI, Scoring Atopic Dermatitis (SCORAD), objective SCORAD, Atopic Dermatitis Severity Index and body surface area in adolescents and adults with atopic dermatitis. Br. J. Dermatol. 2017, 177, 1316–1321.
- Futamura, M.; Leshem, Y.A.; Thomas, K.S.; Nankervis, H.; Williams, H.C.; Simpson, E.L. A systematic review of Investigator Global Assessment (IGA) in atopic dermatitis (AD) trials: Many options, no standards. J. Am. Acad. Dermatol. 2016, 74, 288–294.
- Azizan, N.Z.; Ambrose, D.; Sabeera, B.; Mohsin, S.S.; Pf, W.; Mohd Affandi, A.; Cc, C.; Gopinathan, L.P.; Taib, T.; Tan, W.C.; et al. Management of Atopic Eczema in primary care. Malays. Fam. Physician 2020, 15, 39–43.
- Wollenberg, A.; Barbarot, S.; Bieber, T.; Christen-Zaech, S.; Deleuran, M.; Fink-Wagner, A.; Gieler, U.; Girolomoni, G.; Lau, S.; Muraro, A.; et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: Part II. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 850–878.
- Boehncke, W.H.; Schon, M.P. Psoriasis. Lancet 2015, 386, 983–994.
- Dopytalska, K.; Sobolewski, P.; Blaszczak, A.; Szymanska, E.; Walecka, I. Psoriasis in special localizations. Reumatologia 2018, 56, 392–398.
- Parisi, R.; Symmons, D.P.; Griffiths, C.E.; Ashcroft, D.M. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J. Investig. Dermatol. 2013, 133, 377–385.
- Rendon, A.; Schakel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475.
- Mattei, P.L.; Corey, K.C.; Kimball, A.B. Psoriasis Area Severity Index (PASI) and the Dermatology Life Quality Index (DLQI): The correlation between disease severity and psychological burden in patients treated with biological therapies. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 333–337.
- Gaffen, S.L.; Jain, R.; Garg, A.V.; Cua, D.J. The IL-23-IL-17 immune axis: From mechanisms to therapeutic testing. Nat. Rev. Immunol. 2014, 14, 585–600.
- Kim, W.B.; Jerome, D.; Yeung, J. Diagnosis and management of psoriasis. Can. Fam. Physician 2017, 63, 278–285.
