You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 4 by Conner Chen and Version 3 by Dean Liu.
TATI, also known as pancreatic secretory trypsin inhibitor (PSTI) or serine peptidase inhibitor Kazal 1 type (SPINK1), is a trypsin inhibitor that functions mainly in the pancreas, where it serves as a suppressor of premature trypsinogen activation.
- OPSCC
- HPV
- TATI
- survival
1. Introduction
The incidence of OPSCC has been increasing in recent years, particularly in Western countries [1]. Although over 100,000 new cases of oropharyngeal cancers are diagnosed yearly worldwide, the majority is diagnosed in developed countries [2][3]. Squamous cell carcinomas form over 90% of the newly diagnosed oropharyngeal cancers [2][3][4][5][6]. The median overall 5-year survival in Finland is 64% among men and 70% among women [7]. The most significant risk factors for OPSCC are smoking, heavy alcohol use, and HPV infection. Today, more than half of the new OPSCC cases are associated with HPV [8] and HPV-positive OPSCC and HPV-negative OPSCC are considered as separate disease entities. In HPV-positive OPSCC, the symptom profile and tumor characteristics differ from HPV-negative OPSCC, and the treatment response and the prognosis are usually substantially more favorable in HPV-related disease [9][10][11]. The prognosis for HPV-negative OPSCC remains relatively poor [12][13], and the explanation for poor survival rates remains unclear. To improve treatments and diagnostics, it is important to discover novel information on previously undiscovered prognostic factors and mechanisms affecting survival.
Various biomarkers have previously been associated with OPSCC, such as p16 [9][14][15]. Other potential biomarkers with possible prognostic value in OPSCC include Cyclin D1 and matrix metalloproteinases 1 and 2 [16][17][18][19]. Furthermore, recent studies have shown that HPV16 E6 and E7 serum antibody levels may predict OPSCC in advance [20][21]. However, to our knowledge there are currently no prognostic biomarkers or diagnostic serological biomarkers that are used for individualization of treatments for head and neck squamous cell cancers. Thus, further research on promising biomarkers is warranted. This study is focused on a biomarker known as tumor-associated trypsin inhibitor (TATI), which is associated with multiple malignancies, but has rarely been studied in oropharyngeal cancer [22][23].
TATI, also known as pancreatic secretory trypsin inhibitor (PSTI) or serine peptidase inhibitor Kazal 1 type (SPINK1), is a trypsin inhibitor that functions mainly in the pancreas, where it serves as a suppressor of premature trypsinogen activation [24]. The mechanisms regulating extrapancreatic TATI secretion are only partially known. The reference range of TATI concentration in serum (S-TATI) is 3.2–16 μg/L in healthy individuals [25]. S-TATI has been shown to increase in several non-malignant conditions, such as acute pancreatitis and various other severe inflammatory diseases. Elevated secretion of TATI in cancer patients was first found in the urine of patients with ovarian cancer [26][27] and it has since been detected in serum and tumor tissue in various malignancies [25].
In addition to the role if TATI as a diagnostic tumor marker, it may have value as a prognostic marker [28], and as a target for cancer treatment [29].
2. TATI Serum Concentrations and Clinical Characteristics
S-TATI was determined in 90 serum samples. Based on a selected cut-off value of 17 µg/L, 21 (23.3%) of the patients were considered TATI positive and 69 (76.7%) S-TATI negative. The crosstab comparisons of S-TATI according to clinical parameters are presented in Table 1.Table 1. Clinicopathological data according to S-TATI.
| Variable | S-TATI− | % | S-TATI+ | % | p-Value | Missing/% (n = 90) |
|---|---|---|---|---|---|---|
| 76.1 | ||||||
| 0.6 | ||||||
Abbreviations: TATI: tumor-associated trypsin inhibitor; HPV: Human papillomavirus, TATI immunoexpression was scored in the tumor tissue, TATI in tumor 0–1: mild positivity, TATI in tumor 2–3: moderate-strong positivity, p < 0.05 *.
Table 4. Clinicopathological data according to TATI expression in tumor-adjacent lymphocytes.
| Variable | TATI in Lymphocytes 0–1 | % | TATI in Lymphocytes 2–3 | % | p-Value | Missing/% (n = 76) |
|---|---|---|---|---|---|---|
| Number of patients | 69 | 76.7 | 21 | 23.3 | ||
| Age | 60.8 | 65.1 | 0.054 | |||
| Gender | ||||||
| Male | 52 | 78.8 | 14 | 21.2 | ||
| Female | 17 | 70.8 | 7 | 29.2 | 0.4 | |
| Smoking | ||||||
| Never | 27 | 96.4 | 1 | 3.6 | ||
| Former | 25 | |||||
| Posterior wall of oropharynx | ||||||
| 1 | ||||||
| 20.0 | ||||||
| 4 | ||||||
| 80.0 | ||||||
| <0.001 ** | ||||||
| HPV | ||||||
| HPV− | 21 | 56.8 | 16 | 43.2 | ||
| HPV+ | 48 | 90.6 | 5 | 9.4 | <0.001 ** |
Abbreviations: TATI: Tumor-associated trypsin inhibitor; HPV: Human papillomavirus. p < 0.05 *, p < 0.001 **.
The correlation between S-TATI and HPV status was statistically significant (p < 0.001). Most (90.60%) of the HPV-positive patients were S-TATI negative. Furthermore, S-TATI negativity was linked to lower cancer stage and higher histological grade (82.5% of the patients had cancer stages I-II and 83.3% of the patients were grade III). In addition, S-TATI negativity correlated with tonsil as tumor site (90.6% of the patients).
A majority (96.4%) of the non-smokers were S-TATI negative. Additionally, most (83.3%) of the former smokers were S-TATI negative. Among the smokers, 53.3% were S-TATI negative and 46.6% were S-TATI positive.
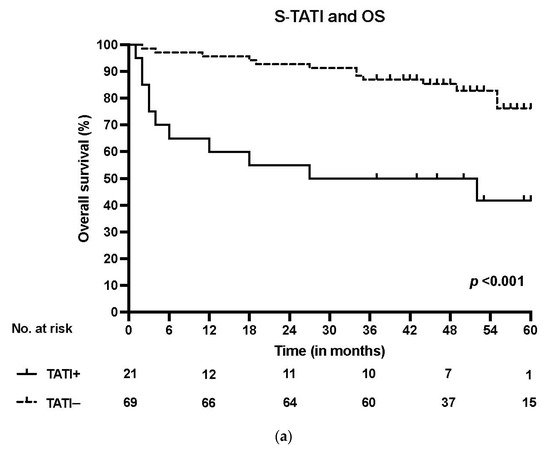
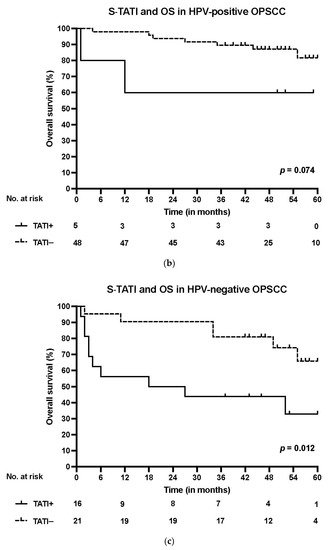
S-TATI positive patients had reduced OS during the 5-year follow-up and S-TATI positivity was linked to poor OS (p < 0.001) and DSS (p = 0.04) in the whole cohort. Furthermore, S-TATI positivity correlated with poor OS (p = 0.01) and DSS (p = 0.05) in HPV-negative patients and with poor DSS (p = 0.01) in HPV-positive patients. The survival curves according to S-TATI and OS are presented in Figure 1.
 In the multivariate Cox regression analysis, poorer OS correlated significantly with age (adjusted Hazard ratio (HR) 1.08, 95% Confidence interval (CI) 0.03–1.13, p = 0.004) and S-TATI (adjusted HR 2.47, 95% CI 1.26–4.84, p = 0.009). In addition, poorer DSS was associated with age (adjusted HR 1.07, 95% CI 0.01–1.14, p = 0.018) and S-TATI (adjusted HR 2.54, 95% CI 1.07–6.02, p = 0.034) in the Cox regression multivariate model. The results of the multivariable analysis are presented in Table 2. In the multivariate Cox regression analysis with p16 as a separate variable, S-TATI correlated significantly with OS (adjusted HR 2.49, 95% CI 1.28–4.83, p = 0.007) and DSS (adjusted HR 2.54, 95% CI 1.09–5.94, p = 0.031) and the results are presented in Table S2.
In the multivariate Cox regression analysis, poorer OS correlated significantly with age (adjusted Hazard ratio (HR) 1.08, 95% Confidence interval (CI) 0.03–1.13, p = 0.004) and S-TATI (adjusted HR 2.47, 95% CI 1.26–4.84, p = 0.009). In addition, poorer DSS was associated with age (adjusted HR 1.07, 95% CI 0.01–1.14, p = 0.018) and S-TATI (adjusted HR 2.54, 95% CI 1.07–6.02, p = 0.034) in the Cox regression multivariate model. The results of the multivariable analysis are presented in Table 2. In the multivariate Cox regression analysis with p16 as a separate variable, S-TATI correlated significantly with OS (adjusted HR 2.49, 95% CI 1.28–4.83, p = 0.007) and DSS (adjusted HR 2.54, 95% CI 1.09–5.94, p = 0.031) and the results are presented in Table S2.


Figure 1. (a) Overall survival (OS) according to positive (>17 µg/L) and negative (<17 µg/L) S-TATI in the whole patient cohort. (b) Overall survival (OS) according to positive and negative S-TATI in HPV-positive OPSCC patients. (c) Overall survival (OS) according to positive and negative S-TATI in HPV-negative OPSCC patients.
Table 2. Multivariate Cox regression analysis for overall-survival (OS) and disease-specific survival (DSS).
| Variable | OS | Tumor 0–1 | DSS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | TATI in | Tumor 2–3 | % | p-Value | Missing/% ( |
HR | 95% CI | p-Value | HR | 95% CI | p-Value | ||
| n | = 77) | ||||||||||||
| Age | 1.08 | 1.03–1.13 | |||||||||||
| Number of patients | 20 | 26.0 | 0.004 * | 1.07 | 1.01–1.14 | 0.018 * | |||||||
| 57 | 63.3 | ||||||||||||
| Number of patients | 30 | 39.5 | 46 | 60.5 | |||||||||
| Smoking | |||||||||||||
| Age | 64.4 | 0.034 * | 0.205 | ||||||||||
| 61.1 | 0.2 | ||||||||||||
| Age | 62.6 | 61.3 | 0.6 | Ex-smoker versus never | 1.20 | 0.27–5.40 | 0.816 | 0.62 | |||||
| Gender | 0.10–3.98 | 0.615 | |||||||||||
| Gender | Current smoker versus never | 4.14 | 1.22–14.06 | 0.023 * | |||||||||
| Male | 15 | 25.4 | 44 | 2.43 | 0.64–9.18 | 0.192 | |||||||
| 74.6 | |||||||||||||
| Male | 23 | 39.7 | 35 | 60.3 | Stage III–IVversusStage I–II | 1.71 | 0.70–4.20 | 0.243 | 2.19 | 0.72–6.64 | 0.168 | ||
| HPV-versus HPV+ | 1.01 | 0.36–2.83 | 0.988 | 0.96 | 0.27–3.43 | 0.955 | |||||||
| S-TATI | 2.47 | 1.26–4.84 | 0.009 * | 2.54 | 1.07–6.02 | 0.034 * | 83.3 | 5 | |||||
HR: Hazard ratio; CI: Confidence interval. S-TATI values are log-transformed. p < 0.05 *.
3. TATI Immunoexpression and Clinical Characteristics
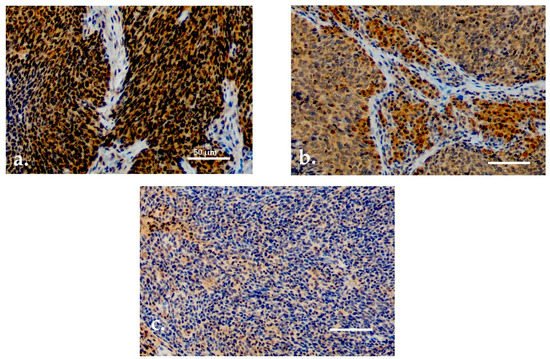
Both serum and tissue data were available for 90 patients. For TATI IHC, adequate samples with HPV status determination were available for 77 (85.6%) patients. TATI was assessed in the tumor tissue in all 77 samples and in the tumor-adjacent lymphocytes in 76 samples (Figure 2a–c). Most of the samples showed moderate or strong IHC staining of TATI in both the tumor (63.3%) and in the peritumoral lymphocytes (60.5%). TATI-immunopositivity was cytoplasmic. TATI expression in IHC was associated with certain patient characteristics but not with S-TATI. Crosstab comparisons of TATI IHC and clinical characteristics are presented in Table 3 and Table 4.
Figure 2. (a) Moderate/strong TATI expression in tumor tissue. (b) Moderate/strong TATI expression in peritumoral lymphocytes and mild expression in the tumor tissue. (c) Negative TATI expression in the tumor tissue. Scale bar length 50 µm. Magnification ×200.
Table 3. Clinicopathological data according to TATI expression in tumor tissue.
| Variable | TATI in | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | 5 | 27.8 | 13 | 72.2 | 0.8 | ||||||||||||||
| Female | 7 | 38.9 | 11 | 61.1 | 0.9 | Smoking | |||||||||||||
| Smoking | Never | 8 | 33.3 | 16 | 66.7 | ||||||||||||||
| Never | 10 | 43.5 | 13 | 56.5 | 16.7 | ||||||||||||||
| Former | 7 | 28.0 | 18 | 72.0 | Current | 17 | 53.1 | 15 | 46.9 | <0.001 ** | |||||||||
| Current | 5 | 17.9 | 23 | 82.1 | 0.4 | Heavy alcohol use | |||||||||||||
| Heavy alcohol use | 16/17.8 | ||||||||||||||||||
| 14/18.2 | Never | 36 | 83.7 | 7 | 16.3 | ||||||||||||||
| Never | 14 | 35.9 | 25 | 64.1 | Former | 6 | 60.0 | 4 | 40.0 | ||||||||||
| Former | 1 | 16.7 | 5 | 83.3 | Current | 13 | 61.9 | 8 | 38.1 | 0.09 | |||||||||
| Current | |||||||||||||||||||
| Former | 5 | 20.0 | 20 | 80.0 | |||||||||||||||
| Current | 15 | 53.6 | 13 | 46.4 | 0.04 * | 4 | 22.2 | 14 | 77.8 | 0.4 | T class | ||||||||
| T class | T1-T2 | 46 | 51.1 | 12 | 13.3 | ||||||||||||||
| T1–T2 | 11 | 22.4 | 38 | 77.6 | T3-T4 | 23 | 25.6 | 9 | 10.0 | 0.4 | |||||||||
| T3–T4 | 9 | 32.1 | |||||||||||||||||
| Heavy alcohol use | 14/18.4 | 19 | |||||||||||||||||
| Never | 17 | 44.7 | 21 | 55.3 | |||||||||||||||
| Former | 1 | 16.7 | 5 | 83.3 | |||||||||||||||
| Current | 9 | 50.0 | 9 | 50.0 | 0.4 | ||||||||||||||
| T Class | 67.9 | ||||||||||||||||||
| T1–T2 | 14 | 28.6 | 35 | 71.4 | 0.4 | ||||||||||||||
| T3–T4 | 16 | 59.3 | 11 | 40.7 | 0.009 * | N class | |||||||||||||
| N class | |||||||||||||||||||
| N Class | N0–N1 | 59 | 79.7 | 15 | 20.3 | ||||||||||||||
| N0–N1 | 12 | 19.0 | 51 | 81.0 | |||||||||||||||
| N0–N1 | 23 | 36.5 | 40 | 63.5 | N2–N3 | 10 | 62.5 | 6 | 37.4 | 0.2 | |||||||||
| 42.9 | Stage | ||||||||||||||||||
| I-II | 52 | 82.5 | 11 | ||||||||||||||||
| N2–N3 | 8 | 57.1 | 0.006 * | ||||||||||||||||
| N2–N3 | 7 | 53.8 | 6 | 46.2 | 0.2 | Stage | |||||||||||||
| Stage | 17.5 | ||||||||||||||||||
| I–II | 9 | 17.0 | 44 | 83.0 | |||||||||||||||
| I–II | 16 | 30.2 | 37 | 69.8 | III-IV | 17 | 63.0 | 10 | 37.0 | 0.04 * | |||||||||
| III–IV | 11 | 45.8 | 13 | 54.1 | 0.007 * | ||||||||||||||
| Grade | |||||||||||||||||||
| III–IV | 14 | 60.9 | 9 | Grade | |||||||||||||||
| 39.1 | 0.01 * | I | |||||||||||||||||
| Grade | I | 1 | 33.3 | 2 | 66.7 | ||||||||||||||
| 0 | 0.0 | 2 | 100.0 | ||||||||||||||||
| I | 1 | 50.0 | 1 | 50.0 | II | 8 | 53.3 | 7 | 46.7 | ||||||||||
| 6 | II | 3 | 23.0 | III | 60 | ||||||||||||||
| II | 6 | 46.2 | 10 | 77.0 | 7 | ||||||||||||||
| 53.8 | 83.3 | 12 | 16.7 | 0.009 * | |||||||||||||||
| III | 17 | 27.4 | 45 | 72.6 | 0.7 | Localization | |||||||||||||
| III | Localization | ||||||||||||||||||
| 23 | 37.7 | 38 | 62.3 | 0.8 | |||||||||||||||
| Localization | |||||||||||||||||||
| Tonsil | 48 | 90.6 | 5 | 9.4 | |||||||||||||||
| Tonsil | 10 | 21.7 | 36 | 78.3 | |||||||||||||||
| Tonsil | 15 | 32.6 | 31 | 67.4 | |||||||||||||||
| Base of tongue | Base of tongue17 | 677.3 | 35.35 | 1122.7 | 64.7 | ||||||||||||||
| Soft palate | |||||||||||||||||||
| Soft palate | |||||||||||||||||||
| Base of tongue | 6 | 37.5 | 10 | 62.5 | 3 | 30.0 | 7 | 70.0 | 3 | 33.3 | 6 | 66.7 | |||||||
| Soft palate | 6 | 66.7 | 3 | 33.3 | Posterior wall of oropharynx | 1 | 20.0 | 4 | 80.0 | 0.7 | |||||||||
| Posterior wall of oropharynx | 3 | 60.0 | 2 | 40.0 | 0.2 | HPV | |||||||||||||
| HPV | HPV− | 9 | 29.0 | 22 | 71.0 | ||||||||||||||
| HPV− | 18 | 60.0 | 12 | 40.0 | HPV+ | 11 | 23.9 | 35 | |||||||||||
| HPV+ | 12 | 33.3 | 34 | 66.6 | 0.003 * |
Abbreviations: TATI: Tumor-associated trypsin inhibitor; HPV: Human papillomavirus, TATI immunoexpression was scored in the inflammatory cells adjacent to the tumor tissue, TATI in lymphocytes 0–1: mild positivity, TATI in lymphocytes 2–3: moderate-strong positivity, p < 0.05 *.
Moderate or strong expression of TATI in tumor tissue and in peritumoral lymphocytes (Figure 2a,b) was observed in 83% and 69.8% of patients with stage I–II disease, respectively. Expression of TATI in tumor tissue did not significantly correlate with other clinical parameters. However, elevated expression of TATI in peritumoral lymphocytes was linked to HPV positivity (66.6%) and lower T class (71.4%). In addition, patients with moderate or strong expression of TATI in peritumoral lymphocytes had a favorable OS (p = 0.025). However, this result was only observed in the whole patient cohort and the comparisons between HPV status and survival was not statistically significant. No correlation was seen between DSS and TATI tissue expression.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018, GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316.
- Lambert, R.; Sauvaget, C.; de Camargo Cancela, M.; Sankaranarayanan, R. Epidemiology of cancer from the oral cavity and oropharynx. Eur. J. Gastroenterol. Hepatol. 2011, 23, 633–641.
- Sinevici, N.; O’sullivan, J. Oral cancer: Deregulated molecular events and their use as biomarkers. Oral Oncol. 2016, 61, 12–18.
- Koneva, L.A.; Zhang, Y.; Virani, S.; Hall, P.B.; McHugh, J.B.; Chepeha, D.B.; Wolf, G.T.; Carey, T.E.; Rozek, L.S.; Sartor, M.A. HPV Integration in HNSCC Correlates with Survival Outcomes, Immune Response Signatures, and Candidate Drivers. Mol. Cancer Res. 2018, 16, 90–102.
- Gooi, Z.; Chan, J.Y.K.; Fakhry, C. The epidemiology of the human papillomavirus related to oropharyngeal head and neck cancer. Laryngoscope 2016, 126, 894–900.
- Pitkäniemi, J.; Malila, N.; Virtanen, A.; Degerlund, H.; Heikkinen, S.; Seppä, K. Syöpä 2018. Tilastoraportti Suomen Syöpätilanteesta; Publication no. 93 of Cancer Society of Finland; Cancer Society of Finland: Helsinki, Finland, 2020; Available online: (accessed on 3 June 2021).
- Chaturvedi, A.K.; Anderson, W.F.; Lortet-Tieulent, J.; Curado, M.P.; Ferlay, J.; Franceschi, S.; Rosenberg, P.S.; Bray, F.; Gillison, M.L. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J. Clin. Oncol. 2013, 31, 4550–4559.
- Gillison, M.L.; D’Souza, G.; Westra, W.; Sugar, E.; Xiao, W.; Begum, S.; Viscidi, L. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J. Natl. Cancer Inst. 2008, 100, 407–420.
- Ramqvist, T.; Dalianis, T. An epidemic of oropharyngeal squamous cell carcinoma (OSCC) due to human papillomavirus (HPV) infection and aspects of treatment and prevention. Anticancer Res. 2011, 31, 1515–1519.
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35.
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Natl. Cancer Inst. 2008, 100, 261–269.
- Elrefaey, S.; Massaro, M.A.; Chiocca, S.; Chiesa, F.; Ansarin, M. HPV in oropharyngeal cancer: The basics to know in clinical practice. Acta Otorhinolaryngol. Ital. 2014, 34, 299–309.
- Sato, F.; Ono, T.; Kawahara, A.; Kawaguchi, T.; Tanaka, H.; Shimamatsu, K.; Kakuma, T.; Akiba, J.; Umeno, H.; Yano, H. Prognostic impact of p16 and PD-L1 expression in patients with oropharyngeal squamous cell carcinoma receiving a definitive treatment. J. Clin. Pathol. 2019, 72, 542–549.
- Adelstein, D.J.; Ismaila, N.; Ku, J.A.; Burtness, B.; Swiecicki, P.L.; Mell, L.; Beitler, J.J.; Gross, N.; Jones, C.U.; Kaufman, M.; et al. Role of Treatment Deintensification in the Management of p16+ Oropharyngeal Cancer: ASCO Provisional Clinical Opinion. J. Clin. Oncol. 2019, 37, 1578–1589.
- Grimminger, C.M.; Danenberg, P.V. Update of prognostic and predictive biomarkers in oropharyngeal squamous cell carcinoma: A review. Eur. Arch. Otorhinolaryngol. 2011, 268, 5–16.
- Vento, S.I.; Jouhi, L.; Mohamed, H.; Haglund, C.; Mäkitie, A.A.; Atula, T.; Hagström, J.; Mäkinen, L.K. MMP-7 expression may influence the rate of distant recurrences and disease-specific survival in HPV-positive oropharyngeal squamous cell carcinoma. Virchows Arc. 2018, 472, 975–981.
- Umbreit, C.; Erben, P.; Faber, A.; Hofheinz, R.-D.; Aderhold, C.; Weiss, C.; Hoermann, K.; Wenzel, A.; Schultz, J.D. MMP9, Cyclin D1 and β-Catenin Are Useful Markers of p16-positive Squamous Cell Carcinoma in Therapeutic EGFR Inhibition In Vitro. Anticancer Res. 2015, 35, 3801–3810.
- Plath, M.; Broglie, M.A.; Förbs, D.; Stoeckli, S.J.; Jochum, W. Prognostic significance of cell cycle-associated proteins p16, pRB, cyclin D1 and p53 in resected oropharyngeal carcinoma. J. Otolaryngol. Head Neck Surg. 2018, 47, 53.
- Lang Kuhs, K.A.; Wood, C.B.; Wiggleton, J.; Aulino, J.M.; Latimer, B.; Smith, D.K.; Bender, N.; Rohde, S.; Mannion, K.; Kim, Y.; et al. Transcervical sonography and human papillomavirus 16 E6 antibodies are sensitive for the detection of oropharyngeal cancer. Cancer 2020, 126, 2658–2665.
- Ren, J.; Xu, W.; Su, J.; Ren, X.; Cheng, D.; Chen, Z.; Bender, N.; Mirshams, M.; Habbous, S.; De Almeida, J.R.; et al. Multiple imputation and clinico-serological models to predict human papillomavirus status in oropharyngeal carcinoma: An alternative when tissue is unavailable. Int. J. Cancer 2020, 146, 2166–2174.
- Rivera, C.; Oliveira, A.K.; Costa, R.A.P.; De Rossi, T.; Paes Leme, A.F. Prognostic biomarkers in oral squamous cell carcinoma: A systematic review. Oral Oncol. 2017, 72, 38–47.
- Almangush, A.; Heikkinen, I.; Mäkitie, A.A.; Coletta, R.D.; Läärä, E.; Leivo, I.; Salo, T. Prognostic biomarkers for oral tongue squamous cell carcinoma: A systematic review and meta-analysis. Br. J. Cancer 2017, 117, 856–866.
- Itkonen, O.; Stenman, U.-H. TATI as a biomarker. Clin. Chim. Acta 2014, 431, 260–269.
- Räsänen, K.; Itkonen, O.; Koistinen, H.; Stenman, U.-H. Emerging Roles of SPINK1 in Cancer. Clin. Chem. 2016, 62, 449–457.
- Eddeland, A.; Ohlsson, K. A radioimmunoassay for measurement of human pancreatic secretory trypsin inhibitor in different body fluids. Hoppe Seylers Z. Physiol. Chem. 1978, 359, 671–675.
- Stenman, U.H.; Huhtala, M.L.; Koistinen, R.; Seppälä, M. Immunochemical demonstration of an ovarian cancer-associated urinary peptide. Int. J. Cancer 1982, 30, 53–57.
- Gaber, A.; Nodin, B.; Hotakainen, K.; Nilsson, E.; Stenman, U.-H.; Bjartell, A.; Birgisson, H.; Jirström, K. Increased serum levels of tumour-associated trypsin inhibitor independently predict a poor prognosis in colorectal cancer patients. BMC Cancer 2010, 10, 498.
- Chen, F.; Long, Q.; Fu, D.; Zhu, D.; Ji, Y.; Han, L.; Zhang, B.; Xu, Q.; Liu, B.; Li, Y.; et al. Targeting SPINK1 in the damaged tumour microenvironment alleviates therapeutic resistance. Nat. Commun. 2018, 9, 4315.
More
