The use of biological templates for the suitable growth of adipose-derived mesenchymal stem cells (AD-MSC) and “neo-tissue” construction has exponentially increased over the last years. The bioengineered scaffolds still have a prominent and biocompatible framework playing a role in tissue regeneration. In order to supply AD-MSCs, biomaterials, as the stem cell niche, are more often supplemented by or stimulate molecular signals that allow differentiation events into several strains, besides their secretion of cytokines and effects of immunomodulation. This systematic review aims to highlight the details of the integration of several types of biomaterials used in association with AD-MSCs, collecting notorious and basic data of in vitro and in vivo assays, taking into account the relevance of the interference of the cell lineage origin and handling cell line protocols for both the replacement and repairing of damaged tissues or organs in clinical application. Our group analyzed the quality and results of the 98 articles selected from PubMed, Scopus and Web of Science. A total of 97% of the articles retrieved demonstrated the potential in clinical applications. The synthetic polymers were the most used biomaterials associated with AD-MSCs and almost half of the selected articles were applied on bone regeneration.
- biomaterial
- stem cells
- tissue engineering
1. Introduction
1.1. Biomaterial: The Biological Generation Template
1.2. Stem Cells: Origin, Design and Differentiation
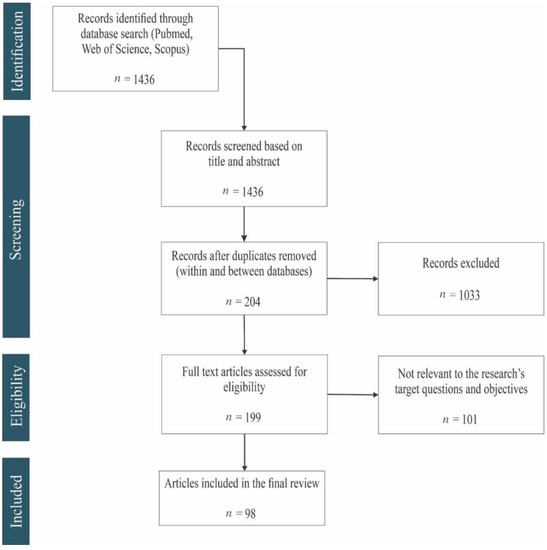
2. General Findings (Flow Diagram Results)
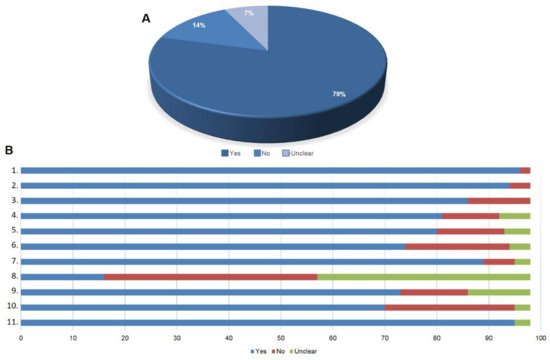
3. Quality of the Selected Articles
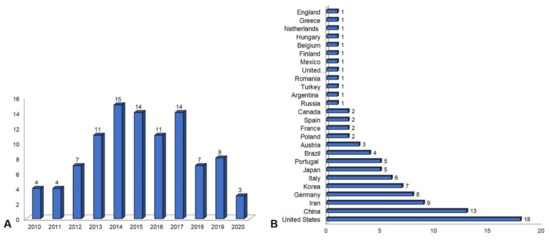
4. Publication Overview between 2010 and 2020
References
- Matichescu, A.; Ardelean, L.C.; Rusu, L.-C.; Craciun, D.; Bratu, E.A.; Babucea, M.; Leretter, M. Advanced Biomaterials and Techniques for Oral Tissue Engineering and Regeneration—A Review. Materials 2020, 13, 5303.
- Raghavendra, G.M.; Varaprasad, K.; Jayaramudu, T. Biomaterials. In Nanotechnology Applications for Tissue Engineering; Elsevier: Amsterdam, The Neterlands, 2015; pp. 21–44.
- Solanki, A.; Kim, J.D.; Lee, K.-B. Nanotechnology for regenerative medicine: Nanomaterials for stem cell imaging. Nanomedicine 2008, 3, 567–578.
- Pryjmaková, J.; Kaimlová, M.; Hubáček, T.; Švorčík, V.; Siegel, J. Nanostructured Materials for Artificial Tissue Replacements. Int. J. Mol. Sci. 2020, 21, 2521.
- Zhang, L.; Webster, T.J. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano Today 2009, 4, 66–80.
- Verma, S.; Domb, A.J.; Kumar, N. Nanomaterials for regenerative medicine. Nanomedicine 2011, 6, 157–181.
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT(R)) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024.
- Harasymiak-Krzyżanowska, I.; Niedojadło, A.; Karwat, J.; Kotuła, L.; Gil-Kulik, P.; Sawiuk, M.; Kocki, J. Adipose tissue-derived stem cells show considerable promise for regenerative medicine applications. Cell. Mol. Biol. Lett. 2013, 18, 479–493.
- Afra, S.; Matin, M.M. Potential of mesenchymal stem cells for bioengineered blood vessels in comparison with other eligible cell sources. Cell Tissue Res. 2020, 380, 1–13.
- Hentze, H.; Soong, P.L.; Wang, S.T.; Phillips, B.W.; Putti, T.C.; Dunn, N.R. Teratoma formation by human embryonic stem cells: Evaluation of essential parameters for future safety studies. Stem Cell Res. 2009, 2, 198–210.
- Brown, C.; McKee, C.; Bakshi, S.; Walker, K.; Hakman, E.; Halassy, S.; Svinarich, D.; Dodds, R.; Govind, C.K.; Chaudhry, G.R. Mesenchymal stem cells: Cell therapy and regeneration potential. J. Tissue Eng. Regen. Med. 2019, 13, 1738–1755.
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells—Current trends and future prospective. Biosci. Rep. 2015, 35.
- Wright, A.; Arthaud-Day, M.L.; Weiss, M.L. Therapeutic Use of Mesenchymal Stromal Cells: The Need for Inclusive Characterization Guidelines to Accommodate All Tissue Sources and Species. Front. Cell Dev. Biol. 2021, 9.
- Salehi-Nik, N.; Rad, M.R.; Kheiri, L.; Nazeman, P.; Nadjmi, N.; Khojasteh, A. Buccal Fat Pad as a Potential Source of Stem Cells for Bone Regeneration: A Literature Review. Stem Cells Int. 2017, 2017, 1–13.
- Cherian, D.S.; Bhuvan, T.; Meagher, L.; Heng, T.S.P. Biological Considerations in Scaling Up Therapeutic Cell Manufacturing. Front. Pharmacol. 2020, 11, 654.
- Zhang, J.; Liu, Y.; Chen, Y.; Yuan, L.; Liu, H.; Wang, J.; Liu, Q.; Zhang, Y. Adipose-Derived Stem Cells: Current Applications and Future Directions in the Regeneration of Multiple Tissues. Stem Cells Int. 2020, 2020, 1–26.
