You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 4 by Rita Xu and Version 3 by Rita Xu.
Endometriosis is a chronic condition that affects women throughout various stages of their lives by causing pain, infertility, and malignant progression.
- endometriosis
- adolescent
- premenarcheal
- obstructive Müllerian anomalies
1. Introduction
Endometriosis is a chronic condition that affects women throughout various stages of their lives by causing pain, infertility, and malignant progression. Most women with endometriosis are diagnosed after their mid-twenties. However, two-thirds of women over twenty years of age who were diagnosed with endometriosis have reported having symptoms such as dysmenorrhea or chronic pelvic pain since they were adolescents. Endometriosis is also considered a progressive disease. Therefore, early diagnosis and long-term management are especially important in younger patients to prevent disease progression and preserve fertility
[1]
. However, endometriosis in adolescents possesses several unique characteristics, which vary from those in adults and should be considered to achieve early diagnosis (
Table 1
). Endometriosis is under-recognized in adolescents, and many adolescents are hesitant to seek medical attention
[2]
. Their diagnoses are complicated due to its atypical symptom presentation. Careful pelvic ultrasound and laparoscopic studies are necessary to detect the subtle signs of endometriosis in adolescents. Moreover, medical and surgical management should be tailored to fit the developmental stage and future plans of adolescents.
Table 1.
Characteristics of adolescent endometriosis.
| Symptoms | —Atypical | ||||
| More often acyclic pain | [3] | ||||
| Frequently resistant to combined oral contraceptive (COC) and NSAIDs | [3] | ||||
| Findings | —Lesions less evident | ||||
| Ultrasound | |||||
| Small endometriomas—persistence beyond at least 3 menstrual cycles may assist in judgement | [4] | ||||
| Earlier-stage superficial tissue invasion—site-specific tenderness and reduced ovarian mobility may aid in diagnosis | [5][6] | ||||
| Laparoscopy | |||||
| Red/clear peritoneal lesions and vesicular lesions—filling pelvis with saline and submersing laparoscope may assist in diagnosis | [7][8] | ||||
| Pathology | —distinct classifications | ||||
| Premenarcheal endometriosis—neonatal uterine bleeding (NUB) considered as potential source | [9] | ||||
| Endometriosis associated with congenital uterine malformation and outflow obstruction—surgical correction is necessary | [10] | ||||
| Treatment | |||||
| Medication | |||||
| COC/NSAIDs—mainstream of treatment | [11] | ||||
| GnRH agonists/antagonists—should be postponed until 17 years of age | [11] | ||||
| Surgery | |||||
| Mostly good prognosis reported on pain | [12][13][14] | ||||
| High recurrence rates | [15][16] | ||||
| No consensus regarding best timing for surgical intervention | [17][18][19][20] | ||||
2. Prevalence and Social Context
The precise age range of “adolescence” is not clearly defined. Some reports limit the age range to teenagers, while others include women until their mid-twenties. However, most reports seem to refer to women under the age of 22 years
2. Prevalence and Social Context
The precise age range of “adolescence” is not clearly defined. Some reports limit the age range to teenagers, while others include women until their mid-twenties. However, most reports seem to refer to women under the age of 22 years[3]
. This is generally the age at which most women graduate from school and become members of society. Therefore, we defined adolescents as women under the age of 22 in this report.
Noninvasive studies such as pelvic ultrasound play a critical role in the early diagnosis of endometriosis, especially in adolescents. In a study of 270 women aged 12–20 years who underwent ultrasound pelvic examination (transvaginal or transrectal), at least one endometriosis feature was identified in 13.3%. Ovarian endometriomas were found in 11%, adenomyosis in 5.2%, and deep infiltrating endometriosis in 3.7%. Among adolescents with dysmenorrhea, the detection of pelvic endometriosis with ultrasound increased to 21%
[4]
.
Laparoscopically diagnosed endometriosis among adolescents is reported to range from 19% to 43%, depending on reports
. In 2013, Jansen et al. reviewed 15 studies including 880 adolescents aged 10–21 years with dysmenorrhea or pelvic pain. Endometriosis was diagnosed in 62% of adolescent women who underwent laparoscopic examinations for pain. The rate of diagnosis was 49% in those experiencing chronic pelvic pain (204 out of 420 women), 70% in those with dysmenorrhea (102 out of 146 women), and 75% in those with chronic pelvic pain resistant to medical therapy (237 out of 314 women)
[3]
.
Despite the relatively high prevalence of endometriosis among adolescents, menstrual stigma and lack of knowledge may hamper adolescents to seek medical attention. Gupta et al. examined how symptoms suggestive of endometriosis among adolescents are perceived at the peer and community levels. Menstruation was often associated with weakness and considered taboo, impeding conversation about menstruation. This leads to a lack of knowledge among adolescents about the variety of menstruation experiences and symptoms suggestive of endometriosis. Lack of training among school personnel in identifying adolescents susceptive of endometriosis was also considered to contribute to help-seeking. Their findings highlighted a need for education/access to information as well as de-stigmatization campaigns
[2].
3. Clinical Findings
Dysmenorrhea and chronic pelvic pain are typical symptoms of endometriosis, and acyclic pain has been reported in adolescents more often than in adults who complain frequently of cyclical pain, implying dysmenorrhea. In adults, the pain commonly precedes the onset of the menstruation and increases during menstruation
.
3. Clinical Findings
Dysmenorrhea and chronic pelvic pain are typical symptoms of endometriosis, and acyclic pain has been reported in adolescents more often than in adults who complain frequently of cyclical pain, implying dysmenorrhea. In adults, the pain commonly precedes the onset of the menstruation and increases during menstruation. In adolescents, Laufer et al. reported that 62.5% had both acyclic and cyclic pain and 28.1% had acyclic pain, whereas only 9.4% of adolescents had the typical cyclic pain with menstruation
[22]
. Although non-steroidal anti-inflammatory drugs (NSAIDs) and combined oral contraceptives (COC) are frequently prescribed as empiric therapy, young patients may frequently report dysmenorrhea resistant to these medications. In a systematic review, the prevalence of endometriosis was higher in girls with chronic pelvic pain resistant to COC and/or NSAIDs (75%; 237/314) than in girls with chronic pelvic pain not resistant to medication (49%; 204/420)
[3]
. Endometriosis should be highly suspected when treatment with these medications fails
. A retrospective cohort study of 900 women who received surgical treatment for endometriosis compared the difference in characteristics between 55 adolescent girls between 13 and 21 years and 845 women aged beyond 22 years. The study showed that adolescents had more risk factors for endometriosis than adults, such as early menarche, first-degree relatives with endometriosis, history of asthma, and congenital anomalies
[24]
.
Ultrasound pelvic examination is noninvasive, and therefore, most accessible for diagnosing endometriosis in adolescents. However, adolescents may have an earlier stage of the disease with smaller endometriotic lesions, which could be difficult to detect using ultrasonography. For small endometriomas, persistence beyond at least three menstrual cycles serves as an indicator
[4]
. Indirect sonographic signs, or “soft markers,” could help in detecting superficial tissue invasions. These “soft markers,” including site-specific tenderness and reduced ovarian mobility, are correlated with endometriosis and adhesions detected during laparoscopy
. Adhesions may be suspected if the ovaries or the uterus move together with adjacent structures, such as the bladder, intestines, pouch of Douglas, and parietal peritoneum, as the sonographer palpates the abdomen
[6]
. Obliteration at the pouch of Douglas may be detected using the sliding sign, where the cervix is gently pressed with the transvaginal sonography probe and/or the uterus is palpated abdominally by hand, to assess whether the rectosigmoid slides independently against the posterior uterine wall
[25]
. Small deep infiltrating endometriosis nodules may be easier to identify by the “tenderness-guided” transvaginal sonography and using additional ultrasound gel in the probe cover, instead of the generally used 4 mL of gel
[26]
.
The findings of laparoscopic surgery are key factors in diagnosing endometriosis. Matalliotakis et al. reported that, during laparoscopic examinations, 45 out of 55 adolescent young girls (81.8%) were diagnosed with endometriosis stage I–II based on the revised American Society for Reproductive Medicine classification, while the remaining 10 (18.2%) were diagnosed as stage III–IV
[24]
. Audebert et al. reported similar results
[27]
. In general, ovarian endometriomas are frequently observed in women aged >20 years. “Powder-burn” lesions consisting of a mixture of black lesions and white scars, and “blueberry spots” referring to blue-black nodules, are typical peritoneal lesions that reflect chronic hemorrhage and fibrosis. In contrast, ovarian endometriomas are not frequently found in adolescents. Peritoneal lesions, especially red and clear peritoneal lesions (
Figure 1
a) and vesicular lesions (
Figure 1
b), which are characteristic of early-stage endometriosis, are the majority of lesions found in adolescents
. These superficial peritoneal lesions are reported to be highly inflammatory. Moreover, it has been shown that clear and red lesions are the most painful
[30]
. Early-stage lesions are difficult to detect even under laparoscopy. These microvascularizations and the filmy, free-floating adhesions on the peritoneal and ovarian surfaces collapse under pneumoperitoneum pressure. Laufer et al. reported that the conformation of these subtle lesions becomes easier to identify by filling the pelvis with normal saline, thereby distending the peritoneum and preventing collapse of lesions. Submersing the laparoscope under water to suppress light reflection also aids in identifying lesions
. The different findings detected in adults versus adolescents (adolescents primarily show early-stage lesions, whereas adults mainly show fibrosis and scarring) demonstrate that endometriosis is a progressive disease. When assessing and diagnosing endometriosis in adolescents, it is essential to direct careful attention to early-stage lesions, as these can be easily missed.
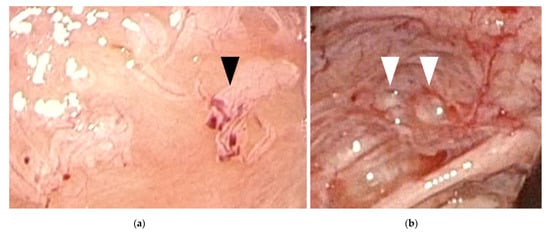

Figure 1.
Early-stage endometriosis lesions found in adolescents. (
a
) Red and clear peritoneal lesions on the peritoneum (black arrowhead); (
b) Vesicular lesions (white arrowhead) found on the peritoneum near the left uterosacral ligament.
) Vesicular lesions (white arrowhead) found on the peritoneum near the left uterosacral ligament.
References
- Stuparich, M.A.; Donnellan, N.M.; Sanfilippo, J.S. Endometriosis in the Adolescent Patient. Semin. Reprod. Med. 2017, 35, 102–109.
- Gupta, J.; Cardoso, L.F.; Harris, C.S.; Dance, A.D.; Seckin, T.; Baker, N.; Ferguson, Y.O. How do adolescent girls and boys perceive symptoms suggestive of endometriosis among their peers? Findings from focus group discussions in New York City. BMJ Open 2018, 8, e020657.
- Janssen, E.; Rijkers, A.; Hoppenbrouwers, K.; Meuleman, C.; D’Hooghe, T. Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: A systematic review. Hum. Reprod. Update 2013, 19, 570–582.
- Martire, F.G.; Lazzeri, L.; Conway, F.; Siciliano, T.; Pietropolli, A.; Piccione, E.; Solima, E.; Centini, G.; Zupi, E.; Exacoustos, C. Adolescence and endometriosis: Symptoms, ultrasound signs and early diagnosis. Fertil. Steril. 2020, 114, 1049–1057.
- Okaro, E.; Condous, G.; Khalid, A.; Timmerman, D.; Ameye, L.; Huffel, S.V.; Bourne, T. The use of ultrasound-based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain--can we reduce the need for laparoscopy? BJOG Int. J. Obstet. Gynaecol. 2006, 113, 251–256.
- Gerges, B.; Lu, C.; Reid, S.; Chou, D.; Chang, T.; Condous, G. Sonographic evaluation of immobility of normal and endometriotic ovary in detection of deep endometriosis. Ultrasound Obstet. Gynecol. 2017, 49, 793–798.
- Redwine, D.B. Age-related evolution in color appearance of endometriosis. Fertil. Steril. 1987, 48, 1062–1063.
- Laufer, M.R. Current approaches to optimizing the treatment of endometriosis in adolescents. Gynecol. Obstet. Investig. 2008, 66 (Suppl. S1), 19–27.
- Benagiano, G.; Guo, S.-W.; Puttemans, P.; Gordts, S.; Brosens, I. Progress in the diagnosis and management of adolescent endometriosis: An opinion. Reprod. Biomed. Online 2018, 36, 102–114.
- Sanfilippo, J.S.; Wakim, N.G.; Schikler, K.N.; Yussman, M.A. Endometriosis in association with uterine anomaly. Am. J. Obstet. Gynecol. 1986, 154, 39–43.
- Saridogan, E. Adolescent endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 46–49.
- Stavroulis, A.; Saridogan, E.; Creighton, S.; Cutner, A. Laparoscopic treatment of endometriosis in teenagers. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 125, 248–250.
- Roman, J.D. Adolescent endometriosis in the Waikato region of New Zealand--a comparative cohort study with a mean follow-up time of 2.6 years. Aust. N. Z. J. Obstet. Gynaecol. 2010, 50, 179–183.
- Yeung, P., Jr.; Sinervo, K.; Winer, W.; Albee, R.B., Jr. Complete laparoscopic excision of endometriosis in teenagers: Is postoperative hormonal suppression necessary? Fertil. Steril. 2011, 95, 1909–1912.e1.
- Lee, S.Y.; Kim, M.-L.; Seong, S.J.; Bae, J.W.; Cho, Y.J. Recurrence of Ovarian Endometrioma in Adolescents after Conservative, Laparoscopic Cyst Enucleation. J. Pediatr. Adolesc. Gynecol. 2017, 30, 228–233.
- Tandoi, I.; Somigliana, E.; Riparini, J.; Ronzoni, S.; Vigano, P.; Candiani, M. High rate of endometriosis recurrence in young women. J. Pediatr. Adolesc. Gynecol. 2011, 24, 376–379.
- Vercellini, P.P.; Fedele, L.; Arcaini, L.; Bianchi, S.; Rognoni, M.T.; Candiani, G.B. Laparoscopy in the diagnosis of chronic pelvic pain in adolescent women. J. Reprod. Med. 1989, 34, 827–830.
- Kitajima, M.; Defrère, S.; Dolmans, M.-M.; Colette, S.; Squifflet, J.; Van Langendonckt, A.; Donnez, J. Endometriomas as a possible cause of reduced ovarian reserve in women with endometriosis. Fertil. Steril. 2011, 96, 685–691.
- Kitajima, M.; Dolmans, M.-M.; Donnez, O.; Masuzaki, H.; Soares, M.; Donnez, J. Enhanced follicular recruitment and atresia in cortex derived from ovaries with endometriomas. Fertil. Steril. 2014, 101, 1031–1037.
- Hirokawa, W.; Iwase, A.; Goto, M.; Takikawa, S.; Nagatomo, Y.; Nakahara, T.; Bayasula, B.; Nakamura, T.; Manabe, S.; Kikkawa, F. The post-operative decline in serum anti-Mullerian hormone correlates with the bilaterality and severity of endometriosis. Hum. Reprod. 2011, 26, 904–910.
- Kontoravdis, A.; Hassan, E.; Hassiakos, D.; Botsis, D.; Kontoravdis, N.; Creatsas, G. Laparoscopic evaluation and management of chronic pelvic pain during adolescence. Clin. Exp. Obstet. Gynecol. 1999, 26, 76–77.
- Laufer, M.; Goitein, L.; Bush, M.; Cramer, D.; Emans, S. Prevalence of endometriosis in adolescent girls with chronic pelvic pain not responding to conventional therapy. J. Pediatr. Adolesc. Gynecol. 1997, 10, 199–202.
- Gałczyński, K.; Jóźwik, M.; Lewkowicz, D.; Semczuk-Sikora, A.; Semczuk, A. Ovarian endometrioma—A possible finding in adolescent girls and young women: A mini-review. J. Ovarian Res. 2019, 12, 104.
- Matalliotakis, M.; Goulielmos, G.N.; Matalliotaki, C.; Trivli, A.; Matalliotakis, I.; Arici, A. Endometriosis in Adolescent and Young Girls: Report on a Series of 55 Cases. J. Pediatr. Adolesc. Gynecol. 2017, 30, 568–570.
- Reid, S.; Lu, C.; Casikar, I.; Reid, G.; Abbott, J.; Cario, G.; Chou, D.; Kowalski, D.; Cooper, M.; Condous, G. Prediction of pouch of Douglas obliteration in women with suspected endometriosis using a new real-time dynamic transvaginal ultrasound technique: The sliding sign. Ultrasound Obstet. Gynecol. 2013, 41, 685–691.
- Guerriero, S.; Ajossa, S.; Gerada, M.; Virgilio, B.; Angioni, S.; Melis, G.B. Diagnostic value of transvaginal ‘tenderness-guided’ ultrasonography for the prediction of location of deep endometriosis. Hum. Reprod. 2008, 23, 2452–2457.
- Audebert, A.; Lecointre, L.; Afors, K.; Koch, A.; Wattiez, A.; Akladios, C. Adolescent Endometriosis: Report of a Series of 55 Cases With a Focus on Clinical Presentation and Long-Term Issues. J. Minim. Invasive Gynecol. 2015, 22, 834–840.
- Redwine, D.B. The distribution of endometriosis in the pelvis by age groups and fertility. Fertil. Steril. 1987, 47, 173–175.
- Davis, G.D.; Thillet, E.; Lindemann, J. Clinical characteristics of adolescent endometriosis. J. Adolesc. Health 1993, 14, 362–368.
- Jansen, R.P.; Russell, P. Nonpigmented endometriosis: Clinical, laparoscopic, and pathologic definition. Am. J. Obstet. Gynecol. 1986, 155, 1154–1159.
- Laufer, M.R. Identification of clear vesicular lesions of atypical endometriosis: A new technique. Fertil. Steril. 1997, 68, 739–740.
More
