Head and neck cancers (HNC) occur in the upper aerodigestive tract and are among the most common cancers. The etiology of HNC is complex, involving many factors, including excessive tobacco and alcohol consumption. Over the last two decades, oncogenic viruses have also been recognized as an important cause of HNC. Major etiological agents of nasopharynx carcinoma and oropharyngeal carcinoma include Epstein-Barr virus (EBV) and human papillomaviruses (HPVs), both of which are able to interfere with cell cycle control. Additionally, the association of hepatitis C and hepatitis B infection was observed in oral cavity, oropharyngeal, laryngeal, and nasopharyngeal cancers. Overall prognoses depend on anatomic site, stage, and viral status. Current treatment options, including radiotherapy, chemotherapy, targeted therapies and immunotherapies, are distributed in order to improve overall patient prognosis and survival rates. However, the interplay between viral genome sequences and the health, disease, geography, and ethnicity of the host are crucial for understanding the role of viruses and for development of potential personalized treatment and prevention strategies.
- head and neck cancer
- human papillomavirus
- Epstein-Barr virus
- Hepatitis C
- Hepatitis B
- clinical trials
1. Introduction
Head and neck cancer (HNC) comprises a heterogeneous group of upper aerodigestive tract neoplasms, and is among the most frequently diagnosed cancer type in the world [1]. According to a report from 2018, HNCs were identified as the eighth most common cancer, comprising 3% of all cancer diagnoses and 1.5% of cancer-related deaths in the U.S. [2][3]. Generally, the most common histologic type, accounting for more than 90% of all HNCs, is head and neck squamous cell carcinoma (HNSCC) [2][3][4]. HNSCC might arise in the mucosal surface lining of the upper aerodigestive tract, including: (i) oral squamous cell carcinomas (OSCC), arising from lips, buccal mucosa, hard palate, anterior tongue, floor of mouth, and retromolar trigone; (ii) oropharyngeal squamous cell carcinomas (OPSCC), which might arise from the base of the tongue, the soft palate, tonsils, uvula, and posterior pharyngeal wall; (iii) laryngeal squamous cell carcinomas (LSCC), arising from the supraglottis, glottis, subglottis; and (iv) nasal squamous cell carcinomas (NSCC), which develop from squamous epithelial cells lining the nasal cavity and paranasal sinuses (Figure 1) [4].
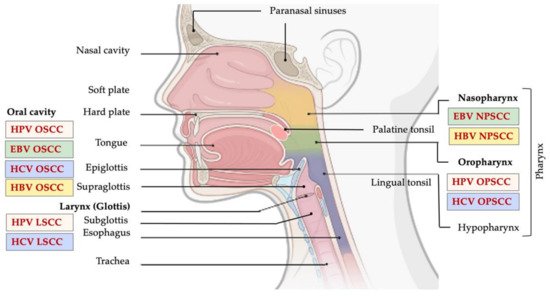
HNSCCs are very heterogeneous in nature and can vary histologically from well-differentiated, keratinized to undifferentiated, nonkeratinizing cancer types [4][5][6]. Around 10% of HNC can develop from lymphocytes, cells of connective tissue or of the salivary glands. The incidence and prevalence of different tumor types is usually influenced by the geographical position, population heterogeneity, and exposure to risk factors.
2. Human Papillomavirus
Papillomaviruses are among the oldest viruses known, dating back from the late Paleozoic era, i.e., 330 million years ago [7]. Over 90 million years ago, papillomaviruses with mucosal tropism started to develop, and during this long period, human papillomaviruses (HPVs), belonging to the Papillomaviridae family, evolved [8]. Almost 280 papillomavirus types have been detected in vertebrates, of which more than 200 types infect humans. HPVs have acquired the ability to utilize human cellular proteins to replicate and control the host cellular and immune systems at several levels in order to stay silent [7].
HPVs are widespread, making them a common risk factor for cancer development. They are classified into five genera, where Alphapapillomavirus, Betapapillomavirus, and Gammapapillomavirus are the most numerous. Clinically, most important mucosal HPVs belong to the alpha genus [7][8][9]. According to the World Health Organization International Agency for Research on Cancer (IARC) (Monograph Volume 100), HPVs are classified as carcinogenic to humans (Group 1), including HPV types 16 and 18; probably carcinogenic to humans (Group 2A) including types 31 and 33 and possibly carcinogenic to humans (Group 2B) including some types other than 16, 18, 31 and 33. The IARC has classified 12 HPV types (HPV-16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58 and 59) as carcinogenic to humans (Group 1) and high-risk (HR) types [10]. On the other hand, low risk (LR)-HPVs (6, 11, 40–44, 54, 61, 72, 81, etc.) are considered to be unrelated to carcinogenesis [6][8][10][11].
Even though the distribution of these most widespread HPVs varies in different geographic areas, HR-HPVs-16, 18, 31, 33 and 45 cause approximately 75% of all HPV-associated squamous cell carcinoma and 94% of all adenocarcinomas [7][12]. The HPV-16 and HPV-18 have the highest prevalence in HPV-positive OPSCC [13][14][15], whereas HPV-31, 33, and 35 are rarely implicated in OPSCC development [15]. LR mucosal HPVs, HPV-6 and HPV-11, generally cause benign papilloma/condyloma, whereas high-risk mucosal HPVs, such as HPV-16 and HPV-18, cause squamous intraepithelial lesions which can evolve to squamous cell carcinoma in the head and neck region and/or anogenital tract [7][12].
2.1. HPV Genome and Mechanism of Infection
HPVs are nonenveloped viruses with circular double-strand DNA of approximately 8000 bp in length (Table 1) [8]. The viral genome has three regions: (i) early (E) region including E1, E2, E4, E5, E6 and E7 oncogenes important in regulating viral DNA transcription and replication; (ii) late (L) region, encoding major (L1) and minor (L2) major and secondary capsid proteins; and (iii) long control region (LCR), a noncoding region positioned between the L1 and E6 open reading frames (ORFs) [8][10]. The LCR contains most of the regulatory elements involved in viral DNA replication and transcription, including the replication origin (ori) [8][10][16].
HPV infection arises through tissue scratches which provide the virus with accessibility to basal keratinocytes. The integration of HPV in host genome is a critical event in cancer progression. In early low-grade lesions, HPV genome is found in the episomal form, whereas integration of the viral genome occurs late in the pathogenesis of cancer [17][18]. The process of HPV genome integration into the host genome is still not completely understood. However, it is believed that DNA damage can facilitate the integration of the viral genome by generating breaks in both the viral and host genomes [18]. Integration breakpoints generally take place in the HPV E1 or E2 genes [17][18]. The E2 ORF is the preferential site of integration due to frequent disruptions and deletions compared to any other site. The E2 protein is a negative regulator of E6 and E7 expression, and loss of E2 ORF during integration enhances expression of the transforming E6 and E7 oncoproteins which are responsible for cellular transformation [18].
The viral oncoproteins E6 and E7 are essential for malignant conversion [16][19]. E6 induces transcription of human telomerase reverse transcriptase (hTERT) which elongates telomeres and prevents end-to-end fusion of chromosomes and apoptosis. Thus, the E6 oncoprotein has a fundamental role in cell immortalization. [7][16][19]. However, the most studied role of E6 oncoprotein is its ability to bind to p53 to form a ternary complex with E6AP ubiquitin ligase and p53 (E6-E6AP-p53), leading to p53 degradation via a proteasome-mediated pathway [7][8][16][19]. The E7 protein binds to the conserved motif of the retinoblastoma (Rb) protein, resulting in the transcriptional factor E2F nuclear translocation, which stimulates uncontrolled replication and cell-division [8][20].
Other cancer-related pathway proteins involved in the cell cycle regulation are also targeted by E6 and E7 oncoproteins. E7 proteins can inactivate cyclin-depend kinase (CDK) cell cycle inhibitors, including p21CIP1 and p27Kip1, and enhance activity of cyclin-dependent kinase 2 (CDK2) [21]. Moreover, E6 and E7 oncoproteins indirectly interfere with noncoding RNAs (microRNAs; miRNA) involved in tumor progression/suppression [16]. E6 and E7 increase oncogenic miRNA-21 levels which decreases expression of tumor suppressor PTEN gene. Based on many studies in HNC and cervical cancer, it was concluded that HPV oncogenes can enhance or decrease miRNA levels in HPV-associated cancer, and their effect depends on targeting either tumor suppressor or proto-oncogenes [7][8][16].
Expression of E6 and E7 oncoproteins can be regulated by epigenetic mechanisms, mostly by DNA methylation [22][23]. Integration of HPV DNA into the host genome during cancer progression causes structural disruption which prevents the transcription of genes positioned at 5’ of the long control region (LCR) and DNA methylation. Selective methylation in the viral LCR can cause overexpression of early viral proteins [24]. E2 is the main HPV regulatory protein which controls the transcription of HPV early proteins through a negative feedback control. LCRs in the mucosal HPV genome contain 4 E2 binding sites (E2BS) [25][26]. E2BS1 has the highest affinity for the E2 protein and is positioned towards the LCR 5’ end. Recruiting E2 to E2BS1 activates the viral early promoter, enhancing the transcription of HPV early proteins, including E6, E7, and E2 itself. Increasing E2 levels forces E2 proteins to bind to the E2BS3 and E2BS4 near the 3’ end of the viral LCR, which represses the early promoter and hinders transcription of early proteins [18][26][27]. However, CpG methylation in the E2BSs inhibits E2 binding, obstructing E2 regulation of viral early promoter. This event leads to overexpression of HPV E6 and E7 oncoproteins, and elevated odds of tumorigenesis [24].
Reports aiming to examine LCR methylation in HNC have described fluctuating CpG methylation levels, suggesting that methylation in LCR is not common to all HPV-driven HNC [23]. In cervical cancer, however, CpG methylation has also been observed in HPV L1 and L2 genes, whereas HPV DNA methylation in these sites has not yet been broadly investigated in HPV-driven HNC, even though current literature proposes similar methylation patterns in HNC [22][23][24]. Many studies have proposed DNA methylation as a biomarker to characterize malignant lesions [24].
The indicators of HPV infections can be numerous, from asymptomatic infections to benign warts or malignant lesions, intraepithelial neoplasia, and invasive carcinoma [7][8]. Generally, oral HPV infections are associated with sexual behavior, but recent evidence supports horizontal, mouth-to-mouth, virus transmission. Also, most HPV infections in newborns are transmitted vertically from the mother during pregnancy, delivery, or later, by saliva [7].
Table 1. Oncogenic viruses and their mode of action in HNC development.
| Oncogenic Virus/Parameter | Human Papillomavirus (HPV) | Herpesvirus | Hepatitis Virus | |||
|---|---|---|---|---|---|---|
| HPV-Positive | HPV-Negative | Epstein-Barr Virus (EBV) | Hepatitis C | Hepatitis B | ||
| Virus-related | Nucleic acid | Circular double stranded DNA [8] | − | Linear double stranded DNA [28] | Single stranded RNA [29] | Double stranded circular DNA [30][31] |
| Genome | Approximately 8 kb in size [8] | − | Approximately 180 kb in size [28] | 9 600 bp in size [29][32] | The smallest genome with 3200 bp in size [30] | |
| Tropism | Kerationocytes and mucosal sufraces [8] | − | B-cells and epithelial cells [33][34] | Hepatocytes, lymphocytes, and salivary gland cells [35] | Hepatocytes and lymphocytes [36] | |
| Major viral oncoproteins | E6, E7 [7][8][16] | − | LMP1, LMP2A, EBNA1 [33][37] | NS3 or NS5A [32][35][38] | S, C, P and X [31][39] | |
| Virus transmission mode | Sexual contact, self-inoculation, vertical and horizontal transmissions [40] | − | Sexual contact, blood or saliva transmission [33] | Vertical transmission, horizontal transmission (sex or sharing of drug-injection needles) [31] | Sexual contact, self-inoculation, vertical and horizontal transmissions [40] | |
| Cancer-related | Anatomic site | Oral cavity, oropharynx, larynx [40][41] | All sites, but mostly oropharynx [28] | Nasopharynx [42] Oral cavity [43] | Oral cavity, oropharynx, larynx [38][44][45][46][47] | Oral cavity [47] Nasopharynx [36][48] |
| Histology | Nonkeratinized [49] | Keratinized [34] | Undifferentiated type NPC, squamous cell and non–keratinizing NPC [50] | Squamous cell [44][46] | Squamous cell or adenocarcinoma [51] | |
| Age | Under age of 50 [6] | Above age of 50 [6] | Above age of 50 [42][52] | Above age of 50 [35][47] | Above age of 50 [36] | |
| Gender | Mostly male [16] | Mostly male [16] | Mosty male [33] | Mostly male, 6.7-fold higher risk in male [35][47] | No significant difference [35][36] | |
| Incidence trend | Increasing | Decreasing | Increasing | Increasing | Increasing | |
2.2. HPV in HNSCC Development
The frequency of HPV-positive HNC is increasing drastically, specifically, that of oropharyngeal cancer (OPC). OPC has the highest incidence among HPV-positive cancers, with different distribution compared to HPV-negative OPC. Data have shown that more than 70% of oropharyngeal cancers are caused by HPV, and that HPV type 16 (HPV-16) causes almost 90% of the HPV-positive OPC in the United States [28].
Studies have shown that approximately 25% of HNC cases worldwide are caused by oral HPV transmission, mainly by oral sexual activities. However, this percentage differs between anatomical sites, i.e., between oral, oropharyngeal and laryngeal regions [40]. Increased number of oral sexual partners drastically increases oral transmission of HPV which ultimately leads to an escalation in infections in the head and neck region. Much higher number of HPV infections of the head and neck region is reported in men compared to women (Table 1) [40]. Globally, HPVs cause roughly 33.6% of nonkeratinized OPSCC, and 22.2% and 20.2% of OSCC and LSCC, respectively [49] (Figure 1). However, it is still completely unclear why the prevalence of HPV-positive HNC differs between anatomical sites [40].
HPV-positive OPC is considered entirely different than the traditional tobacco and alcohol-related OPCs, with a better prognosis compared to HPV-negative OPC [4]. It is important to understand that HPV status determines the criteria by which to analyze the cancer stage and provide an overall prognosis. Since pathology reports for OPC lacking HPV status can be misinterpreted, HPV testing is now suggested for all patients with newly diagnosed OPSCC [4]. Patients diagnosed with HPV-positive HNCs are younger, have more sexual partners and earlier ages of onset of sexual activity. Additionally, these patients are characterized with favorable health profiles and statistically low disease comorbidities than those observed in long-term smokers and heavy alcohol users [6]. Currently, there is a significant difference in HPV-positive and -negative OPC prognoses. However, this is not the same for HPV-positive and -negative oral cancers, and the role of HPV in oral cancers has not yet been fully elucidated [53].
Additionally, HPV-16 infection of the antral tissue of the nasal cavity has been suggested to be involved in the onset of Killian polyps (KP) [54]. KP is a benign lesion arising from the maxillary sinus region with still unknown etiology. Between 2000 and 2014, only a few studies were published on HPV association with nasal polyps, with highly inconsistent results, i.e., HPV-DNA prevalences ranging from 0% to 40% [54]. These discrepancies may be attributable to factors such as race, geographical area, laboratory procedures, tissue fixation, number of samples, and choice of controls [54].
However, there is no evidence claiming neoplastic transformation of KP, except reported incidents mimicking malignant transformation [55]. It is difficult to report HPV carcinogenesis in KP, as patients remove them immediately after presentation, and carcinogenic events caused by HPV infection require longer to develop and to be detected [56]. A study by Knör and colleagues indicated the existence of HPV in nasal and antrochoanal polyps. However, the association did not reflect any oncogene transformation [54]. Similarly, Oton-Gonzalez et al. (2021) reported the presence of low HPV-16 DNA amounts in both episomal and integrated forms in antral KP. However, they did not report viral expression of E2, E5, E6, and E7 sequences in the HPV-positive KP samples [56].
Detecting HPV DNA only is not adequate to define tumor causality. Thus, the expression of several markers is required to distinguish between HPV and non-HPV-related cases of head and neck carcinoma [11]. It has been suggested that tumor suppressor genes p16INK4a and E6/E7 mRNA are sensitive biomarkers in detecting HPV-positive OPSCC in comparison to OSCC and LSCC. The weak sensitivity in detecting HPV-positive OSCC and HPV-positive LSCC contributes to the discrepancy in the prevalence of HPV-positive HNC between the three anatomical regions [40].
2.3. Current Treatment Options and Update on Clinical Trials
Treatment approaches for HNC subtypes, specifically OPSCC, are also dependent on HPV status, with more favorable outcomes and overall survival (OS) rates for HPV-positive patients [57][58][59]. Accordingly, clinical studies suggest a three-year OS of 82.4% and 57.1% in HPV-positive compared to HPV-negative patients, respectively [60][61]. Although the molecular profiles of the two groups of patients are different, treatment strategies for HNC patients usually follow the same therapeutic regimens, suggesting a need for novel treatment approaches that reflect the molecular background of HPV-positive and -negative patients [61].
Currently, RT is one of the most frequently used treatments for HNSCC patients in early and advanced disease stages, with 50% OS rate in early diagnosed cases [62] and 10–25% OS in advanced stages [63]. Interestingly, HPV-positive HNSCC patients are found to be more sensitive to RT, possibly due to the hypoxic status where low oxygen levels reduce therapeutic efficacy [61].
In past decades, surgical procedures of HNC have been challenging due to the complex anatomical structures and the inability to reach distal organ portions, resulting in surgery complications [64]. Advances in surgical technology have led to two approaches, i.e., transoral laser microsurgery (TLM) and transoral robotic surgery (TORS), with clinically significant outcomes [65][66]. Compared to TLM, TORS uses the Da Vinci Robot, allowing more precise tissue dissection that minimizes surgical morbidity. Considering safety, flexibility, and improved functional outcome of the patients compared to other surgical procedures, TORS was approved by the Food and Drug Administration (FDA) in 2009 for transoral resections of head and neck tumor lesions, with major application in patients diagnosed with OPSCC [65][67]. Recently, surgical procedures involving TLM or TORS are also preferred for oral cavity SCC, as well as for advanced stages of laryngeal and hypopharyngeal cancer [68][69]. Despite promising clinical outcomes of patients after TORS, however, RT and CT cannot be avoided in advanced disease stages [70].
Several clinical trials for TORS have been registered, mainly regarding to OPSCC. Moore et al. (2018) evaluated TORS outcomes for OPSCC patients (n = 314) ± adjuvant RT or CT [71]. From the total number of patients with known HPV status (n = 309), 93% (n = 286) were HPV-positive OPSCC. In this study, 24% of patients were treated with surgery alone, while postsurgical RT and CRT were applied to 28% and 48%, respectively. Five years after the surgical procedure, the study results indicated recurrence-free survival (RFS), distant-metastasis-free survival (DMFS), OS, and cancer-specific survival (CSS) rates of 92%, 90%, 86%, and 94%, respectively (Table 2). The five-year recurrence rate of the patients enrolled in this study was 8%, with increased risk in the group that underwent surgery alone [71]. Additionally, it is important to consider that this procedure might cause serious complications, including postoperative hemorrhage in 3–8% of the patients [67][72]. Even though HPV-16-positive patients have high post-therapeutic survival rates, still certain factors such as swallowing function after surgery and long-term toxicity significantly affect patient quality of life (QoL). An ongoing clinical trial (NCT02215265, PATHOS) with an estimated enrollment of 1100 patients is evaluating whether reduction of RT intensity in intermediate-risk OPSCC patients or CT dispense in higher-risk patients will cause favorable swallowing function after minimally invasive surgery. The estimated completion time of the study is 2027 [73].
According to recent findings, 80% of the patients who underwent TORS received RT which, at the same time, improved OS rates. Importantly, the radiation dose is usually low (60–66 Gy vs. 70 Gy) in cases receiving TORS as the initial treatment [74]. A number of clinical trials evaluating TORS following RT and CRT are currently ongoing (Table 2) [75]. In a randomized, phase II ECOG-ACRIN 3311 clinical trial, Ferris et al. (2020) evaluated the effects of low dose RT in HPV-positive, stage III-IVA OPSCC patients (n = 511) after TORS procedure. Patients were divided into four experimental groups (Arm A–D) (Table 2). The results indicated two-year progression free survival (PFS) > 90% in all study groups (detailed results in Table 2). Former data suggest favorable oncogenic outcome of TORS in p16-positive OPSCC patients with intermediate recurrence risk, while low-risk disease patients do not require postsurgical therapeutic interventions [70].
With the aim of decreasing high RT toxicity rates of nearby tissues and improving clinical outcomes, different approaches for HNC treatment have been recently applied, either as single treatment or in combination with other therapies, including several chemotherapeutic agents [63]. So far, FDA approved CT agents for the treatment of HNC include platinum-based therapy cisplatin and carboplatin, as well as 5-fluorouracil (5-FU), bleomycin, docetaxel and methotrexate with response rates up to 40% [76]. The results of meta-analyses and a number of clinical trials have suggested that the use of combinatorial therapy containing cisplatin, 5-FU, and docetaxel (TPF regimen) improves clinical outcomes with acceptable toxic rates among HNSCC patients [76][59].
| Clinical Trial/NCT Number/Year | Phase | Disease Stage | Patient Number (n) | Treatment Arms | Outcome |
|---|---|---|---|---|---|
| 2007–2015 [71] |
N/A | OPSCC, stage I-IVb | n = 314 (n = 286 HPV-positive) | Arm 1: TORS4; Arm 2: TORS + RT6 (50–70 Gy); Arm 3: TORS + RT (30–70 Gy) + adjuvant CT (CDDP1/Carboplatin/Docetaxel/Cetuximab) |
5-year after surgery LR13 RFS17: 92% DMFS18: 90% OS8: 86% CSS3: 94% |
| ORATOR NCT01590355 2012–2019 [75] |
Phase II | Early stage OPSCC | n = 68 (n = 60 p16-positive) | Arm 1: RT ± CT7 (70/63/56 Gy in 35 fxs for 7 weeks); Arm 2: TORS + neck dissection |
Median follow-up (27 months): Arm 1: 25 months Arm 2: 29 months QoL11 (MDADI score) Arm 1: 86.9 Arm 2: 80.1 |
| ECOG-ACRIN 3311 NCT01898494 2013–2020 [70] |
Phase II | HPV-positive, stage III-Iva OPSCC | n = 353 | Arm A: TORS; Arm B: TORS, low-dose IMRT (50 Gy); Arm C: TORS, standard-dose IMRT (60 Gy); Arm D: TORS, standard-dose IMRT (60–66 Gy) + CT (weekly CDDP 40 mg/m2) |
2-year PFS12: Arm A: 93.9% Arm B: 95.0% Arm C: 95.9% Arm D: 90.5% |
| ADEPT NCT01687413 2012–2020 [77] |
Phase III | p16-positive OPSCC | n = 42 | Experimental group: postoperative IMRT (60 Gy in 30 fxs); Active comparator: RT (60 Gy in 30 fxs) + cisplatin (40 mg/m2 × 6 doses) |
1-year DFS: 100% vs. 90.9% 2-year LR control: 96.3% vs. 81.8% 2-year DM: 7.4% vs. 0% |
| ECOG-E1308 NCT01084083 2010–2015 [78] |
Phase II | HPV-positive and/or p16-positive stage III-IV OPSCC | n = 80 | Group 1: CDDP (75 mg/m2) and paclitaxel (90 mg/m2), low dose IMRT (54 Gy in 27 fxs × 5 weeks), cetuximab (400 mg/m2 → 250 mg/m2); Group 2: CDDP (75 mg/m2) and paclitaxel (90 mg/m2), standard dose IMRT (69.3 Gy in 33 fxs × 6 week), cetuximab (400 mg/m2 → 250 mg/m2) |
2-year PFS and OS: 64% vs. 91% (IMRT 54 Gy); Primary CRR19: 73% |
| NCT01530997 2012–2020 [74] |
Phase II | HPV-positive and/or p16-positive OPSCC, T0-T3, N0-N2c, M0 | n = 43; HPV+/p16+: 63.6% HPV−/p16+: 36.4% |
De-intensification chemoradiation therapy; IMRT (54–60 Gy) + CDDP (30 mg/m2 × 6 doses + limited surgical evaluation |
pCR20: 86% 2-year LC14: 100% |
| NCT02281955 2014–2020 [74][79] |
Phase II | HPV-positive and/or p16-positive OPSCC, T0-T3, N0-N2c, M0 | n = 113; HPV+/p16+: 40.4 % HPV−/p16+: 10.5% HPV unknown/p16+: 49.1% |
IMRT (60 Gy, 2Gy/fx) + CDDP (30–40 mg/m2 × 6 doses) or cetuximab (250 mg/m2) or Carboplatin (AUC 1.5 and paclitaxel 45 mg/m2) or Carboplatin AUC 3 + surgical evaluation |
2-year outcome: PFS: 88.4% LC: 96.4% RC15: 98.2% LRC16: 94.6% DMFS: 92.0% OS: 93.0% |
| RTOG 0129 NCT00047008 2003–2014 [60] |
Phase III | Stage III-IV SCC of oral cavity, oropharynx, hypohparynx, larynx, T2, N2-3, M0 or T3-4) | n = 721; HPV+ (n = 206), HPV− (n = 117) |
Arm 1: Standard fractionation RT (70 Gy in 35 fx, 2 Gy/fx) + CDDP (100 mg/m2); Arm 2: Accelerated fractionation RT (72 Gy in 42 fx) + CDDP (100 mg/m2) |
3-year outcome: OS (Arm 1 and Arm 2): 64.3% vs. 70.3%; OS (HPV-positive and HPV-negative group): 82.4% vs. 57.1%; PFS (HPV-positive and HPV-negative group): 73.7% vs. 43.4% |
| NCT01663259 2012–2020 (www.clinicaltrial.gov; accessed on 23 March 2021) |
N/A | Stage III-IV (excluding N3 or T4), HPV-positive and/or p16-positive OPSCC | n = 42; HPV-positive (n = 42) |
Cetuximab (400 mg/m2 → 250 mg/m2 concurrent with RT (70 Gy in 35 fx, 50–60 Gy) | 2-year outcome: RR21: 19% DFS: 81% OS: 95.2% FFLRP22: 87.9% |
| CheckMate 141 NCT02105636 2014–2019 [80] |
Phase III | Platinum-refractory, recurrent HNSCC | n = 361; Arm 1 (n = 240): p16-positive (n = 63) p16-negative (n = 50); Arm 2 (n = 121): p16+ (n = 29) p16− (n = 36) |
Arm 1: Nivolumab (3 mg/kg, IV, every 2 weeks); Arm 2: Cetuximab/Methotrexate/Docetaxel (Cetuximab 400 mg/m2 → 250 mg/m2 or methotrexate 40–60 mg/m2 or docetaxel 30–40 mg/m2, weekly) |
18-month OS (Arm 1 and Arm 2): 7.49 vs. 5.06 months; 1-year OS: 36.0% vs. 16.6%; 6-month PFS: 19.7% vs. 9.9%; RR: 13.3% vs. 5.8%; Median OS in p16-positive patients: 9.1 vs. 4.4 months; Median OS in p16-negative patients: 7.5 vs. 5.8 months |
| KEYNOTE-012 NCT01848834 2013–2020 [81] |
Phase Ib | PD-L1-positive, R/M5 HNSCC | n = 60; HPV-positive (n = 23), HPV-negative (n = 37) |
Pembrolizumab (10 mg/kg, once every 2 weeks) | OR9 (central vs. investigator review): 18% vs. 21%; OS: 13 months |
| KEYNOTE-040 NCT02252042 2014–2020 [82] |
Phase III | R/M HNSCC | n = 495;HPV-positive (n = 119), HPV-negative (n = 376) | Pembrolizumab group (200 mg, 3-week cycle); Active comparator group (Methotrexate 40–60 mg/m2 or docetaxel 75 mg/m2 or cetuximab 400 mg/m2 → 250 mg/m2) |
2-year outcome: OS (pembrolizumab vs. active comparator group): 8.4 vs. 6.9 months; PFS: 2.1 vs. 2.3 months ORR10: 14.6% vs. 10.1%; DOR 23: 18.4 vs. 5.0 months |
| KEYNOTE-048 NCT02358031 2015–2020 [83] |
Phase III | R/M HNSCC | n = 882; HPV-positive (n = 190), HPV-negative (n = 692) |
Pembrolizumab monotherapy (200 mg of 3-week cycle for 2 years); Pembrolizumab + CT (200 mg of 3-week cycle for 2 years + cisplatin 100 mg/m2 or carboplatin (AUC 5 + 5-FU 2 1000 mg/m2 up to 6 cycles); Cetuximab + CT (Control) (400 mg/m2 → 250 mg/m2 + cisplatin 100 mg/m2 or carboplatin (AUC 5 + 5-FU 1000 mg/m2 up to 6 cycles) |
47 months outcome: OS (Pembrolizumab + CT group vs. control group): 13.0 vs. 10.7 months; OS in PD-L1 CPS > 1 participants: 13.6 vs. 10.4 months; OS (Pembrolizumab monotherapy vs. control group): 11.5 vs. 10.7 months |
Patients who fail to receive appropriate systemic agents or belong to the category not eligible of undergoing surgery or concurrent CT are usually treated with RT alone [63]. In a clinical study involving HPV-positive stage I–IV OPSCC, O’Sullivan et al. (2012) evaluated the outcome in patients treated with RT alone (n = 148 HPV+, n = 59 HPV−). The results have indicated increased three-year OS in HPV-positive patients (81%) versus HPV-negative patients (44%). Interestingly, OS in p16-positive patients with a less than 10-pack-year smoking history was similar in the RT-alone treatment and CT treatment groups (86% vs. 88%), respectively [84].
As mentioned previously, HPV-positive patients have better prognoses and OS rates compared to HPV-patients [60][61]. In accordance with this, treatment de-intensification strategies are considered in HNC patients with HPV-positive status in order to reduce long-term toxicity but also improve therapeutic outcomes [63]. In a phase III RTOG 0129 clinical trial, Ang and colleagues evaluated the association of OS rates and HPV status in stage III-IV OPSCC patients. Patients were treated with accelerated-fractionation RT and standard-fractionation RT, both in combination with cisplatin. The results indicated nonsignificant change in three-year OS between accelerated-fractionation RT and standard-fractionation RT, 70.3% vs. 64.3%, respectively. However, HPV-positive OPSCC patients (n = 206 of 323) had a better three-year OS (82.4%) compared to HPV-negative OPSCC patients (57.1%), as well as reduction in risk of death for 58% [60].
Moreover, a postoperative adjuvant de-intensification treatment strategy was evaluated in phase III clinical trial (ADEPT, NCT01687413) with HPV-related, p16-positive OPSCC patients (n = 42). The patients were divided into two treatment arms, i.e., IMRT or IMRT in combination with standard cisplatin dose (Table 2). The results indicated one-year DFS of 100% in IMRT group compared to IMRT in combination with cisplatin (90.9%). In addition, two-year loco-regional control (LRC) between the two treatment groups were 96.3% vs.81.8%, respectively. Although more favorable treatment outcomes in regard to abovementioned parameters were observed in patients receiving IMRT only, two-year distant metastasis did not occur in IMRT and cisplatin-treated group (0%), while 7.4% of the patients in IMRT group experienced distant metastasis [77].
An open-label, phase II study (E1308, NCT01084083) enrolling patients of stage III–IV HPV-positive OPSCC has evaluated the ability of reduction in radiation dose in patients with complete CR to induction CT. The patients received induction CT including cisplatin, paclitaxel and cetuximab, following IMRT (54 Gy and 69.3 Gy) with cetuximab. Patients characterized with primary-site CR and treated with lower IMRT have shown two-year PFS and OS of 80% and 94%, respectively. Additionally, after one-year follow up, patients receiving 54 Gy radiation dose had less swallowing difficulties (40% vs. 89%) [85]. Dose de-escalation of adjuvant RT aiming to lower long-term toxic rates has been evaluated in multiple clinical trials (NCT01560997, NCT02281955) [86], as summarized in Table 2.
Despite the treatment advances in HNSCC patients implicating surgery, RT, and CT, the prognosis still remains poor, especially in recurrent/metastatic HNSCC, regardless of HPV status [87]. Consequently, cetuximab, an IgG1-subclass anti-EGFR monoclonal antibody that blocks the activation of tyrosine kinase –dependent molecular signaling pathway by binding to extracellular domain of epidermal growth factor receptor (EGFR) was approved by FDA for the treatment of locoregionally-advanced (LA), as well as recurrent/metastatic disease [88]. Overexpression of EGFR has been detected in majority of HNC patients (approximately 90%), and is in the same time correlated to decreased survival rate and RT resistance [89]. Clinical studies have demonstrated significant improvement in OS with cetuximab addition to the EXTREME regimen (cisplatin or carboplatin, and 5-FU), thus becoming a standard of care for R/M HNC patients in the past decade [88][90]. So far, cetuximab is the only FDA approved anti-EGFR antibody for the treatment of HNC patients [91].
Similarly, taking into consideration good prognosis of HPV-negative OPSCC patients with <10 pack-year smoking history, long-term toxicity of patients was evaluated in a clinical trial (NCT01663259) where concurrent CT was replaced with cetuximab. The patients were treated with concurrent RT of 70 Gy in 35 fractions together with cetuximab. Following treatment regimen, the study results have indicated two-year RR, PFS, and OS of 19%, 81%, 95.2%, respectively, with two-year freedom from loco-regional progression (LRP) of 87.9%. Cetuximab in combination with RT has resulted in mild to moderate adverse events (AE) in 33.3% of the patients, while 66.7% of the patients have developed severe AE. On the contrary to favorable therapeutic outcomes reflecting OS, PFS, still AE were observed in patients, with all grade toxicities including oral mucositis (100%), dysphagia (88.1%), and hematologic toxicity (31%). Several on-going clinical trials (De-ESCALaTE, NCT01874171, TROG 12.01 NCT01855451) including treatment with RT and cetuximab are evaluating optimum treatment strategies for HPV-negative OPSCC patients in order to decrease the toxicity rate and improve patient QoL.
Besides cetuximab and other monoclonal antibodies (mAbs) targeting EGFR in HNSCC patients, immunotherapy in the treatment of HNSCC has been recognized as a potential approach for improvement of clinical outcomes in patients [87]. In immunotherapy, host immune system cells recognize cancer cells as foreign, triggering destruction mechanisms to eliminate the target cells [92]. Importantly, cancer cells activate different mechanism to promote self-survival, including immune escape, as well as T-cell exhaustion [93]. One of the key mechanisms of cancer immune escape is the activation of programmed death-1 or PD-1/PD-L1 signaling pathway [58][83]. Overexpression of PD-1 has been observed on cluster domain 8 (CD8+) tumor-infiltrating lymphocytes in HPV-positive patients and it is in the same time linked with better five-year OS compared to HNSCC patients with low PD-1 expression (93.9% vs. 63.6%), respectively [92][94]. Except PD-1 checkpoint, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) is also potential target for HNSCC immunotherapy [93]. Thus, inhibition of PD-1/PD-L1 signaling with anti-PD1/PD-L1 agents in order to inhibit immunosuppressive response of tumor cells represents a promising approach in the treatment of HNSCC patients [58]. So far, two anti-PD-1 antibodies, nivolumab and pembrolizumab are approved by FDA for the treatment of R/M HNSCC patients. However, response rates to immunotherapeutic agents in HNSCC patients range from 13–20%, with significantly improved OS rate in one out of ten patients [58][87]. Nivolumab and pembrolizumab monoclonal anti-PD-1 antibodies have been investigated through clinical studies in the treatment of patients with HNSCC [63]. The effects of nivolumab were investigated in a randomized phase III clinical trial (CheckMate 141, NCT02105636) for the treatment of platinum-refractory HNSCC [93]. The results have shown OS rate of 7.49 months compared to the standard of care treatment arm receiving cetuximab, methotrexate or docetaxel, where OS of patients was 5.06 months. Based on clinical outcomes, nivolumab obtained FDA approval in 2016 as the first anti-PD1 mAb for the treatment of R/M HNSCC refractory to platinum-based therapy [80].
In an open-label, phase 1b clinical trial (KEYNOTE-012), safety, tolerability and anti-cancer effects of pembrolizumab in patients with PD-1+ R/M HNSCC were evaluated (NCT01848834). From the total number of PD-1+ patients (n = 60), 38% were HPV-positive patients. OR by central imaging review was achieved in 18% of all patients, with greater response in HPV-positive patients. Additionally, pembrolizumab treatment was well-tolerated, where 17% of the cohort had stage 3–4 drug related AE, and 45% of the patients experienced serious adverse events (SAE). Showing significant clinical outcome in R/M HNSCC patients with high PD-1 expression, the effects of pembrolizumab on R/M HNSCC was evaluated in a follow-up, phase III KEYNOTE-040 clinical trial (NCT02252042) [81]. The patients were divided into two treatment groups, including pembrolizumab treatment, as well as comparator group receiving methotrexate, docetaxel or cetuximab. Two-year OS rate in pembrolizumab treatment group was 8.4 months, while OS in active comparator group was 6.9 months. Additionally, objective response rate (ORR) by RECIST 1.1 central imaging was 14.6 and 10.1 months, respectively. Moreover, in a randomized, phase III clinical trial (KEYNOTE-048, NCT02358031), OS in participants treated with pembrolizumab and CT in combination was 13 months, compared to control group receiving cetuximab and CT with OS of 10.7 months. Based on PD-1 combined positive score (CPS) > 1, OS rate in pembrolizumab mono-treatment arm was 13.6 months in contrast to control group receiving cetuximab and CT (10.4 months). Finally, pembrolizumab was approved by FDA in 2019 as a first-line treatment option for R/M HNSCC patients with PD-1 combined positive score > 1 (www.fda.gov; accessed on 23 March 2021). These data emphasize the exigency for evaluation of novel biomarkers in HNSCC patients with an aim of improving response and OS rates of the patients.
In the recent years, prevention of HPV-related infections that might lead to cancer has been emphasized. Currently available vaccines Cervarix, Gardasil-4 and Gardasil-9 are approved for prevention of cervical cancer, vaginal cancer, penile cancer, and genital warts caused by HPV viruses [40]. Bivalent vaccine Cervarix is used for the prevention of HPV-16 and 18, Gardasil-4 is quadrivalent vaccines targeting HPV-16, 18, 6, and 11, while Gardasil-9 is 9-valent vaccine that, besides the aforementioned HPV subtypes, also targets HPV-31, 33, 45, 52, 58 [95]. Although recent research suggests that vaccines might be effective in the prevention of oral HPV infections, more clinical trials examining the decrease in OPSCC after vaccination are urgently required [96]. The efficacy of the Cervarix vaccine in preventing cervical and oral infections four years after vaccination was evaluated in a phase III clinical trial (NCT00128661). Among 91.9% of eligible patients (n = 5840), oral prevalence of HPV was detected in 1.7% of individuals, with the vaccine efficacy of 93.3% [97]. Similarly, in another study, oral HPV infection incidence in vaccinated population was decreased in comparison to unvaccinated patients [98], with prevalence of HPV infection of only 5.6%. Moreover, Chaturvedi et al. (2018) showed reduction of HPV16, 18, 6, 11 oral infection prevalence for 88.2% in vaccinated US population [28]. Finally, these data suggest significant decrease in oral HPV infections in vaccinated individuals, underlining the importance of prevention strategies for HPV-induced OPSCC [95].
3. Epstein-Barr Virus
Epstein–Barr virus (EBV), also known as human herpes virus type 4 (HHV4), is one of the nine identified human herpesvirus types in the herpes family, and one of the most abundant viruses in human population [34]. EBV was discovered by Epstein’s group in a Burkitt’s lymphoma cell line in 1964 using electron microscopy as the first human tumor-related virus [33][34][43]. According to the International Agency for Research on Cancer (IARC), EBV is graded as a Group I carcinogen since its relation to certain lymphoid and epithelial malignancies. Globally, many people are infected by EBV and carry the virus throughout their life, usually without any harsh symptoms. However, persistent EBV infection can lead to the development of malignancies [11]. EBV infects roughly 90–95% of all adults globally and causes approximately 1% of all cancers, including Burkitt’s lymphoma (BL), diffuse large B cell lymphoma (DLBCL), gastric carcinoma (GC), Hodgkin’s lymphoma (HL) and nasopharyngeal carcinoma (NPC) [34][99][100]. The EBV has long been correlated only to NPC development, but recent meta-analysis study showed association of EBV with OSCCs development [43].
3.1. EBV Genome and Mechanism of Infection
The EBV genome consists of linear double-stranded DNA, approximately 180 kb pairs long, containing 85 genes (Table 1) [33]. The EBV exhibits dual tropism, infecting both, B cells and epithelial cells, due to the ability to alternate its cell entry mechanism by switching envelop proteins. EBV is known to use the glycoprotein gp350 envelope protein for binding to the complement receptor type 2 protein present on the membrane surface of B-cells [33]. However, while infecting epithelial cells, it switches to the gp40 envelop protein and binds to the surface integrins. The proteins used in different ways of infection and cell entry mechanisms are very important trait of EBV’s perseverance in humans [33][101]. Infection of B-cells starts by binding of EBV glycoprotein gp350 to cell receptor CD21 and interaction of viral glycoprotein gp42 with MHC class II molecules. This triggers fusion of the viral envelope with the cell membrane, allowing EBV to enter the B cell [101][102]. In CD21 negative human cells, CD35, also called complement receptor 1 (CR1), represents another binding factor for gp350/220, allowing the entry of EBV [101]. In order to evade epithelial cells, viral proteins BMRF-2 and gH/gL interact with cellular β1 and αvβ6/αvβ8 integrins, respectively. This promotes fusion of the viral envelope with the epithelial cell membrane, permitting EBV entrance to the epithelial cell [101][102]. In contrast to B-cell entry, epithelial-cell entry is obstructed by viral glycoprotein gp42 [102].
Upon encountering host cells, the viral capsid dissolves and the viral genome is transported to the cell nucleus. EBV is able to undergo both latent and lytic phases of infection. In latent infection, the EBV genome replication occurs only once with each cell cycle. During lytic infection, the EBV genome is replicated to produce a high number of viral genomes, packaged in infectious particles for transmission. EBV infection in normal epithelium follows the lytic infection mode, whereas latent infection is the principal mode of EBV infection in epithelial cancers such as NPC and EBV-associated gastric carcinoma. Consequently, the latent EBV infection represents a critical hallmark of the pathogenesis of EBV-associated epithelial cancers. Most probably, some somatic mutations and changes in cell signaling in premalignant epithelial cells enhance the switch of the default lytic infection to latent infection, enabling the persistent infection of EBV in epithelial cells [34].
Proteins expressed by the EBV genome, including LMP1, LMP2, and EBNA1, are involved in maintaining the oncogenic properties of the virus and control cancer at every stage, from oncogenesis to progression and metastasis [103][104].
LMP1 has three functional domains in its C-terminal region, C-terminal activating regions 1, 2, and 3 (CTAR1, CTAR2, and CTAR3) that can activate NF-κB, JNK (c-Jun N-terminal kinase), p38 MAPK, JAK/STAT, and PI3K/Akt signal pathways involved in cell cycle progression [103][104]. Cells expressing LMP1 have nonfunctional G2 cell cycle checkpoint and progressive tumorigenesis mediated by the matrix metalloproteases (MMPs). MMPs help degrade extracellular matrix, making the cells vulnerable to the virus. LMP1 can regulate p53 and epidermal growth factor receptor (EGFR) resulting in promoted cell cycle progression through downregulation of the CDK inhibitor p27Kip1, CDK2, and Rb [104]. Additionally, LMP1 can regulate telomerase activity via the p16INK4A/Rb, PI3K-AKT and JNK signaling pathways to stimulate cell immortalization [105] and to regulate angiogenesis and INF-γ pathways important in oncogenic transformation of cells via mediated immune escape [103].
LMP2 viral proteins include LMP2A and LMP2B transmembrane proteins which can hinder tyrosine kinase signaling. LMP2 imitates activated B-cell receptor (BCR), obstructing regular B-cell signal transduction and can associate with Src family protein tyrosine kinases (PTKs) as well as spleen tyrosine kinase (Syk), both related with BCR signal transduction [106]. LMP-2A can influence transformation of epithelial cells through activation of the PI3-kinase–Akt pathway [107]. Like LMP1, LMP2 can also control INF-γ signaling to assure immune escape in cancer. EBNA1 protein is mostly expressed in dividing memory B cells, but due to its preservation of the viral genome in latent infections, EBNA1 is found all EBV-associated cancers including NPSCC. It assists replication of the viral episomes and promotes the survival of cells with damaged DNA [104][108]. EBNA1 can modulate pathways such as invasion, cell proliferation, survival, and DNA damage repair [104]. LMP1, LMP2, and EBNA1proteins represent relevant targets for therapeutic research in last decades, particularly in the field of immunotherapy [37].
3.2. EBV in HNSCC Development
EBV infection is reported in 100% of nonkeratinizing nasopharyngeal carcinomas (NPCs) [34]. Clinically, nasopharyngeal carcinoma (NPC) is exceedingly invasive and metastatic cancer type that is widely predominant in southern China [42][109]. Epidemiology, pathological types, and treatment options of NPC differs from other cancers arising from head and neck [110]. Patients diagnosed with early clinical stage disease have circa 90% OS rate. However, most patients are diagnosed in advanced stages, and the survival rate is decreased to <50% [50].
According to the numerous data, EBV is closely linked to NPC. Zheng et al. detected EBV genomes in almost 100% of tumor lesions in undifferentiated type NPC, squamous cell and non–keratinizing NPC in endemic areas [50]. However, low levels of EBV DNA load were also detected in some NP brushing samples from normal NP, which contradicts to the hypothesis that healthy NP epithelial cells are negative for EBV infection. Zheng et al. detected the EBV DNA load in 87.8% of NP brushing samples (n = 82) from the control group in the high-risk area [50].
Extensive efforts were made to find a method to simplify NPC diagnosis and screening by testing EBV-related biomarkers. Since 1990s, detecting EBV DNA load in plasma or serum was established as a strong biomarker of NPC [50]. The blood plasma EBV DNA is detected in the tumor cells of nearly all anaplastic nasopharyngeal cancers (NPCs) [42]. This method is considered the most precise molecular predictive biomarker in diagnosis and treatment [42][50][110]. Additionally, nasopharyngeal (NP) brushing/swab samples can also be used for qualitative and quantitative detection of EBV DNA. In addition, tumor makers mRNA,18 microRNA22 and tumor suppressor gene methylation can be evaluated by NP brush sampling [50].
3.3. Current Treatment Options and Update on Clinical Trials
EBV-positive NPC is recognized as one of the most aggressive and metastatic head and neck cancers, with a cure rate of 90% in early I–II stages [111]. As the majority of patients are asymptomatic, diagnosis usually occurs in advanced III-IV EBV-positive NPC stages, resulting in five-year survival rate of 50–60% [111][112]. Several characteristics of nonkeratinizing EBV-positive NPC exist, including high expression of PD-L1 [113], dense lymphocytic infiltration [114] and prevalence of specific genetic alterations (CCDN1, PI3K/Akt, NF-kB, and epigenetic deregulations), providing potentially novel targets for immunotherapeutic and personalized medicine approaches [112]. However, current treatment options are cancer stage-dependent: I-II NPC stage patients are treated with RT and concurrent CT, while advance stage patients are treated with CT [115]. Although first line treatment options including cisplatin and gemcitabine have resulted in high response rates, PFS remains at just seven months [116]. No drug for EBV-positive NPC has been approved to date.
The expression of EBV-specific proteins represents a promising approach for treatment strategies with checkpoint inhibitors, potentially resulting in favorable tolerance and clinical outcome [114][117]. The effects of nivolumab, an anti-PD1 antibody, were evaluated in a clinical trial with R/M NPC patients (NCT02339558) (Table 3). Following nivolumab treatment, the results indicated ORR of 20.5% and a disease control rate of 54.5%. Moreover, one-year OS and PFS among the patients were 59% and 19.3%, respectively. In regard to PD-1 expression, 33% of PD-L1-positive NPC patients responded to nivolumab treatment, compared to PD-L1-negative NPC patients, where response did not exceed 13%. In addition, patients with loss of human leukocyte antigen A (HLA-A) and/or HLA-B expression showed statistical significance in one-year PFS (30.9%) compared to patients with HLA-A/HLA-B tumor expression (5.6%). Considering promising outcomes of nivolumab treatment in pretreated NPC patients, its activity and correlation to biomarker expression should be further evaluated [112]. Therefore, anti-tumor activity of nivolumab and ipilimumab, an anti-CTLA-4 antibody as combinatorial therapy is currently under evaluation in phase II clinical trial in patients with rare tumor types, including NPC (NCT02834013) (Table 3). Importantly, the efficacy of nivolumab and other immunotherapeutic candidates is currently under phase II clinical investigation (CheckMate358, NCT02488759) in patients with virus-associated cancer, including EBV-positive NPC (Table 3). Activity of another anti-PD1 monoclonal antibody, pembrolizumab has been evaluated in multi-cohort, phase Ib clinical trial (KEYNOTE-028, NCT02054806) in patients with R/M NPC expressing PD-L1 (93.2%) (Table 3). In this study, ORR by investigator review was achieved in 25.9% of the patients, with one-year PFS rate of 33.4%. Conclusively, pembrolizumab has demonstrated favorable anti-cancer activity and acceptable safety profile in NPC patients [118].
In addition to immunotherapy, the adoptive transfer of cytotoxic T-lymphocytes (CTLs) specific to EBV that target LMP1, LMP2, and EBNA1 viral antigens has been recognized as potential approach for endemic NPC patients [37]. In order to improve the patient’s clinical outcome, CT including gemcitabine and carboplatin (four cycles) was administered to NPC patients (n = 35), following EBV-CTL (six doses). Therapeutic response rate was 71.4%, with two-year OS of 62.9% and three-year OS of 37.1%. Thus, addition of CTL with T-cells specific for EBV LMP2 were significantly correlated to OS rates. Interestingly, after adoptive CTL transfer, 14.3% of the patients did not require further CT treatment for 34 months. As a result of outstanding OS rates in locally recurrent or metastatic EBV-positive NPC patients [119], currently ongoing phase III clinical trial (NCT02578641) will evaluate therapeutic outcome of carboplatin and gemcitabine with or without EBV CTL therapy in patients with metastatic EBV-positive NPC. CTLs have drawn a great attention in the treatment of EBV-positive NPC. Multiple clinical trials are evaluating the efficacy of specific CTLs, including LMP1 and LMP2 (NCT00516087), CTL treatment with prior treatment with monoclonal CD45 antibody (NCT00706316), however, the results are not available yet (Table 3). Multiple treatment strategies for NPC have been investigated through clinical trials, including EGFR inhibitors, PI3K/Akt inhibitors, inhibitors of angiogenesis, and other, however no drug has been approved for the treatment of NPC. However, EBV-positive NPC has a specific molecular signature where immunotherapy represents a promising approach in EBV-positive NPC management, especially adoptive EBV CTL that are under clinical investigation [37][112].
Furthermore, high expression of EBV-specific proteins, such as LMP1, LMP2, and most importantly, EBNA1, represents a promising target for the vaccine development [33]. Among the crucial factors for EBNA1 as an immunotherapeutic target are its involvement in the regulation of molecular signaling, maintenance of EBV DNA, and the presence of multiple CD4+ T-cell epitopes. Immunological competence of LMP2 is observed through its expression of large number of CD8+ T-cell epitopes [33][120].
| Clinical Trial/NCT Number/Year | Phase | Disease Stage | Patient Number (n) | Treatment Arms | Outcome |
|---|---|---|---|---|---|
| NCI-9742 NCT02339558 2015–2019 [114] |
Phase II | Nonkeratinizing, R/M NPC, stage III-IVc | n = 45; Plasma EBV DNA detection (n = 44) |
Nivolumab (3 mg/kg for 4 weeks) | ORR: 20.5% one-year outcome: OS: 59% PFS:19.3% |
| KEYNOTE-028 NCT02054806 2014–2020 [118] |
Phase I | PD-L1-positive, R/M NPC | n = 27 | Pembrolizumab (10 mg/kg every 2-week cycle for 24 months) | ORR: 25.9%; one-year PFS: 33.4% |
| Adoptive T-cell transfer NCT02578641 2008–2011 [119] |
Phase II | EBV-positive R/M NPC | n = 38 | Venesection → CT (gemcitabine 1000 mg/m2 and carboplatin AUC 2 every 4 weeks for 4 cycles) + EBV-CTLs 1 (1 × 108 cells/m2 on weeks 0, 2, 8, 16, 24, 32) | RR: 71.4%; Median OS: 29.9 months; two- and three-year OS: 62.9% vs. 37.1% |
| MVA-EBNA1/LMP2 (MVA-EL) NCT01256853 2006–2010 [121] |
Phase I | EBV-positive NPC | n = 18 | MVA-EL vaccine (3 intradermal vaccinations at 3-week period, with doses of 5 × 107, 1 × 108, 2 × 108, 3.3 × 108, 5 × 108 plaque forming units (pfu) | T-cell response (one or both antigens): 15 patients |
| MVA-EL NCT01147991 2005–2010 [120] |
Phase Ia | EBV-positive NPC | n = 16 | MVA-EL vaccine (3 intradermal vaccinations at 3-week period, doses of 5 × 107–5 × 108 pfu) | T-cell response (one or both antigens): 8 patients (7/14, EBNA1; 6/14 LMP2) |
The first clinical trials evaluating vaccination strategies for NPC used LMP2 CD8+ T-cell epitope peptides loaded into autologous monocyte-derived dendritic cells. Out of 16 patients, LMP2 T-cells were detected in 9, with partial CR in 2 patients [122]. The presence of the abovementioned EBV targets has drawn interest in the implementation of immunotherapeutic strategies in the treatment of NPC. Unfortunately, vaccination with autologous monocyte-derived DCs is limited to specialized centers with trained personnel, thus excluding treatment on a large scale [33]. Therefore, Hui and colleagues (2013) presented a distinct immunotherapeutic approach based on vaccination with recombinant vectors, known as Modified Vaccinia Ankara (MVA). MVA virus encodes inactivated fusion protein with half of EBNA1 C-terminal coding for epitopes but lacks specific repeat that results in failure in sequence presentation to CD8+ T-cells. Additionally, the MVA virus also encodes LMP2A [121]. In phase I clinical study (NCT01256853), patients in remission having EBV-positive NPC (n = 18) were treated with three MVA-EL vaccines at escalating doses. Out of eighteen patients, T-cell response mapped to certain CD4 and CD8 EBNA1 and LMP2 epitopes was observed in fifteen patients. Importantly, strong responses were observed in patients treated with the highest dose, suggesting a good safety profile and immunogenicity of MVA-EL. Considering its safe profile and favorable treatment outcome, the safety and immunogenicity of MVA-EL was evaluated in a follow-up phase I clinical trial (NCT01147991) among fourteen patients in remission with EBV-positive NPC. The results revealed good tolerance of the vaccine, as well as the development of immunogenicity to at least one antigen in eight patients [123]. Based on promising results, the clinical benefit rate of the MVA-EL vaccine is currently under evaluation in a phase II clinical trial with R/M NPC patients with residual EBV DNA load following conventional treatment regimen (NCT01094405) (Table 3) [33].
References
- Pezzuto, F.; Buonaguro, L.; Caponigro, F.; Ionna, F.; Starita, N.; Annunziata, C.; Buonaguro, F.M.; Tornesello, M.L. Update on Head and Neck Cancer: Current Knowledge on Epidemiology, Risk Factors, Molecular Features and Novel Therapies. Oncology 2015, 89, 125–136.
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72.
- Suresh, G.M.; Koppad, R.; Prakash, B.V.; Sabitha, K.S.; Dhara, P.S. Prognostic Indicators of Oral Squamous Cell Carcinoma. Ann. Maxillofac. Surg. 2019, 9, 364–370.
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and Neck Squamous Cell Carcinoma. Nat. Rev. Dis. Primer 2020, 6, 92.
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The Molecular Landscape of Head and Neck Cancer. Nat. Rev. Cancer 2018, 18, 269–282.
- Marur, S.; D’Souza, G.; Westra, W.H.; Forastiere, A.A. HPV-Associated Head and Neck Cancer: A Virus-Related Cancer Epidemic. Lancet Oncol. 2010, 11, 781–789.
- Syrjänen, S. Oral Manifestations of Human Papillomavirus Infections. Eur. J. Oral Sci. 2018, 126 (Suppl. 1), 49–66.
- Araldi, R.P.; Sant’Ana, T.A.; Módolo, D.G.; de Melo, T.C.; Spadacci-Morena, D.D.; de Cassia Stocco, R.; Cerutti, J.M.; de Souza, E.B. The Human Papillomavirus (HPV)-Related Cancer Biology: An Overview. Biomed. Pharmacother. 2018, 106, 1537–1556.
- Brianti, P.; De Flammineis, E.; Mercuri, S.R. Review of HPV-Related Diseases and Cancers. New Microbiol. 2017, 40, 80–85.
- Tommasino, M. The Human Papillomavirus Family and Its Role in Carcinogenesis. Semin. Cancer Biol. 2014, 26, 13–21.
- Broccolo, F.; Ciccarese, G.; Rossi, A.; Anselmi, L.; Drago, F.; Toniolo, A. Human Papillomavirus (HPV) and Epstein-Barr Virus (EBV) in Keratinizing versus Non- Keratinizing Squamous Cell Carcinoma of the Oropharynx. Infect. Agent. Cancer 2018, 13, 32.
- Burd, E.M. Human Papillomavirus and Cervical Cancer. Clin. Microbiol. Rev. 2003, 16, 1–17.
- Sathish, N.; Wang, X.; Yuan, Y. Human Papillomavirus (HPV)-Associated Oral Cancers and Treatment Strategies. J. Dent. Res. 2014, 93, 29S–36S.
- Jiang, S.; Dong, Y. Human Papillomavirus and Oral Squamous Cell Carcinoma: A Review of HPV-Positive Oral Squamous Cell Carcinoma and Possible Strategies for Future. Curr. Probl. Cancer 2017, 41, 323–327.
- Budu, V.A.; Decuseară, T.; Balica, N.C.; Mogoantă, C.A.; Rădulescu, L.M.; Chirilă, M.; Maniu, A.A.; Mistra, D.M.; Muşat, G.C.; Oprişcan, I.C.; et al. The Role of HPV Infection in Oropharyngeal Cancer. Rom. J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2019, 60, 769–773.
- Blitzer, G.C.; Smith, M.A.; Harris, S.L.; Kimple, R.J. Review of the Clinical and Biologic Aspects of Human Papillomavirus-Positive Squamous Cell Carcinomas of the Head and Neck. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 761–770.
- Williams, V.M.; Filippova, M.; Soto, U.; Duerksen-Hughes, P.J. HPV-DNA Integration and Carcinogenesis: Putative Roles for Inflammation and Oxidative Stress. Future Virol. 2011, 6, 45–57.
- Parfenov, M.; Pedamallu, C.S.; Gehlenborg, N.; Freeman, S.S.; Danilova, L.; Bristow, C.A.; Lee, S.; Hadjipanayis, A.G.; Ivanova, E.V.; Wilkerson, M.D.; et al. Characterization of HPV and Host Genome Interactions in Primary Head and Neck Cancers. Proc. Natl. Acad. Sci. USA 2014, 111, 15544–15549.
- Schiller, J.T.; Day, P.M.; Kines, R.C. Current Understanding of the Mechanism of HPV Infection. Gynecol. Oncol. 2010, 118, S12–S17.
- Yim, E.-K.; Park, J.-S. The Role of HPV E6 and E7 Oncoproteins in HPV-Associated Cervical Carcinogenesis. Cancer Res. Treat. 2005, 37, 319–324.
- Roman, A.; Munger, K. The Papillomavirus E7 Proteins. Virology 2013, 445, 138–168.
- Dong, L.; Zhang, L.; Hu, S.-Y.; Feng, R.-M.; Zhao, X.-L.; Zhang, Q.; Pan, Q.-J.; Zhang, X.; Qiao, Y.-L.; Zhao, F.-H. Risk Stratification of HPV 16 DNA Methylation Combined with E6 Oncoprotein in Cervical Cancer Screening: A 10-Year Prospective Cohort Study. Clin. Epigenetics 2020, 12, 62.
- Balderas-Loaeza, A.; Anaya-Saavedra, G.; Ramirez-Amador, V.A.; Guido-Jimenez, M.C.; Kalantari, M.; Calleja-Macias, I.E.; Bernard, H.-U.; Garcia-Carranca, A. Human Papillomavirus-16 DNA Methylation Patterns Support a Causal Association of the Virus with Oral Squamous Cell Carcinomas. Int. J. Cancer 2007, 120, 2165–2169.
- Ekanayake Weeramange, C.; Tang, K.D.; Vasani, S.; Langton-Lockton, J.; Kenny, L.; Punyadeera, C. DNA Methylation Changes in Human Papillomavirus-Driven Head and Neck Cancers. Cells 2020, 9, 61359.
- Laaneväli, A.; Ustav, M.; Ustav, E.; Piirsoo, M. E2 Protein Is the Major Determinant of Specificity at the Human Papillomavirus Origin of Replication. PLoS ONE 2019, 14, e0224334.
- Amaro-Filho, S.M.; Pereira Chaves, C.B.; Felix, S.P.; Basto, D.L.; de Almeida, L.M.; Moreira, M.A.M. HPV DNA Methylation at the Early Promoter and E1/E2 Integrity: A Comparison between HPV16, HPV18 and HPV45 in Cervical Cancer. Papillomavirus Res. 2018, 5, 172–179.
- Khanal, S.; Shumway, B.S.; Zahin, M.; Redman, R.A.; Strickley, J.D.; Trainor, P.J.; Rai, S.N.; Ghim, S.-J.; Jenson, A.B.; Joh, J. Viral DNA Integration and Methylation of Human Papillomavirus Type 16 in High-Grade Oral Epithelial Dysplasia and Head and Neck Squamous Cell Carcinoma. Oncotarget 2018, 9, 30419–30433.
- Chaturvedi, A.K.; Graubard, B.I.; Broutian, T.; Pickard, R.K.L.; Tong, Z.-Y.; Xiao, W.; Kahle, L.; Gillison, M.L. Effect of Prophylactic Human Papillomavirus (HPV) Vaccination on Oral HPV Infections Among Young Adults in the United States. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 262–267.
- Kato, N. Genome of Human Hepatitis C Virus (HCV): Gene Organization, Sequence Diversity, and Variation. Microb. Comp. Genom. 2000, 5, 129–151.
- Liang, T.J. Hepatitis B: The Virus and Disease. Hepatol. Baltim. Md 2009, 49, S13–S21.
- Tsai, K.-N.; Kuo, C.-F.; Ou, J.-H.J. Mechanisms of Hepatitis B Virus Persistence. Trends Microbiol. 2018, 26, 33–42.
- Wang, Y.; Wang, J.; Wu, S.; Zhu, H. The Unexpected Structures of Hepatitis C Virus Envelope Proteins. Exp. Ther. Med. 2017, 14, 1859–1865.
- Fernandes, Q.; Merhi, M.; Raza, A.; Inchakalody, V.P.; Abdelouahab, N.; Zar Gul, A.R.; Uddin, S.; Dermime, S. Role of Epstein-Barr Virus in the Pathogenesis of Head and Neck Cancers and Its Potential as an Immunotherapeutic Target. Front. Oncol. 2018, 8, 257.
- Tsao, S.W.; Tsang, C.M.; Lo, K.W. Epstein-Barr Virus Infection and Nasopharyngeal Carcinoma. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372.
- Su, F.-H.; Chang, S.-N.; Chen, P.-C.; Sung, F.-C.; Huang, S.-F.; Chiou, H.-Y.; Su, C.-T.; Lin, C.-C.; Yeh, C.-C. Positive Association Between Hepatitis C Infection and Oral Cavity Cancer: A Nationwide Population-Based Cohort Study in Taiwan. PLoS ONE 2012, 7, e48109.
- Weng, J.-J.; Wei, J.-Z.; Li, M.; Lu, J.-L.; Qin, Y.-D.; Jiang, H.; Qu, S.-H. Effects of Hepatitis B Virus Infection and Antiviral Therapy on the Clinical Prognosis of Nasopharyngeal Carcinoma. Cancer Med. 2020, 9, 541–551.
- Louis, C.U.; Straathof, K.; Bollard, C.M.; Ennamuri, S.; Gerken, C.; Lopez, T.T.; Huls, M.H.; Sheehan, A.; Wu, M.-F.; Liu, H.; et al. Adoptive Transfer of EBV-Specific T Cells Results in Sustained Clinical Responses in Patients With Locoregional Nasopharyngeal Carcinoma. J. Immunother. 2010, 33, 983–990.
- Mahale, P.; Sturgis, E.M.; Tweardy, D.J.; Ariza-Heredia, E.J.; Torres, H.A. Association Between Hepatitis C Virus and Head and Neck Cancers. J. Natl. Cancer Inst. 2016, 108.
- Shih, C.; Yang, C.-C.; Choijilsuren, G.; Chang, C.-H.; Liou, A.-T. Hepatitis B Virus. Trends Microbiol. 2018, 26, 386–387.
- Tumban, E. A Current Update on Human Papillomavirus-Associated Head and Neck Cancers. Viruses 2019, 11, 922.
- D’souza, G.; Carey, T.E.; William, W.N.; Nguyen, M.L.; Ko, E.C.; Riddell, J.; Pai, S.I.; Gupta, V.; Walline, H.M.; Lee, J.J.; et al. Epidemiology of Head and Neck Squamous Cell Cancer among HIV-Infected Patients. J. Acquir. Immune Defic. Syndr. 1999 2014, 65, 603–610.
- Tan, E.L.; Looi, L.M.; Sam, C.K. Evaluation of Plasma Epstein-Barr Virus DNA Load as a Prognostic Marker for Nasopharyngeal Carcinoma. Singap. Med. J. 2006, 47, 803–807.
- She, Y.; Nong, X.; Zhang, M.; Wang, M. Epstein-Barr Virus Infection and Oral Squamous Cell Carcinoma Risk: A Meta-Analysis. PLoS ONE 2017, 12, e0186860.
- Borsetto, D.; Fussey, J.; Fabris, L.; Bandolin, L.; Gaudioso, P.; Phillips, V.; Polesel, J.; Boscolo-Rizzo, P. HCV Infection and the Risk of Head and Neck Cancer: A Meta-Analysis. Oral Oncol. 2020, 109, 104869.
- Donà, S.; Borsetto, D.; Fussey, J.; Biscaro, V.; Vian, E.; Spinato, G.; Menegaldo, A.; Da Mosto, M.C.; Rigoli, R.; Polesel, J.; et al. Association between Hepatitis C and B Viruses and Head and Neck Squamous Cell Carcinoma. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2019, 121, 104209.
- Nagao, Y.; Sata, M. High Incidence of Multiple Primary Carcinomas in HCV-Infected Patients with Oral Squamous Cell Carcinoma. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2009, 15, CR453–CR459.
- Nayyar, S.S.; Thiagarajan, S.; Malik, A.; D’Cruz, A.; Chaukar, D.; Patil, P.; Alahari, A.D.; Lashkar, S.G.; Prabhash, K. Head and Neck Squamous Cell Carcinoma in HIV, HBV and HCV Seropositive Patients—Prognosis and Its Predictors. J. Cancer Res. Ther. 2020, 16, 619–623.
- Ye, Y.-F.; Xiang, Y.-Q.; Fang, F.; Gao, R.; Zhang, L.-F.; Xie, S.-H.; Liu, Z.; Du, J.-L.; Chen, S.-H.; Hong, M.-H.; et al. Hepatitis B Virus Infection and Risk of Nasopharyngeal Carcinoma in Southern China. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2015, 24, 1766–1773.
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide Burden of Cancer Attributable to HPV by Site, Country and HPV Type. Int. J. Cancer 2017, 141, 664–670.
- Zheng, X.-H.; Wang, R.-Z.; Li, X.-Z.; Zhou, T.; Zhang, J.-B.; Zhang, P.-F.; Lu, L.-X.; Jia, W.-H. Detection of Methylation Status of Epstein-Barr Virus DNA C Promoter in the Diagnosis of Nasopharyngeal Carcinoma. Cancer Sci. 2020, 111, 592–600.
- Komori, M.F.; Kimura, T.; Kariya, S.; Onoda, T.; Takeda, S.; Mizukawa, N.; Iida, S.; Kimata, Y.; Nishizaki, K. Epidemiological Correlations between Head and Neck Cancer and Hepatitis B Core Antibody Positivity. Anticancer Res. 2020, 40, 2393–2403.
- Prabhu, S.R.; Wilson, D.F. Evidence of Epstein-Barr Virus Association with Head and Neck Cancers: A Review. J. Can. Dent. Assoc. 2016, 82, g2.
- Lydiatt, W.; O’Sullivan, B.; Patel, S. Major Changes in Head and Neck Staging for 2018. Am. Soc. Clin. Oncol. Educ. Book Am. Soc. Clin. Oncol. Annu. Meet. 2018, 38, 505–514.
- Knör, M.; Tziridis, K.; Agaimy, A.; Zenk, J.; Wendler, O. Human Papillomavirus (HPV) Prevalence in Nasal and Antrochoanal Polyps and Association with Clinical Data. PLoS ONE 2015, 10, e0141722.
- Thakur, J.S.; Chaitanya, A.; Minhas, R.S.; Azad, R.K.; Sharma, D.R.; Mohindroo, N.K. Killian’s Polyp Mimicking Malignant Tumor. Ann. Maxillofac. Surg. 2015, 5, 281–283.
- Oton-Gonzalez, L.; Rotondo, J.C.; Cerritelli, L.; Malagutti, N.; Lanzillotti, C.; Bononi, I.; Ciorba, A.; Bianchini, C.; Mazziotta, C.; De Mattei, M.; et al. Association between Oncogenic Human Papillomavirus Type 16 and Killian Polyp. Infect. Agent. Cancer 2021, 16, 3.
- Cramer, J.D.; Burtness, B.; Le, Q.T.; Ferris, R.L. The Changing Therapeutic Landscape of Head and Neck Cancer. Nat. Rev. Clin. Oncol. 2019, 16, 669–683.
- Cramer, J.D.; Burtness, B.; Ferris, R.L. Immunotherapy for Head and Neck Cancer: Recent Advances and Future Directions. Oral Oncol. 2019, 99, 104460.
- Sindhu, S.K.; Bauman, J.E. Current Concepts in Chemotherapy for Head and Neck Cancer. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 145–154.
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N. Engl. J. Med. 2010, 363, 24–35.
- Göttgens, E.L.; Ostheimer, C.; Span, P.N.; Bussink, J.; Hammond, E.M. HPV, Hypoxia and Radiation Response in Head and Neck Cancer. Br. J. Radiol. 2018, 92, 20180047.
- Alterio, D.; Marvaso, G.; Ferrari, A.; Volpe, S.; Orecchia, R.; Jereczek-Fossa, B.A. Modern Radiotherapy for Head and Neck Cancer. Semin. Oncol. 2019, 46, 233–245.
- Schwartz, D.L.; Hayes, D.N. The Evolving Role of Radiotherapy for Head and Neck Cancer. Hematol. Oncol. Clin. N. Am. 2020, 34, 91–108.
- Ionna, F.; Guida, A.; Califano, L.; Motta, G.; Salzano, G.; Pavone, E.; Aversa, C.; Longo, F.; Villano, S.; Ponzo, L.M.; et al. Transoral Robotic Surgery in Head and Neck District: A Retrospective Study on 67 Patients Treated in a Single Center. Infect. Agent. Cancer 2020, 15, 40.
- Mydlarz, W.K.; Chan, J.Y.K.; Richmon, J.D. The Role of Surgery for HPV-Associated Head and Neck Cancer. Oral Oncol. 2015, 51, 305–313.
- Fundakowski, C.E.; Lango, M. Considerations in Surgical versus Non-Surgical Management of HPV Positive Oropharyngeal Cancer. Cancers Head Neck 2016, 1, 6.
- Golusiński, W. Functional Organ Preservation Surgery in Head and Neck Cancer: Transoral Robotic Surgery and Beyond. Front. Oncol. 2019, 9, 293.
- Colevas, A.D.; Yom, S.S.; Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Brizel, D.M.; Burtness, B.; Busse, P.M.; Caudell, J.J.; et al. NCCN Guidelines Insights: Head and Neck Cancers, Version 1.2018. J. Natl. Compr. Canc. Netw. 2018, 16, 479–490.
- Chinn, S.B.; Myers, J.N. Oral Cavity Carcinoma: Current Management, Controversies, and Future Directions. J. Clin. Oncol. 2015, 33, 3269–3276.
- Ferris, R.L.; Flamand, Y.; Weinstein, G.S.; Li, S.; Quon, H.; Mehra, R.; Garcia, J.J.; Chung, C.H.; Gillison, M.L.; Duvvuri, U.; et al. Transoral Robotic Surgical Resection Followed by Randomization to Low- or Standard-Dose IMRT in Resectable P16+ Locally Advanced Oropharynx Cancer: A Trial of the ECOG-ACRIN Cancer Research Group (E3311). J. Clin. Oncol. 2020, 38, 6500.
- Moore, E.J.; Van Abel, K.M.; Price, D.L.; Lohse, C.M.; Olsen, K.D.; Jackson, R.S.; Martin, E.J. Transoral Robotic Surgery for Oropharyngeal Carcinoma: Surgical Margins and Oncologic Outcomes. Head Neck 2018, 40, 747–755.
- Zenga, J.; Suko, J.; Kallogjeri, D.; Pipkorn, P.; Nussenbaum, B.; Jackson, R.S. Postoperative Hemorrhage and Hospital Revisit after Transoral Robotic Surgery: Postoperative Hemorrhage After TORS. Laryngoscope 2017, 127, 2287–2292.
- Owadally, W.; Hurt, C.; Timmins, H.; Parsons, E.; Townsend, S.; Patterson, J.; Hutcheson, K.; Powell, N.; Beasley, M.; Palaniappan, N.; et al. PATHOS: A Phase II/III Trial of Risk-Stratified, Reduced Intensity Adjuvant Treatment in Patients Undergoing Transoral Surgery for Human Papillomavirus (HPV) Positive Oropharyngeal Cancer. BMC Cancer 2015, 15, 602.
- Chera, B.S.; Amdur, R.J.; Tepper, J.; Qaqish, B.; Green, R.; Aumer, S.L.; Hayes, N.; Weiss, J.; Grilley-Olson, J.; Zanation, A.; et al. Phase 2 Trial of De-Intensified Chemoradiation Therapy for Favorable-Risk Human Papillomavirus–Associated Oropharyngeal Squamous Cell Carcinoma. Int. J. Radiat. Oncol. 2015, 93, 976–985.
- Nichols, A.C.; Theurer, J.; Prisman, E.; Read, N.; Berthelet, E.; Tran, E.; Fung, K.; de Almeida, J.R.; Bayley, A.; Goldstein, D.P.; et al. Radiotherapy versus Transoral Robotic Surgery and Neck Dissection for Oropharyngeal Squamous Cell Carcinoma (ORATOR): An Open-Label, Phase 2, Randomised Trial. Lancet Oncol. 2019, 20, 1349–1359.
- Wen, Y.; Grandis, J.R. Emerging Drugs for Head and Neck Cancer. Expert Opin. Emerg. Drugs 2015, 20, 313–329.
- Psyrri, A.; Rampias, T.; Vermorken, J.B. The Current and Future Impact of Human Papillomavirus on Treatment of Squamous Cell Carcinoma of the Head and Neck. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 2101–2115.
- Marur, S.; Li, S.; Cmelak, A.J.; Gillison, M.L.; Zhao, W.J.; Ferris, R.L.; Westra, W.H.; Gilbert, J.; Bauman, J.E.; Wagner, L.I.; et al. E1308: Phase II Trial of Induction Chemotherapy Followed by Reduced-Dose Radiation and Weekly Cetuximab in Patients With HPV-Associated Resectable Squamous Cell Carcinoma of the Oropharynx—ECOG-ACRIN Cancer Research Group. J. Clin. Oncol. 2017, 35, 490–497.
- Chera, B.S.; Kumar, S.; Shen, C.; Amdur, R.; Dagan, R.; Green, R.; Goldman, E.; Weiss, J.; Grilley-Olson, J.; Patel, S.; et al. Plasma Circulating Tumor HPV DNA for the Surveillance of Cancer Recurrence in HPV-Associated Oropharyngeal Cancer. J. Clin. Oncol. 2020, 38, 1050–1058.
- Ferris, R.L.; Blumenschein, G.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867.
- Seiwert, T.Y.; Burtness, B.; Mehra, R.; Weiss, J.; Berger, R.; Eder, J.P.; Heath, K.; McClanahan, T.; Lunceford, J.; Gause, C.; et al. Safety and Clinical Activity of Pembrolizumab for Treatment of Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (KEYNOTE-012): An Open-Label, Multicentre, Phase 1b Trial. Lancet Oncol. 2016, 17, 956–965.
- Cohen, E.E.W.; Soulières, D.; Le Tourneau, C.; Dinis, J.; Licitra, L.; Ahn, M.-J.; Soria, A.; Machiels, J.-P.; Mach, N.; Mehra, R.; et al. Pembrolizumab versus Methotrexate, Docetaxel, or Cetuximab for Recurrent or Metastatic Head-and-Neck Squamous Cell Carcinoma (KEYNOTE-040): A Randomised, Open-Label, Phase 3 Study. Lancet Lond. Engl. 2019, 393, 156–167.
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab Alone or with Chemotherapy versus Cetuximab with Chemotherapy for Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (KEYNOTE-048): A Randomised, Open-Label, Phase 3 Study. Lancet 2019, 394, 1915–1928.
- O’Sullivan, B.; Huang, S.H.; Perez-Ordonez, B.; Massey, C.; Siu, L.L.; Weinreb, I.; Hope, A.; Kim, J.; Bayley, A.J.; Cummings, B.; et al. Outcomes of HPV-Related Oropharyngeal Cancer Patients Treated by Radiotherapy Alone Using Altered Fractionation. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2012, 103, 49–56.
- Marur, S.; Forastiere, A.A. Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. Mayo Clin. Proc. 2016, 91, 386–396.
- Ma, D.J.; Price, K.A.; Moore, E.J.; Patel, S.H.; Hinni, M.L.; Garcia, J.J.; Graner, D.E.; Foster, N.R.; Ginos, B.; Neben-Wittich, M.; et al. Phase II Evaluation of Aggressive Dose De-Escalation for Adjuvant Chemoradiotherapy in Human Papillomavirus-Associated Oropharynx Squamous Cell Carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 1909–1918.
- Sim, F.; Leidner, R.; Bell, R.B. Immunotherapy for Head and Neck Cancer. Hematol. Oncol. Clin. N. Am. 2019, 33, 301–321.
- Taberna, M.; Oliva, M.; Mesía, R. Cetuximab-Containing Combinations in Locally Advanced and Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2019, 9, 383.
- Kabolizadeh, P.; Kubicek, G.J.; Heron, D.E.; Ferris, R.L.; Gibson, M.K. The Role of Cetuximab in the Management of Head and Neck Cancers. Expert Opin. Biol. Ther. 2012, 12, 517–528.
- Sano, D.; Fujisawa, T.; Tokuhisa, M.; Shimizu, M.; Sakagami, T.; Hatano, T.; Nishimura, G.; Ichikawa, Y.; Iwai, H.; Oridate, N. Real-World Treatment Outcomes of the EXTREME Regimen as First-Line Therapy for Recurrent/Metastatic Squamous Cell Carcinoma of the Head and Neck: A Multi-Center Retrospective Cohort Study in Japan. Anticancer Res. 2019, 39, 6819–6827.
- Kozakiewicz, P.; Grzybowska-Szatkowska, L. Application of Molecular Targeted Therapies in the Treatment of Head and Neck Squamous Cell Carcinoma (Review). Oncol. Lett. 2018.
- Gavrielatou, N.; Doumas, S.; Economopoulou, P.; Foukas, P.G.; Psyrri, A. Biomarkers for Immunotherapy Response in Head and Neck Cancer. Cancer Treat. Rev. 2020, 84, 101977.
- Kao, H.-F.; Lou, P.-J. Immune Checkpoint Inhibitors for Head and Neck Squamous Cell Carcinoma: Current Landscape and Future Directions. Head Neck 2019, 41 (Suppl. 1), 4–18.
- Ferris, R.L. Immunology and Immunotherapy of Head and Neck Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 3293–3304.
- Berman, T.A.; Schiller, J.T. Human Papillomavirus in Cervical Cancer and Oropharyngeal Cancer: One Cause, Two Diseases: HPV in Cervical and Oropharyngeal Ca. Cancer 2017, 123, 2219–2229.
- Timbang, M.R.; Sim, M.W.; Bewley, A.F.; Farwell, D.G.; Mantravadi, A.; Moore, M.G. HPV-Related Oropharyngeal Cancer: A Review on Burden of the Disease and Opportunities for Prevention and Early Detection. Hum. Vaccines Immunother. 2019, 15, 1920–1928.
- Herrero, R.; Quint, W.; Hildesheim, A.; Gonzalez, P.; Struijk, L.; Katki, H.A.; Porras, C.; Schiffman, M.; Rodriguez, A.C.; Solomon, D.; et al. Reduced Prevalence of Oral Human Papillomavirus (HPV) 4 Years after Bivalent HPV Vaccination in a Randomized Clinical Trial in Costa Rica. PLoS ONE 2013, 8, e68329.
- Hirth, J.M.; Chang, M.; Resto, V.A.; Guo, F.; Berenson, A.B. Prevalence of Oral Human Papillomavirus by Vaccination Status among Young Adults (18–30 Years Old). Vaccine 2017, 35, 3446–3451.
- Bakkalci, D.; Jia, Y.; Winter, J.R.; Lewis, J.E.; Taylor, G.S.; Stagg, H.R. Risk Factors for Epstein Barr Virus-Associated Cancers: A Systematic Review, Critical Appraisal, and Mapping of the Epidemiological Evidence. J. Glob. Health 2020, 10, 010405.
- Correia, S.; Palser, A.; Elgueta Karstegl, C.; Middeldorp, J.M.; Ramayanti, O.; Cohen, J.I.; Hildesheim, A.; Fellner, M.D.; Wiels, J.; White, R.E.; et al. Natural Variation of Epstein-Barr Virus Genes, Proteins, and Primary MicroRNA. J. Virol. 2017, 91.
- Ogembo, J.G.; Kannan, L.; Ghiran, I.; Nicholson-Weller, A.; Finberg, R.W.; Tsokos, G.C.; Fingeroth, J.D. Human Complement Receptor Type 1/CD35 Is an Epstein-Barr Virus Receptor. Cell Rep. 2013, 3, 371–385.
- Odumade, O.A.; Hogquist, K.A.; Balfour, H.H. Progress and Problems in Understanding and Managing Primary Epstein-Barr Virus Infections. Clin. Microbiol. Rev. 2011, 24, 193–209.
- Wang, L.W.; Jiang, S.; Gewurz, B.E. Epstein-Barr Virus LMP1-Mediated Oncogenicity. J. Virol. 2017, 91.
- Yin, H.; Qu, J.; Peng, Q.; Gan, R. Molecular Mechanisms of EBV-Driven Cell Cycle Progression and Oncogenesis. Med. Microbiol. Immunol. (Berl.) 2019, 208, 573–583.
- Mainou, B.A.; Everly, D.N.; Raab-Traub, N. Unique Signaling Properties of CTAR1 in LMP1-Mediated Transformation. J. Virol. 2007, 81, 9680–9692.
- Fotheringham, J.A.; Coalson, N.E.; Raab-Traub, N. Epstein-Barr Virus Latent Membrane Protein-2A Induces ITAM/Syk- and Akt-Dependent Epithelial Migration through Av-Integrin Membrane Translocation. J. Virol. 2012, 86, 10308–10320.
- Fukuda, M.; Longnecker, R. Epstein-Barr Virus Latent Membrane Protein 2A Mediates Transformation through Constitutive Activation of the Ras/PI3-K/Akt Pathway. J. Virol. 2007, 81, 9299–9306.
- Wilson, J.B.; Manet, E.; Gruffat, H.; Busson, P.; Blondel, M.; Fahraeus, R. EBNA1: Oncogenic Activity, Immune Evasion and Biochemical Functions Provide Targets for Novel Therapeutic Strategies against Epstein-Barr Virus- Associated Cancers. Cancers 2018, 10, 109.
- Lung, M.L.; Cheung, A.K.L.; Ko, J.M.Y.; Lung, H.L.; Cheng, Y.; Dai, W. The Interplay of Host Genetic Factors and Epstein-Barr Virus in the Development of Nasopharyngeal Carcinoma. Chin. J. Cancer 2014, 33, 556–568.
- Liu, S.-L.; Sun, X.-S.; Li, X.-Y.; Tang, L.-Q.; Chen, Q.-Y.; Lin, H.-X.; Liang, Y.-J.; Yan, J.-J.; Lin, C.; Guo, S.-S.; et al. The Diagnostic and Prognostic Values of Plasma Epstein-Barr Virus DNA for Residual Cervical Lymphadenopathy in Nasopharyngeal Carcinoma Patients: A Retrospective Study. Cancer Commun. Lond. Engl. 2019, 39, 14.
- Ngan, H.-L.; Wang, L.; Lo, K.-W.; Lui, V.W.Y. Genomic Landscapes of EBV-Associated Nasopharyngeal Carcinoma vs. HPV-Associated Head and Neck Cancer. Cancers 2018, 10, 210.
- Ma, B.B.Y.; Hui, E.P.; Chan, A.T.C. Investigational Drugs for Nasopharyngeal Carcinoma. Expert Opin. Investig. Drugs 2017, 26, 677–685.
- Chen, B.J.; Chapuy, B.; Ouyang, J.; Sun, H.H.; Roemer, M.G.M.; Xu, M.L.; Yu, H.; Fletcher, C.D.M.; Freeman, G.J.; Shipp, M.A.; et al. PD-L1 Expression Is Characteristic of a Subset of Aggressive B-Cell Lymphomas and Virus-Associated Malignancies. Clin. Cancer Res. 2013, 19, 3462–3473.
- Ma, B.B.Y.; Lim, W.-T.; Goh, B.-C.; Hui, E.P.; Lo, K.-W.; Pettinger, A.; Foster, N.R.; Riess, J.W.; Agulnik, M.; Chang, A.Y.C.; et al. Antitumor Activity of Nivolumab in Recurrent and Metastatic Nasopharyngeal Carcinoma: An International, Multicenter Study of the Mayo Clinic Phase 2 Consortium (NCI-9742). J. Clin. Oncol. 2018, 36, 1412–1418.
- Lee, A.W.M.; Ma, B.B.Y.; Ng, W.T.; Chan, A.T.C. Management of Nasopharyngeal Carcinoma: Current Practice and Future Perspective. J. Clin. Oncol. 2015, 33, 3356–3364.
- Zhang, L.; Huang, Y.; Hong, S.; Yang, Y.; Yu, G.; Jia, J.; Peng, P.; Wu, X.; Lin, Q.; Xi, X.; et al. Gemcitabine plus Cisplatin versus Fluorouracil plus Cisplatin in Recurrent or Metastatic Nasopharyngeal Carcinoma: A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet 2016, 388, 1883–1892.
- Fang, W.; Zhang, J.; Hong, S.; Zhan, J.; Chen, N.; Qin, T.; Tang, Y.; Zhang, Y.; Kang, S.; Zhou, T.; et al. EBV-Driven LMP1 and IFN-γ up-Regulate PD-L1 in Nasopharyngeal Carcinoma: Implications for Oncotargeted Therapy. Oncotarget 2014, 5, 12189–12202.
- Hsu, C.; Lee, S.-H.; Ejadi, S.; Even, C.; Cohen, R.B.; Le Tourneau, C.; Mehnert, J.M.; Algazi, A.; van Brummelen, E.M.J.; Saraf, S.; et al. Safety and Antitumor Activity of Pembrolizumab in Patients With Programmed Death-Ligand 1-Positive Nasopharyngeal Carcinoma: Results of the KEYNOTE-028 Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 4050–4056.
- Chia, W.-K.; Teo, M.; Wang, W.-W.; Lee, B.; Ang, S.-F.; Tai, W.-M.; Chee, C.-L.; Ng, J.; Kan, R.; Lim, W.-T.; et al. Adoptive T-Cell Transfer and Chemotherapy in the First-Line Treatment of Metastatic and/or Locally Recurrent Nasopharyngeal Carcinoma. Mol. Ther. 2014, 22, 132–139.
- Taylor, G.S.; Steven, N.M. Therapeutic Vaccination Strategies to Treat Nasopharyngeal Carcinoma. Chin. Clin. Oncol. 2016, 5, 23.
- Hui, E.P.; Taylor, G.S.; Jia, H.; Ma, B.B.Y.; Chan, S.L.; Ho, R.; Wong, W.-L.; Wilson, S.; Johnson, B.F.; Edwards, C.; et al. Phase I Trial of Recombinant Modified Vaccinia Ankara Encoding Epstein–Barr Viral Tumor Antigens in Nasopharyngeal Carcinoma Patients. Cancer Res. 2013, 73, 1676–1688.
- Lin, C.-L.; Lo, W.-F.; Lee, T.-H.; Ren, Y.; Hwang, S.-L.; Cheng, Y.-F.; Chen, C.-L.; Chang, Y.-S.; Lee, S.P.; Rickinson, A.B.; et al. Immunization with Epstein-Barr Virus (EBV) Peptide-Pulsed Dendritic Cells Induces Functional CD8+ T-Cell Immunity and May Lead to Tumor Regression in Patients with EBV-Positive Nasopharyngeal Carcinoma. Cancer Res. 2002, 62, 6952–6958.
- Taylor, G.S.; Jia, H.; Harrington, K.; Lee, L.W.; Turner, J.; Ladell, K.; Price, D.A.; Tanday, M.; Matthews, J.; Roberts, C.; et al. A Recombinant Modified Vaccinia Ankara Vaccine Encoding Epstein–Barr Virus (EBV) Target Antigens: A Phase I Trial in UK Patients with EBV-Positive Cancer. Clin. Cancer Res. 2014, 20, 5009–5022.
