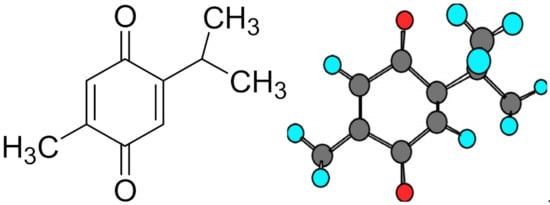
2. Neoplasm and Its Pathogenesis
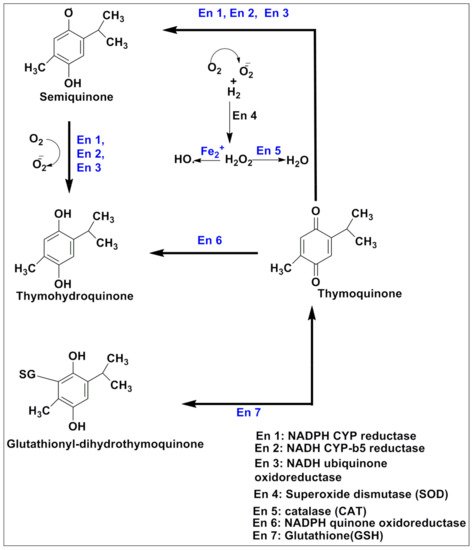
A large group of individuals are diagnosed with cancer annually, being the second leading cause of mortality worldwide [20]. Its pathogenesis is very complex and is often difficult to identify, and most of the time, it is multifactorial. The tendency to multiply some groups of cells beyond their limit leads to abnormal development in a specific body part, which is called neoplasm or cancer [21]. Generally, metastasis-suppressor genes are involved in the inhibition of motility, invasiveness, colony formation, growth arrest, differentiation, proliferation, adhesion to extracellular matrix components, cell-cell adhesion and aggregation, and the immune sensitivity of cells [22][23]. All of these tasks require precise timing, which is controlled by a variety of cellular functions. Signaling, transcriptional activation, integrin expression, and signaling, cell adhesion, and motility, cell communication, cytokine stress-induced signaling, serine protease expression, and nucleotide diphosphate kinase activity are among these functions [24]. Failing any of the above-said factors or group of factors may initiate cancer genesis [25]. Epigenetic changes also play a crucial role in disease initiation. Lower levels of H3K4me2, H3K18ac, and H3K9me are linked to a poor prognosis in prostate, lung, and kidney cancers, respectively; similarly, higher levels of H3K9ac expression in lung cancer patients are linked to a shorter survival period [26][27]. Thymoquinone has recently been shown to modulate epigenetic machinery, such as histone acetylation and deacetylation, DNA methylation, and demethylation, all of which are significant epigenetic changes that may lead to carcinogenesis [28]. TQ has antineoplastic activity against human tumors, antioxidant effects and anti-inflammation in animal models and cell culture systems, chemopreventive effects, and most notably, anti-multidrug-resistant variants of human malignant cell [29].
2.1. The Mechanistic Approach to Treat Cancer Using TQ Drug Molecule
The pharmacological effects of TQ on different cell lines and animal models demonstrated substantial antineoplastic activities in numerous cancers, including breast, prostate, brain, pancreas, gastric, colon, bladder, lungs, bone, cervical, and many more [30]. Mechanistically, it can suppress various properties, including multiplication in cancer cells, apoptosis, activation of detoxifying enzymes, metastasis, suppression of tumor-angiogenesis invasion, and cell cycle control [31][32][33][34][35][36][37][38][39].
Kinases are cellular enzyme stimuli, essential for cellular metabolic functions, and their overexpression is closely linked with cancer [40]. TQ effectively targets many phosphoinositides, including 3-kinase (PI3K) [41], mitogen-activated protein kinase (MAPK)/Janus kinase signal transducers and transcription (JAK/STAT) [42][43], polo-like kinase 1 (PLC1) [44] and tyrosine kinase [45].
Responsive and resistive MCF-07 breast cancer cell lines displayed good anticarcinogenic activities with TQ analogs such as caryophyllyl and germacrylic conjugates as well as fatty acid conjugates [46]. The TQ neutralizes oxidative free radicals and ameliorates doxorubicin-induced nephrotoxicity [47]. The carcinogenesis produces eicosanoids, and peroxidizes membrane lipid suppressive activities [48]. Furthermore, TQ displayed a hyperproliferative effect in rats and also abrogated Fe (III) nitrilotriacetic acid (Fe-NTA) induced oxidative stress [30]. TQ reduced Cyclin A, Cyclin B1, Cyclin D1 and Cyclin E [49][50][51][52] expression and increased levels of p21 and p53 [53][54]. TQ is capable of decreasing Bcl-2 and increasing cleaved caspase-3, 9, and 7, and Bax proteins, as well as modulating the expression of microRNA (miRNA) and long non-coding RNAs (lncRNA), acetylation/deacetylation of histone along with methylation/demethylation of DNA, resulting in mitochondrial apoptosis induction [28][30][55][56]. TQ also halts the PI3K/AKT signaling pathway by upregulating PTEN, thus interfering with GSK-3β activity, enhancing β-catenin degradation, and decreasing MMP-9 and MMP-2 levels in esophageal cancer cells (Eca109 cells) [50]. MicroRNA-34a (miR-34a) expression is vital to cancer development and metastasis [57], and its expression is reduced by TQ in human metastatic breast cancers (MBC) compared to normal breast tissues [58]. Altogether, microRNA-34a can act as therapy either alone or in combination with TQ, and synergize therapeutic potential [59]. TQ exerts antiproliferative activities in cancer cells by modulating the structure of DNA [60][61]. TQ synergized pancreatic cancer cells (MIA Paca-2 cells) cytotoxicity along with juglone via ferroptosis, an iron-dependent mechanism [62]. The mechanistic approach of TQ for cancer treatment is depicted in Figure 3 and in vitro and in vivo applications of TQ are reported in Table 1 and Table 2, respectively.
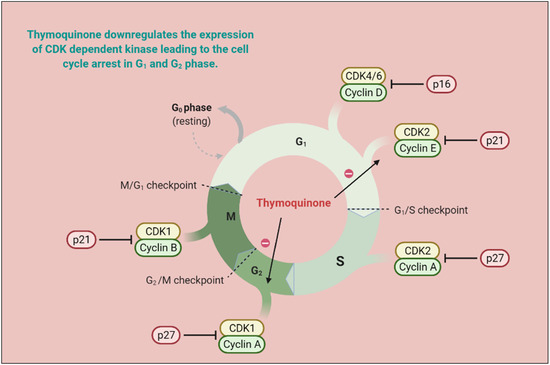
Figure 3. TQ prevents carcinogenic intermediate synthesis by inhibiting the G2/M phase of the cell cycle. It also inhibits ROS-mediated DNA damage to prevent tumorigenesis. TQ upregulates pro-apoptotic genes (p21 and p27) and downregulates the anti-apoptotic gene (Bcl-2), thereby arresting the G2/M phase of the cell cycle. (CDK—cyclin-dependent kinases; CYP—cytochrome P; TQ—Thymoquinone).
Table 1. In vitro applications of thymoquinone in the treatment of cancer (↓: decreases, ↑: increase).
| S.N | Drug and Dose | Cell Line | Molecular Target | Outcome | Ref. |
|---|---|---|---|---|---|
| 1 | TQ (25–75µM) |
||||
| ↓elongation factor 2 kinase, ↓Src/FAK, ↓Akt, ↑miR-603, ↓NF-kB | |||||
| ↓tumor growth | |||||
| [ | |||||
| 65 | |||||
| ] | |||||
| S.N | Drug and Dose | Animal Model | Molecular Target | Outcome | Ref. |
|---|
| S.N | Formulations | Animal Model/Cell Line | Major Finding | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Eca109 cells | ↑p21, and p53 levels; ↓Cyclin A, Cyclin B1, and Cyclin E expression; ↑β-catenin degradation, and ↓MMP-2, 9 levels; ↓in Bcl-2 and ↑caspase-3,7 and 9 cleavages, ↑Bax, ↑PTEN | Induced cell cycle arrest in the G2/M phase; ↓cell proliferation and invasion | [ | ||||||
| 1 | 50 | ] | TQ, DOX, and TQ+DOX | [ | 63] | ||||
| 1 | Chitosan- (CS)-coated poly(d,l-lactide-co-glycolide) NPs | Wistar albino rats | MDA-MB-231 MCF-7 | ↑Intestinal permeation; ↑BA; ↓dose and dosing frequency, ↑antioxidant potential | [172] | ||||
| 2 | TQ (511.19 µM) and juglone (40.90 µM) | MIA PaCa-2, BXPC-3, and Panc-1 pancreatic cancer cells | Ferroptosis | ||||||
| 2 | Anisamide coated TQ loaded lipidic core nanocapsules shell of eudragit S100 | Synergism in anticancer potential | 124 | ] | |||||
| HT-29, | HCT-116, | [ | 62 | ] | |||||
| Caco-2 | Anisamide coating ↑colonic delivery of TQ due to specific binding with overexpressed sigma receptor | [ | 173] | 3 | TQ (2.5–200 μM) | C6 rat glioma cells | Induced DNA damage, apoptosis, and ↑iROS. ↓GSH; ↑intracellular calcium level which initiates apoptosis ↓Bcl-2 and pSTAT3; ↑Bax, ↑Caspase-3,9; ↓MMP and GSH levels | Dose-dependent apoptosis induction | |
| 3 | RNA aptamer A10 coated TQ loaded planetary ball-milled NPs of starch PCL, and PEG for specific bindings to prostate-specific membrane antigen overexpressed ABC transporter genes | DOX resistant C4-2B-R and LNCaP-R cells with a high expression of Hh | ↑targeted delivery ↑circulations time, resensitized cancer cells for DOX |
[174] | [64] | ||||
.
2.2.2. Active Targeting
Receptors Based Active Targeting
A variety of surface receptors have been found to be upregulated in certain physiological conditions, including cancer, and are widely utilized for delivery via surface-decorated nanoparticles (NPs). The surface-coated NPs can target those cells which overexpress specific receptors on their surface and because of this, the nanoparticles attach to these [10]. The same is shown in Figure 5. The ligands which are used for surface modification include hyaluronic acid, anisamide, transferrin, folic acid, and many more utilized for active targeting of TQ into cancer. These have been reported in the following sections. This receptor is overexpressed in various types of cancers, including colon, brain, breast, lung, prostate, and kidney [187][188]. Anisamide is a benzamide analog, which exhibits a higher affinity towards sigma receptor-expressing cells [189] Anisamide-conjugated polymeric nanocapsules of eudragit-S100 delivered TQ into the colon-specific region through binding with overexpressed colonic sigma receptor [173]. The RNA aptamer, A10-coated planetary ball-milled starch NPs of TQ exclusively delivered drug into docetaxel-resistant prostate cancer cell lines (C4-2B-R and LNCaP-R) through overexpressed prostate-specific membrane antigen and inhibited drug efflux, which improves cancer potential [174]. The PEG and PCL, in the ball-milled NPs, decrease non-specific binding to the cell membrane and allow prolonged circulations. Hyaluronic acid (HA)-decorated Pluronic® NPs of TQ accumulated in TNBC cells through selective binding with overexpressed CD44 receptor of cancer cells [176]. Pluronic-enhanced TQ encapsulation and HA facilitate CD44 targeting and make it have prolonged circulation, which reduced the dose for cell migration by modulating both miR-361/Rac1 and RhoA/actin stress fibers and the miR-361/VEGF-A mechanism that attenuate angiogenesis and metastasis of TNBC cells. Radio-iodinated NPs of folic acid-chitosan specifically bind to overexpressed folate receptors of human ovarian cancer cells (SKOV3) and improve anticancer efficacy through improved cellular internalization and retention [145]. A PEGylated-PLGA-TQ-NP surface decorated with transferrin potentiated anticancer efficacy of TQ through specific binding with the overexpressed transferring receptor on tumor cells, which decreases dose and improved cellular accumulations of NPs through EPR, as investigated in lung carcinoma A549 cells [182]. The as1411-conjugated nanodroplets delivered TQ into cancer cells through specific binding with overexpressed nucleolin on the cancer cells surface as investigated in MDA-MB-231 cells [183]. The as1411-conjugation facilitates rapid cellular uptake and dose-dependent cytotoxicity via nucleolin-stimulated Rac1 activation [190].
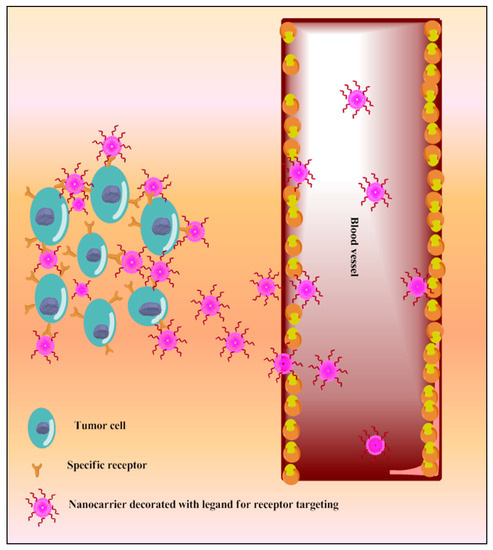
Figure 5. Schematic diagram of TQ nanocarriers for receptor-based active targeting.
PI3K/Akt activation in cancer cells leads to resistance to traditional chemotherapeutics [191]. pH-sensitive gold niosomes of TQ along with Akt-siRNA were utilized to deliver TQ into tamoxifen-resistant breast cancer cells as well as knockdown of Akt-overexpression [63][166]. These niosomes resensitized cancer cells to TQ through Akt silencing and enhanced apoptosis by inhibiting MDM2 expression as well as inducing p53 [166].
Stimulus-Responsive NPs for Active Targeting
Designing stimuli-responsive NPs for active targeted drug delivery is dependent upon tumor microenvironments such as pH, hyperthermia, catalytic enzymes, or external stimuli such as pressure, ultrasonication, or magnetic field. The stimuli-responsive NPs retain their physicochemical properties, including structure, during their circulation. They are stimulated upon exposure to small changes in the tumor microenvironment or external stimuli and undergo rapid changes (aggregation, permeability, and disruption) to release the encapsulated drug. Various TQ-loaded stimuli-responsive NPs with enhanced anticancer potentials have been discussed in the following sections. A TQ-loaded Fe3SO4 NPs surface decorated with ethylene glycol and polyvinylpyrrolidone (PVP) pH-dependently delivered TQ in TNBC cells (MDA-MB-231) [175]. PVP surface decoration improved water solubility and delivered drugs in the acidic environment, which maximized tumoricidal efficiency.
Eudragit L-100-coated nanoconjugates of chitosan, HPMC, and PVA pH dependently delivered TQ into the colon for cancer management [180]. This study finds that at pH 7 concentration, eudragit L-100 dissolves and chitosan becomes degraded by anaerobic bacteria. The bacterial fermentation end-product butyrate forms polysaccharides with anticancer potential; TQ is released with butyrate and reaches into cancer cells, showing higher cytotoxicity. A technetium-99m (99mTc)-labeled TQ formulation was designed for theranostic application against skeletal muscle malignancy (rhabdomyosarcoma) [146]. The 99mTC with TQ synergizes anticancer potential through rapid internalization and slower externalization, which enhanced theranostic applications. A fluorescent liposome co-delivered TQ and curcumin into lung cancer cells (A549) and potentially inhibited cellular proliferation compared with TQ or curcumin alone or the lipidic formulation of either of them, probably due to improved internalization [152]. A TQ-capped magnetic nanoparticle of iron oxide improved endocytotic internalization in breast cancer cells (MDA-MB-231 cells) and displayed a potent synergistic chemo-photothermal effect compared with free TQ [144]. Guar gum microvehicles rapidly release TQ in the intracellular acidic environment of cancer cells (pH~ 5.5) compared to physiological pH (~7.4), due to breakdown of the interlinking bonds in an acidic environment, leading to prolonged TQ release, with synergistic anticancer activity, as investigated in HepG2 cell line [159].
3. Role of TQ in Toxicity Reduction
TQ is systemically well-tolerated with a large safety profile dose (LD50, 2.5 g/kg) [3] and has the potential to reduce oxidative stress and systemic toxicity as the dose increases. The intravenous dose of 25 mg/kg thymoquinone nanostructured lipid carrier (TQ-NLC) was found safe in female Sprague Dawley rats [192]. It shows antiproliferative effect at 20 µM, genotoxicity at concentration ≥1.25 µM, and cellular narcosis at between 2.5 and 20 µM concentrations in the rat hepatocyte [193]. TQ (10 mg/kg) ameliorated sodium arsenate (20 mg/kg)-induced neurotoxicity by increasing the levels of norepinephrine, dopamine, superoxide dismutase, and catalase, and decreases serotonin, nitrate, and tumor necrosis factor-alpha (TNF-α) levels in the cerebellum, cortex, and brain stem regions [194]. In another study, the neuroprotective effect of TQ (10 mg/kg/day) was observed on electromagnetic radiation-induced oxidative stress [195]. Similarly, glutamate and iron oxide nanoparticle-induced toxicity were also attenuated by TQ [5]. A combined formulation of Costus speciosus, Fumaria indica, Cichorium intybus, and TQ (CFCT) (25 mg/kg per oral) decrease cisplatin-induced hepatorenal toxicity in rats through membrane stabilization and decreasing aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase serum levels [196].
4. Recent Update on Patents of Thymoquinone
The latest patent literature search on thymoquinone and its loaded nanocarriers reported potential applications in the prevention, balancing, and treatment of multiple physiological conditions such as cancer, inflammations, dermal disorders, anxiety, and stress-related disorders; treatment of female urinary tract infections; and management of immunological diseases, etc. TQ was patented alone and in combinations for the treatment of inflammatory symptoms, including the eicosapentaenoic acid pathway [197]. Additionally, TQ and H5WYG peptide-loaded nano-micelles were also patented for targeted cancer drug delivery [198] and TQ-loaded nanodroplet emulsions for cancer targeting [199]. TQ-loaded nanocarriers are not limited to cancer targeting. Aminoglycoside-thymoquinone-loaded nano-liposomal formulations have been patented for aminoglycoside antibiotic delivery [200]. Authors rightfully assume an increase in patent outcomes when pure thymoquinone is converted to nanocarrier-loaded thymoquinone for various pharmacological applications. The patents illustrating the pharmacological significance of thymoquinone and related nanocarriers are recorded in Table 5.
Table 5. Patents of thymoquinone (TQ) and their nanocarrier systems related to inflammation and cancer (↓: decrease, ↑: increase).
| S.N | Patent no | Type of Formulations | Product Claim and Activity | Outcome | Reference | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical Trial ID | Title | Trial Status | Age and Patient Inclusion Criteria | Intervention | Conditions | Sponsor | Target Size | Source | |||||||||||
| 1 | WO2016024145A1WIPO (PCT) | TQ derivative | Cancer treatment | ↑Anticancer effects | |||||||||||||||
| 1 | NCT03208790 | Clinical and immunohistochemical evaluation of the cancer chemopreventive effect of thymoquinone compared to placebo on oral potentially malignant lesions among an Egyptian population: a randomized clinical trial | Phase 2 | 18–25 years Patients with any known potentially malignant lesion confirmed histologically | [201] | ||||||||||||||
| 100 mg | 200 mg | Placebo oral capsule | Premalignant Lesion | Cairo University, Egypt | 81 | https://clinicaltrials.gov/show/NCT03208790 | ; accessed on 12 January 2021 | 2 | WO2018134852A1WIPO (PCT) | Vesicular formulations | Treatment of dermal inflammatory disorders | ↑Bioavailability | |||||||
| 2 | IRCT2016100914106N5 | [ | Preparation of oral gel-made from thymoquinone (TQ), and a clinical study investigating the efficacy of it on patients with aphtha | 202 | ] | ||||||||||||||
| 2 | Patient possessing aphthous ulcer | Recurrent Aphthous Stomatitis | Kermanshah University of Medical Science, Iran | 56 | http://en.irct.ir/trial/13800 | ; accessed on 12 January 2021 | 3 | WO2013030669A4WIPO (PCT) | |||||||||||
| 3 | IRCT2016021826637N1 | TQ, TQ + eicosapentaenoic acid | Inflammation management including eicosapentaenoic acid | ↓Inflammatory symptoms | Evaluation effect of mucoadhesive NS in the treatment of chronic periodontitis | 2[203] | |||||||||||||
| Patients who had not undertaken periodontal therapy in the past 3 months | Mucoadhesive Locally Delivery NS extract 0.2% and Thymoquinone 0.02%. | Chronic periodontitis | The ethics committee of Kermanshah University of Medical Science, Iran | 20 | http://en.irct.ir/trial/22014 | ; accessed on 12 January 2021 | 3 | TQ (1–25 µM) | Caki-1 cells, xenograft mouse model | ||||||||||
| 4 | TQ loaded porous PVPylated Fe3O4 nanostructures | ↑p53; ↑Bax; ↓Bcl-2; ↓Bcl-xl, ↓cyclin D1, ↓cyclin D2, and ↓survivin via suppression of JAK2/STAT3 signaling pathway | MDA-MB-231 | ↑ROS related cell death, ↑water-solubility, pH-dependent cellular delivery, ↑apoptosis | [175] | ||||||||||||||
| 4 | WO2016167730A1WIPO (PCT) | Nanomicelles | Nanomicelles loaded with drug and H5WYG peptides for anticancer activity | 5 | TQ loaded hyaluronic acid-decorated Pluronic® NPs | MDA-MB-231, MDA-MB-468), murine (4T1), chick embryos |
↓cell migration at a low dose; ↑circulation time; ↑cancer cells targeting | [176] | |||||||||||
| 6 | PEGylated vitamin-E TPGS-lipidic nanocapsules for co-delivery of DTX and TQ | MCF-7 and MDA-MB-231 |
|||||||||||||||||
| ↓Bcl-2, ↓Bcl-xl, ↑Bax, ↑release of cytochrome C and AIF, ↑cleaved subunits of caspase-3, 8, 7, and PARP | |||||||||||||||||||
| Induce proliferation and apoptosis | |||||||||||||||||||
| [ | |||||||||||||||||||
| 116 | |||||||||||||||||||
| ] | |||||||||||||||||||
| Induces apoptosis via accumulation of ROS, ↓tumor growth | [ | ||||||||||||||||||
| 57 | |||||||||||||||||||
| TQ (20–80 µM) | |||||||||||||||||||
| U87MG, U118MG, and A172 | |||||||||||||||||||
| ↑Par-4, ↑p53, ↑p21, ↑Rb, ↓lamin B1, ↓cyclin E, ↓cyclin-dependent kinase-2 (CDK-2) | |||||||||||||||||||
| ↓Glioblastoma | |||||||||||||||||||
| [ | |||||||||||||||||||
| 117 | |||||||||||||||||||
| ] | |||||||||||||||||||
| 58 | |||||||||||||||||||
| Temozolomide (100 μM) + TQ (50 μM) | |||||||||||||||||||
| U87MG cell line. | |||||||||||||||||||
| ↓MMP 2, ↓MMP-9 | |||||||||||||||||||
| ↑cytotoxicity, ↓cells invasion | |||||||||||||||||||
| [ | |||||||||||||||||||
| 118 | |||||||||||||||||||
| ] | |||||||||||||||||||
| ↑apoptotic index, caspase 3, and HSP90 expressions in the DOX group | ↓DOX toxicity | [ | 121 | ] | |||||||||||||||
| 2 | Cisplatin+ TQ+ vitamin E | Wistar rats | ↓Catalase, ↓glutathione peroxidase, ↓SOD, and ↓reduced glutathione levels | ↑cisplatin effect, ↓oxidative stress, ↓cisplatin toxicity | [122] | ||||||||||||||
| 2 | TQ (5–25 μM) | LPS/D-galactosamine induced acute hepatitis and HCl/EtOH-induced gastritis mouse model | ↓(AP)-1/NF-κB pathways, ↓iNOS; ↓NO, ↓TNF-α; ↓COX-2, ↓IL-6, ↓PGE2, ↓IL-1β; ↓IRAK1 | ↓inflammatory response | [123][ | 4 | TQ (1–50 µM) | MDA-MB-231, MDA-MB-436, and BT-20 | ↓expression of eEF-2K, Src/FAK, and Akt; ↓NF-κB/miR-603 signaling axis | Dose-dependent ↓cell proliferation and migration | [65] | ||||||||
| ↑Targeted delivery for cancer cells | 51 | ] | [ | 204 | |||||||||||||||
| 4 | ] | 5 | |||||||||||||||||
| NCT03776448 | The effect of 2 g daily supplementation of thymoquinone -containing sativa nigra oil on blood glucose levels of adults: a placebo-controlled double-blinded randomized controlled trial | N/A | 18–60 years of regular Student or Faculty in Sulaiman Al Rajhi Colleges | 18–60 years | Diabetes mellitus | Sulaiman Al Rajhi Colleges, Saudi Arabia | 30 | https://clinicaltrials.gov/show/NCT03776448 | ; accessed on 12 January 2021 | TQ, artemisinin hybrids | CCRF-CEM and Multidrug-Resistant CEM/ADR500 Leukemia Cells | Specifically inhibit cancer cells | 5 | US20160101124A1Low toxicity/high selectivity profile | [ | Nanoliposome loaded with TQ and aminoglycoside | Nano-liposomal aminoglycoside-TQ formulations for administration to the mammal66] | ||
| ↑bactericidal activity, ↓renal toxicity | [ | 205 | ] | 6 | TQ(5µg/mL) and Emodin (25µg/mL) | MCF-7, MDA-MB 231, MDA-MB 468 and T47D | ↑ROS generation; ↓FAK and Integrins, ↑p53, ↑Bax, and ↑cleaved caspase 3 expressions; ↓Bcl-2 | ↑apoptosis, ↓cell migration, and ↓stemness efficiently in breast cancer | PEGylation ↑circulation time; Re-sensitized the resistant TNBC cells; ↓side effects; ↑anti-metastatic effects[67] |
||||||||||
| [ | 177 | ] | 7 | TQ, TQ+cisplatin TQ+DOX |
HCC HepG2 and SMMC-7721 HL-7702 cells |
↑ROS, ↑caspase 3 | ↑apoptosis and selectively ↓cell viability | [68] | |||||||||||
| 7 | Chitosan coated PLGA-NPs for TQ delivery | A375 | ↑cellular accumulation; sustained delivery ↑cytotoxicity, | ||||||||||||||||
| 6 | [ | 178 | ] | ↓intravascular diseases such as in-stent restenosis | [207] | 8 | TQ (2–150 μM) | A375, B16F10 | ↓NLRP3 (NACHT, LRR, and pyrin domain-containing protein 3); ↓proteolytic cleavage of caspase-1; ↓IL-1β and ↓IL-18, ↓NF-κB, ↓ROS | Inactivation of caspase-1, ↓melanoma cells migration | |||||||||
| 8 | Poly-L-lysine and PEG-coated polysaccharide nanocontainers of diethylaminoethyl dextran/xanthan gum for TQ delivery | MCF-7 cells | ↑cellular accumulation ↑cytotoxicity | [179 | [69] | ||||||||||||||
| ] | 9 | TQ 20 gm/kg | HCT116 | ↓CD44, ↓EpCAM, ↓Ki67, ↑p53, ↑p21, ↓PCNA, ↑TUNEL positivity, ↓γ-H2AX | ↓viability of 5FU-sensitive and resistant HCT116 | [70] | |||||||||||||
| 9 | Eudragit L-100 chitosan, HPMC, and PVA NPs of TQ for colon cancer treatment | Caco-2 | ↑colonic drug delivery ↑cytotoxicity | [180] | 10 | DOX, TQ, TQ/DOX | |||||||||||||
| 10 | HepG2, | PEGylated liposome of dihexadecanoyl-sn-glycero-3-phosphocholine for co-delivery of DTX and TQHuh7 | MCF-7 | ↑drug encapsulation ↓docetaxel dose, ↑cancer cells cytotoxicity |
[181] | ||||||||||||||
| 11 | 59 | TQ (1–30 μM) | Jurkat, HL60 and HeLa cell line | ↑UHRF1 degradation, ↑cleaved caspase-3 and ↑p73 | ↑apoptosis | [119] | |||||||||||||
| 60 | TQ (10 mg/kg 5–200 µM) | ||||||||||||||||||
| 4 | TQ (20 mg/kg) and pentoxifylline (15 mg/kg) | female albino mice | ↓Notch1, ↓Hes1, ↓Jagged1, ↓β-catenin, ↓TNF-α, ↓IL-6, ↓IFN-γ, and ↓VEGF with ↑in IL-2, ↑CD4, ↑CD8, and↑apoptotic cells | ↑chemotherapeutic effect of cisplatin by targeting Notch signaling pathway, ↓tumor growth |
[125] | ||||||||||||||
| 4 | TQ 50 mg/kg | Colorectal cancer in SD rats | ↑Antioxidant activity | Protective and preventive measure in cancer management | [126] | ||||||||||||||
| 8 | US10485837B2 | black cumin extract. | NS seeds component for management of anxiety, stress, and sleep disorders | Improve cognitive function | [208] | ||||||||||||||
| 9 | WO-2011126544-A2 | TQ+ gemcitabine/oxaliplatin, | TQ analogs for the treatment of pancreatic cancer | ↓drug resistance, ↑chemotherapeutic activity against pancreatic cancer | [209] | ||||||||||||||
| 10 | |||||||||||||||||||
| 5 | CTRI/2018/11/016334 | A randomized, open-label, prospective, three-arm, parallel, multicenter study to evaluate efficacy and safety of metformin with/without concomitant administration of thymoquinone in patients with type 2 diabetes mellitus. | 2 | Patients aged 18–65 years with type 2 diabetes mellitus and (BMI) between 18–30 kg per meter square | Type 2 diabetes mellitus without complications | Intas Pharmaceuticals Ltd., India | 60 | http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=28562; accessed on 12 January 2021 | WO-2016005786-A1 | The liposome of TQ and taxane, | Liposomal formulations comprising TQ and taxane, and methods of treating cancer using the same | ||||||||
| 6 | Synergize anticancer effect, ↑capsulation efficiency of the taxane ↑liposomes stability | CTRI/2020/05/025167 | Evaluation of efficacy and safety of thymoquinone compared to best supportive care in patients with covid-19 | Phase 2 | Confirmed COVID-19 patient (either sex) aged 18–65 years[206] | ||||||||||||||
| 50 mg tablet for 14 days as an add-on to best supportive as per guidelines of clinical management of COVID-19 as issued by MOHFW | RR < 20, HR < 90, oxygen saturation (pulse oximetry) >93% on room air at screening | Intas Pharmaceuticals Ltd., India | 100 | http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=43378&EncHid=&userName=thymoquinone | ; accessed on 12 January 2021 | 5 | 7TQ (20 mg/kg) | CN-110420203-ASD rat | ↑TRAIL/TRAILR2, ↑caspase-3, and ↓Bcl-2 downregulation, ↓TGF-β1 gene expression level. ↑hepatic GSH level and marked ↓hepatic MDA level, ↓alpha-fetoprotein level | ↓HCC progression, ↑apoptosis | [127] | ||||||||
| TQ | Application of the TQ in preparation prevention intravascular stent restenosis medicaments | ||||||||||||||||||
| 7 | NCT04476420 | Comparison of NS oil with conventional management on clinical outcomes in oral submucous fibrosis | Phase 3 | 18 years clinically diagnosed patients (either sex) of oral submucous fibrosis | Topical application of N. sativa seed oil over buccal mucosa (1 mL) three times a day for 10 min (3–5 min on each side) | Oral submucous fibrosis | Ziauddin University, Pakistan | 40 | https://clinicaltrials.gov/ct2/show/NCT04476420; accessed on 12 January 2021 | 6 | 20 mg/kg BW | Diethylnitrosamine induced HCC in rats. | ↓EGFR/ERK1/2 activation | protective effect against HCC | [128] | ||||
| 8 | NC T04292314 | Impact of combination therapy between hydroxyurea, omega 3, NS, and honey on antioxidant-oxidant status and reduction of iron overload in pediatric major thalassemia | Phase 3 | Any case with the full manifestation of β-Thalassemia major disease Aged from 7–15 years old |
1 g black seed oil contains 1% thymoquinone per day for 8 consecutive months up to 10 months | Beni-Suef University (Egypt)Maternity and Children Hospital, Makkah University of Arizona (Saudi Arabia) | 350 | https://clinicaltrials.gov/ct2/show/NCT04292314?cond=thymoquinone&draw=2&rank=3; accessed on 12 January 2021 | ↑miR-16 and miR-375, ↑caspase 3; ↓Bcl-2 |
↓apoptosis; ↓cell viability | |||||||||
| 7 | TQ | Hamster oral cancer Induced by DMBA |
↓PI3K/AKT/mTOR signaling pathways ↓the mRNA expression level of NF-κBp50/p65 |
↑chemopreventive activity | [129] | ||||||||||||||
| 9 | CTRI/2020/12/029514 | An open-label, balanced, randomized, three-treatment, single-period, single oral dose, parallel, exploratory pharmacokinetic study of thymoquinone tablet 12.5 mg, 25 mg, 50 mg in normal, healthy, adult, human subjects under fasting condition | 8 | TQ(5 mg), 6-MP (5 mg/kg) | Albino rats | ↑spermatogenesis, ↓P53, ↓caspase-3 apoptotic pathway, ↑PI3K; ↓TNF-α[71] | |||||||||||||
| ↓6-MP induced testicular damage, ↑its anticancer potential | [ | 130 | ] | 11 | TQ, cisplatin, geraniol | MCF-7 | ↑SOD, ↓myeloperoxidase, ↓lipid peroxidation; ↓8-isoprostane levels | ↓cisplatin neurotoxicity | [72] | ||||||||||
| Transferrin decorated TQ loaded PEG-PLGA-NPs | ↑cellular accumulation | 12 | TQ (8 μM) | HEp-2 | ↓MMP; ↓mitochondrial cytochrome c release | ↑apoptosis of tumor cells | [73] | ||||||||||||
| ↓therapeutic dose | ↓onset time, ↑cytotoxicity | [182] | |||||||||||||||||
| 12 | AS1411-conjugated nanodroplets of phospholipids 1,2-dipalmitoyl-sn-glycero-3-phosphocholine |
MDA-MB-231 | Specific binding with overexpressed nucleolin on to cancer cell surface, ↑cytotoxic potential |
[183] | 13 | TQ (20 mM or 40 mM) | Human glioblastoma cells T98G and U87MG, Gli36DEGFR | ↑recruitment and accumulation of the microtubule-associated protein light chain 3-II (LC3-II); accumulation of the LC3-associated protein p62 |
↑autophagy and induces cathepsin-mediated, caspase-independent cell death |
[74] | |||||||||
| 13 | 14 | TQ (10–40 mM)) | HaCaT, HEK001 HeLa | ↑GSN levels, ↑p27, ↑cleaved PARP; ↑UHRF1 by HPV E6/E7 causes GSN silencing | ↑apoptosis and cell cycle arrest in early stage | [75] | |||||||||||||
| 15 | Indirubin-3-monoxime and TQ | A549 | ↓Bcl-2/Bax ratio, ↓p-AKT, ↓p-mTOR, ↓Caspase-3, ↓p-53, ↓NFκB, ↓Akt/mTOR/NFκB, ↑p38, ↑ROS; ↓tumor growth by targeting NF-κB; ↑PPAR-γ activation; ↓Akt, 4E-BP1, ↓eIF4E, S6R and ↓p70S6K phosphorylation | ↓metastasis, ↑cell cycle arrest; ↓tumor growth | [52][76] | ||||||||||||||
| Not yet recruiting | 18.00–45.00 year(s) | A normal, healthy, adult having a Body Mass Index between 18.5 to 30.0 | Dose—12.5 mg; 25 mg; 50 mg | Frequency—a single oral dose; Dosage form— tablet; Route of administration—oral |
Not having any significant diseases | Intas Pharmaceuticals Limited, Corporate House, Ahmedabad—380054, Gujarat, India | 12 | http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=49799&EncHid=&userName=thymoquinone; accessed on 12 January 2021 | US-6218434-B1 | TQ and dithymoquinone | Use of the naturally occurring quinones TQ and dithymoquinone as antineoplastic and cytotoxic agents | ↓drug sensitivity against multi-drug resistant human cancers | 16 | TQ (5 μM-10 μM) | clone E6-1, HL-60, K-562 |
↑thymine glycol metabolite; induce DNA damage; ↓guanine levels | ↑antiproliferation, ↑apoptosis | [77] | |
| [ | 17 | TQ (10 μM) | OVCA429, SKOV3, HeyA8, OVCAR3, OVCAR8 | ↓JNK, ↓Src, ↓FAK are involved in LPA-induced invasive cell migration | ↓migration of cancer cells in a dose-dependent manner | [78] | |||||||||||||
| PEGylated LMW TQ-loaded chitosan nanocapsules | 18 | TQ (20- 40 μmol/L) | T24, 253J SV-HUC-1 | ↓activation of Wnt/β-catenin signaling pathway, ↑E-cadherin, and ↓N-cadherin, ↓vimentin, ↓MYC, ↓Axin-2, ↓MMP7, ↓CyclinD1, ↓β-catenin | ↓epithelial–mesenchymal transition in bladder cancer cells | [79] | |||||||||||||
| 19 | TQ (5 μM) and alpha-hederin (50 μM) | PC3, HT-29, HCT116 | Zinc level modulations | Dose-dependent cytotoxicity | [80] | ||||||||||||||
| 20 | TQ (1–100 μM) | 786-O cells | ↑sub-G1 population and % of apoptotic cells. ↓collective migration | Induces dose and time-dependent cytotoxicity, ↓invasive potential | [81] | ||||||||||||||
| 21 | TQ and paclitaxel | MCF-7, T47D | ↑Pre-G phase cells, ↓TWIST-1 gene, and ↑SNAIL-1, ↑SNAIL-2 genes. | ↓paclitaxel resistance, ↑apoptosis, ↑necrosis, | [82] | ||||||||||||||
| 22 | TQ (50 µM), Cur (15 µM), Caff (10 mM), DOX |
HCT116, MCF7 | ↓bromodeoxyuridine incorporation, ↑accumulation of senescence-associated β-galactosidase (SA-β-gal), ↑cell cycle arrest, and ↑p53, ↑P-p53, and ↑p21 proteins | ↑DOX sensitivity and apoptosis towards proliferative cells | [83] | ||||||||||||||
| 23 | TQ | MDA-MB-231 | ↓Beclin-1, ↓VEGF, ↓Integrin-β1, ↓MMP-2,9 | ↓proliferation and migration, ↓Autophagy, ↓colony formation | [84] | ||||||||||||||
| 24 | TQ | DU-145, PC-3, LNCaP |
↓p-Akt, ↓NF-κB↓MMP-3, ↓MMP-7 | ↓IL-7-induced tumor progression and metastatic invasion in PC-3 cells | [85] | ||||||||||||||
| 25 | TQ (50, 100 μM) | MCF-7, HepG2 | ↓sphingosine-1-phosphate (S1P), ↓ceramide-1-phosphate (C1P), ↓NF-κB1 mRNA, ↓NF-κB, ↓p65 protein levels, ↑neutral sphingomyelinase (N-SMase) enzyme activity, ↑cellular levels of C16-C24 ceramides and ↑cleaved caspase-3; ↑glucose-regulated protein 78-kd (GRP78) mRNA and protein | ↑ceramide accumulation and ER stress in conjunction with ↓S1P, C1P, and NF-κB mediated cell survival ↑cancer cell death by triggering apoptosis | [86] | ||||||||||||||
| 26 | TQ (10 mM) + Difluoromethylornithine (0.5 mM) | T lymphoblastic leukemia (ALL) Jurkat cell line | |||||||||||||||||
| 10 | NCT04686461 | Effect of TQ extracted from NS in the treatment of arsenical keratosis | Not applicable | Age: 19–65 years Arsenical keratosis: Presence of moderate to severe keratosis (>5 mm) in both palms and soles | 210] | ||||||||||||||
| NS seeds extract containing ointment | dose—twice daily for 12 weeks | The patient did not receive a topical application of any drug for the last three months.Drinking arsenic-contaminated water (>50 µg/L) for at least more than 6 months | Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh | 34 | https://clinicaltrials.gov/ct2/show/NCT04686461?cond=NCT04686461&draw=2&rank=1 | ; accessed on 12 January 2021 | 11 | CN-103288618-A | TQ synthesis method | A synthesis method of TQ serving as blood vessel inhibition medicament | A Synthesis method of TQ serving for blood vessel inhibition drug | [ | ↓UHRF1, ↓DNMT1, ↓HDAC1 | Synergism, ↓cancer cell viability and ↑apoptosis | [87] | ||||
| 211 | ] | 27 | TQ and Cur | NLF, NB69, SK-N-BE(2) | ↓proliferation, ↑apoptosis | [88] | |||||||||||||
| 28 | TQ (50–100 µM) + FA (450 µM) | MDA-MB 231 | ↓PI3K/Akt pathway | Synergism in ↓cancer cell proliferation | [89] | ||||||||||||||
| 29 | TQ (20–100 μM) | C6 glioma cells | ↑H2O2 generation, ↑microconidial ROS, ↓intracellular GSH level, ↓NF-κB, ↓PI3K, and AKT activation | ↑apoptosis, ↓proliferation, and ↓glioma cell viability | [90] | ||||||||||||||
| 30 | TQ (20–60 μM) | 786-O, 786-O-SI3, BFTC-909 |
↓Nanog, ↓Nestin, ↓Bid, ↑RO ↓CD44, ↓Oct-4, ↓Bcl-2, ↑cytochrome c, ↓phosphorylation of mTOR (Ser2448 and 2481) and AKT (Ser473) | ↓the proliferation of renal cell carcinoma cells via ROS-induced apoptosis |
[91] | ||||||||||||||
| 12 | 31 | TQ (0.5 μM) | HeLa cells | ↓ROS generation | ↓cancer cells proliferation | [92] | |||||||||||||
| 32 | TQ (1–30 μM) | A431 cells | ↑intracellular ROS, ↑p53, ↑Bax, ↓Mdm2, ↓Bcl-2, ↓Bcl-xl, ↓STAT3, ↑caspase-9,7 and 3; ↓phosphorylation of the upstream kinase, ↓Src, ↓cyclin D1, ↓survivin | ↑apoptosis, ↓cell viability in dose-dependent manner | [93] | ||||||||||||||
| 33 | TQ (20 μmol/L TQ) | LoVo | ↑p-PI3K, ↓p-Akt, ↓p-GSK3β, ↓β-catenin, ↓COX-2 expression; ↓PGE2 levels and the suppression of EP2 and EP4 activation | ↓cancer cell proliferation. ↓cell migration |
[94] | ||||||||||||||
| 34 | TQ (5 μM) | A549 | ↑Bax and ↓Bcl2 and ↑Bax/Bcl2 ratio, ↓cyclin D and ↑p21, ↑TRAIL receptor 1 and 2, ↓NFκB, ↓IKK1 | ↑G2/M cell cycle arrest, ↑apoptosis | [95] | ||||||||||||||
| 35 | TQ + DTX | DU145, C4-2B |
↓PI3K/AKT, ↑BAX and ↑BID, ↑caspase-3, ↑PARP and ↓BCL-XL | ↑cytotoxicity and ↑apoptosis | [96] | ||||||||||||||
| 36 | TQ (10–40 μM) +Dox (50–100 nM) | HTLV-1 positive (HuT-102) and HTLV-1 negative (Jurkat) CD4+ malignant T-cell lines | ↑ROS, ↓tumor volume, ↓MMP | ↓cell viability, induced apoptosis | [97] | ||||||||||||||
| 37 | TQ (2 μM,) | Irinotecan-resistant (CPT-11-R) LoVo colon cancer cells | Activate JNK and P38 and MOMP | ↑the total cell death index and ↑apoptosis | [98] | ||||||||||||||
| 38 | TQ (2–100 µM) | A431 and Hep2 | ↑Bax/Bcl-2 ratio, ↓Akt and JNK phosphorylations | ↓tumor volume and mass; ↑apoptosis; ↓cell proliferation | [99] | ||||||||||||||
| 39 | TQ (10–60 mM) | B16-F10 | ↓p-STAT3, p-JAK2 expression, and p-STAT3, ↑Bax and ↑caspase-3, ↓VEGF-A, ↓MCP-1, ↓TGF-b1, ↓RANTES, and↓IL-1β | ↑cytotoxicity; ↑apoptosis | [100] | ||||||||||||||
| 40 | TQ (10 mM) | A549 | ↑Bax/Bcl-2, ↑p53; ↑caspases-3 and 9 | ↓cells viability; ↑apoptosis | [101] | ||||||||||||||
| 41 | 5-FU + TQ | HCT116 | ↓WNT/ß-Catenin and PI3K/AKT, ß-Catenin | ↓angiogenesis | [102] | ||||||||||||||
| 42 | TQ (10 mg/kg) | MDA-MB-231 | ↑E-cadherin mRNA expression | ↓proliferation, migration, ↓invasion of cancer cells. | [103] | ||||||||||||||
| 43 | TQ (36 μg/mL) + tylophorine (88 μg/mL) | Hela cells | ↑cell arrest in the G2/M phase | [104] | |||||||||||||||
| 44 | TQ (20 µM) | Jurkat cells, MDAMB-468 cells | ↓UHRF1), ↓DNMT1 G9A, ↓HDAC, DNA methylation and histone post-translational modifications | ↑tumor suppressor genes | [31] | ||||||||||||||
| 45 | TQ (40 µM) | A498 | ↑Bax, ↓Bcl-2, ↓Akt phosphorylation | ↓proliferative, ↑apoptosis | [105] | ||||||||||||||
| 46 | TQ (1–10 µM) | HEK293 cells, Caki-1, A498 | ↓HIF-1α-mediated glycolysis via ubiquitination-proteasome dependent pathway | ↓cancer cell angiogenesis | [106] | ||||||||||||||
| 47 | TQ (10–100 µM) | HeLa cells (Cancer) |
↓dose-dependent cellular viability | [107] | |||||||||||||||
| 48 | TQ (0.5 mM) + cyclophosphamide (20 µM) | Her2+SKBR-3 and Her2- MDA-231 | ↓PI3K/Akt signaling, ↑PTEN, ↓cyclin D | synergistic cells death | [108] | ||||||||||||||
| 49 | TQ (0.003 mg/mL) | HSC-3, HSC-4, oral fibroblast, HACAT cell line | Dose and time-dependent cytotoxicity | [109] | |||||||||||||||
| 50 | TQ (0–80 µM) | PC3 cell line | ↑ROS, ↓MCL-1, ↓MCL-XL, ↑BAX, ↑AIF, ↑cytochrome c | induced apoptosis | [110] | ||||||||||||||
| 51 | TQ | AGS(CRL-1739) cell line | VEGF-A gene expression | induced apoptosis | [111] | ||||||||||||||
| 52 | TQ | KB cells | ↓activation of PI3K/Akt pathway. | ↓proliferation, ↓migration, and invasion | [112] | ||||||||||||||
| 53 | TQ (60 μmol/L) | 786-O, ACHN | ↑p-AMPK w, ↓p-mTOR, ↑p-S6K | ↓metastasis, induce autophagy | [113] | ||||||||||||||
| 54 | TQ+ gemcitabine | MCF-7, T47D | ↓CD44+/CD24− cell clone | Potentiate gemcitabine efficacy | [114] | ||||||||||||||
| 55 | TQ (0.5–20 µM) | 769-P and 786-O | ↑E-cadherin, ↓Snail, ↓ZEB1 expression, ↑LKB1 phosphorylation, ↑AMPK | ↓metastasis | [115] | ||||||||||||||
| 56 | TQ (40–80 µM) | T24 and 253J bladder cancer cell | B16, F10 | ↓p-STAT3, ↑DNA damage, and ↑ intracellular ROS | ↑apoptosis | [120] | |||||||||||||
| 61 | TQ (20, 100 mg/kg) IV | MDA-MB-231, MDA-MB-436, |
2.2. TQ Nanocarrier for the Treatment of Cancer
Many drugs do not reach the antineoplastic drug pipeline because of low aqueous solubility, high toxicity, large doses, and shorter half-life. Nanoformualtions provide opportunities to improve the pharmacokinetics of these drugs for precise treatment at the molecular level with reduced off-target effect [131][132][133]. The tumor tissues that exhibit enhanced permeability and retention (EPR) and hypoxia-like properties could be utilized for targeted drug delivery. The NPs take advantage of the EPR effect and accumulate in the cancer cells, providing maximum therapeutic efficacy with minimum off-target effect [134]. The nanoformulations, including polymeric (natural/synthetic), lipidic (liposomes, niosomes, ethosomes, cubosomes, solid lipid nanoparticles (SLN), nanoemulsion, and microemulsion), protentious (bovine serum albumin, human serum albumin) and metallic (silver, gold, iron, etc.), in combination with surface modification, are utilized for targeted delivery of therapeutic drugs in tumor sites [135][136][137]. NPs deliver drugs at the selective tumor site utilizing multiple approaches, including passive targeting and active targeting. Some of them are explained in the following sections to deliver TQ at the target site. Applications of TQ nanocarriers and surface-modified TQ nanocarriers for the management of cancer and inflammation are reported in Table 3 and Table 4, respectively. Moreover, the therapeutic importance of TQ-loaded nanoparticulate-based therapies for RA management is also reported in Table 3 with comparison to the conventional formulations and pure TQ.
Table 3. TQ nanocarrier in the management of cancer
| MCF 7, HEK 293 | |||||
| ↑absorption, ↑BA | |||||
| ↑cancer cells targeting | |||||
| [ | |||||
| 184 | |||||
| ] | |||||
| CN-103833871-A | |||||
| Hyaluronic acid-adipodihydrazide-TQ-grafted polymer | |||||
| TQ grafted polymer for tumors specific delivery | |||||
| ↑tumors targeting, pH-dependent drug release | |||||
| [ | |||||
| 212 | |||||
| ] | |||||
| 13 | US-8029831-B2 | TQ containing NS seed extract + cranberry fruit extract/ | Management of microbial infections of the female urinary tract. | ↓Urine pH, ↑antimicrobial activity, ↓inflammation and pain, ↓physiological stress. | [213] |
| 14 | DE-19844022-C1 | Iron-binding glyco proteins (lactoferrin) and/or 10-hydroxy- 2-decenoic acid + TQ | use of iron-binding glycoproteins and/or 10-hydroxy-2-decenoic acid in combination with TQ for treatment of AIDS and other immunodeficiency diseases. | ↓HIV plaques | [214] |
| 15 | US20190192686A1 | Nanodroplet micelle | Cancer management | ↑targeted delivery of anticancer drugs, ↓systemic toxicity. | [215] |
| S.N | Formulations | Animal Model/Cell Line | Major Finding | Ref. |
|---|---|---|---|---|
| 1 | Core-shell NPs of mesoporous silica | SW1088, A172, HCN2 |
pH driven TQ release in tumor acidic environment ↑cell cycle arrest | [138] |
| 2 | Docetaxel (DTX) and TQ in borage oil-based nanoemulsion | MCF-7 MDA-MB-231 |
↑DTX anticancer potential; ↓dose, ↑apoptosis | [139] |
| 3 | TQ-loaded Soluplus-Solutol HS15 mixed micelles 2 | SH-SY5Y | ↑solubility (10-fold), ↑neuroblastoma cell migration | [140] |
| 4 | TQ- Chitosan NPs (12.5–200 µg/mL) | HepG2 | ↓cancer cells proliferation, ↑antimetastasis | [141] |
| 5 | TQ-loaded methoxy poly (ethylene glycol)-b-poly(“-caprolactone-NPs | MCF-7, PANC-1, Caco-2 Balb/c mice |
↑oral BA (1.3-fold), ↑Solubility, ↑cancer cells selectivity | [142] |
| 6 | TQ loaded Soy phytosomes | A549 | Improved release pattern; ↑the dose-dependent anticancer effect, ↑apoptotic induction | [143] |
| 7 | TQ-capped iron oxide NPs (TQ-IONPs) | MDA-MB-231 | ↑BA; ↑cellular uptake of TQ-IONPs; synergize the chemo-photothermal effect | [144] |
| 8 | TQ loaded radio-iodinated folic acid-chitosan NPs | SKOV-3 Caco-2 |
Folate receptor-mediated NPs ↑cellular internalization, ↑targeting to ovarian cancer cell | [145] |
| 9 | TQ loaded technetium-99m based NPs (99mTc-TQ-NPs) | Rhabdo-myosarcoma cancer cells line | ↑internalization and ↓externalization of radiopharmaceuticals; ↑anticancer potential | [146] |
| 10 | Cockle-shell-derived aragonite CaCl3 NPs for co-delivery of DOX and TQ | MBA MD231 3D | Co-delivery ↓cellular migration and invasion, | [147] |
| 11 | Glyceryl monooleate, cubosome for TQ delivery | MCF-7 MDA-MB-231 |
↑cytoplasmic accumulation; ↓cancer cells viability; ↑antitumor activity, ↑apoptosis | [148] |
| 12 | PLGA-PEG-Pluronic-TQ-NPs | Tamoxifen resistant breast cancer cells UACC 732, MCF-7 | ↑EE, sustained release, ↑targeted delivery, selective cytotoxicity to UACC 732 | [149] |
| 13 | Vitamin-E-TPGS lipospheres for codelivery of cabazitaxel and TQ | MCF-7 MDA-MB-231 |
↑cellular internalization ↑anticancer potential, |
[150] |
| 14 | Chitosan grafted lipidic nanocapsules for co-delivery of DTX and TQ | TNBC MCF-7 |
↑intracellular dual drug payload, escape endosomal effect, ↑anti-angiogenic effect, ↑cytotoxicity | [151] |
| 15 | Carum- and TQ loaded niosomes for target breast cancer cells | MCF-7, CaSki, SiHa |
↑solubility, ↑BA and ↑permeability, ↓Cell Migration, ↑cytotoxicity | [10] |
| 16 | TQ and Cur loaded fluorescent liposomes | A549 | ↑cellular internalization ↓cellular proliferation, ↑cancer cells cytotoxicity |
[152] |
| 17 | TQ loaded mesoporous silica NPs | HeLa MCF-7 |
↓effective dose (8-fold), ↑aqueous solubility, ↑cellular internalization ↓cell migration, ↑cytotoxicity, ↑apoptosis |
[153] |
| 18 | TQ-NLC | HepG2 3T3 |
↑cellular accumulation driven by time and dose; modulate cellular morphology, ↑anticancer potential | [154] |
| 19 | TQ loaded SLN of phospholipon 90G | Carrageenan induced paw edema in rat | ↑BA, ↑anti-inflammatory potential ↓paw edema, ↑antioxidant potential |
|
| 20 | Ethosomes for topical TQ delivery |
5. Clinical Trials OF Thymoquinone
TQ has the potential to correct various physiological conditions of the body. It is widely investigated from dietary supplementation to chemoprevention. To date, a total of 10 clinical trials (Table 6) of thymoquinone claiming its effect on malignant lesions, aphtha, chronic periodontitis, type 2 diabetes mellitus, oral submucous fibrosis, pediatric major thalassemia, and supportive care in patients with COVID-19 are ongoing worldwide, the details of which are mentioned in Table 6. Moreover, recently, a clinical trial of TQ was registered to analyze efficacy and safety for best supportive measures (Guidelines on Clinical Management of COVID-19 issued by MOHFW, India) against COVID-19 patients. The confirmed COVID-19 patients were assigned as Cohort A and Cohort B. Cohort A patients received 50 mg TQ once a day for 14 days along with the best supportive measure, while Cohort B patients received the best supportive measure only. The trial was primarily evaluated for virologic (change in positive COVID-19 status on days 8 and 15) and clinical outcomes (proportion of patients on WHO progression scale 0 to 10 on days 8 and 15). A human trial (CTRI/2020/12/029514) of TQ tablets (dose of 50 mg; 25 mg; 12.5 mg) was registered to measure safety and tolerability and to analyze pharmacokinetic behavior in normal healthy adults under fasting conditions. A trial (NCT04686461) of thymoquinone extract is underway to investigate its effects against arsenical keratosis. In this trial, TQ-loaded topical ointment was used to treat 34 patients with arsenical keratosis at two-week intervals. The TQ ointment formulation was found to reduce the keratotic nodular size as well as improvement of the lesion calculated using the Likert Scale.
Table 6. Some recent thymoquinone clinical trials.
| S.N |
|---|
| Carrageenan rat paw edema | ||||
| ↑EE, ↑skin deposition | ||||
| ↓skin irritation | ||||
| [ | ||||
| 155 | ||||
| ] | ||||
| 21 | ||||
| TQ loaded chitosan, pluronic F127 liposome for topical delivery | ||||
| Carrageenan-induced paw edema | ||||
| ↑EE, ↑skin penetration | ||||
| ↑anti-inflammatory activity | ||||
| [ | 156 | ] | ||
| 22 | SNEDDSs containing black seed oil and cur | Carrageenan-induced paw edema | ↑entrapment efficiency, ↑transdermal penetration ↑anti-inflammatory activity |
[157] |
| 23 | black seed oil loaded egg yolk liposomes | Eddy hot plate method in Swiss albino mice | ↑BA; ↑EE, ↑anti-inflammatory activity |
[158] |
| 24 | TQ and piperine loaded micro vehicle of guar gum | HepG2 cell lines | pH-responsive delivery ↓lethal dose ↑bactericidal activity ↓minimum inhibitory dose |
[159] |
| 25 | Bio-SNEDDSs for co-delivery of cur and TQ | MCF-7 cells | ↑drug loading, ↓cell viability | [160] |
| 26 | Fluorescent organic NPs | A549, HeLa SiHa, HEK-293T | ↑BA, theranostic applications | [161] |
| 27 | TQ and resveratrol loaded silica NPs | HeLa cell line | ↑EE, ↑drug loading, ↑apoptosis | [162] |
| 28 | chitosan-based nanocarrier for the encapsulation of NS oil | HCT 116 (colorectal carcinoma), PC3 (prostatic cancer) | dose-dependent ↓cell viability | [163] |
| 29 | TQ Pluronic NPs | MCF7 cells | ↑TQ encapsulation, ↑cytotoxicity | [164] |
| 30 | TQ-NP of polystyrene-block-poly(ethylene oxide) diblock polymer | MCF-10-A cells MCF-7 cells, MDA-MB-231 cells |
↑cellular uptake; ↑cytotoxicity |
[165] |
| 31 | pH-sensitive multilamellar gold niosomes along with Akt-siRNA | tamoxifen-resistant T-47D and Akt-overexpressing MCF-7 cells | ↑TQ delivery at cancer cell; ↑anticancer potential, resensitized T-47D cells |
[166] |
| 32 | polysaccharide microcontainers of chitosan, xanthan gum soybean oil, and Nile red for TQ delivery | mouse melanoma M-3 cell |
↑cellular uptake, ↓nonspecific toxicity; ↑antitumor effect | [167] |
| 33 | Myristic acid-chitosan nanogels | MCF-7 | ↑solubility, ↑cellular uptake | [168] |
| 34 | ketoprofen and TQ loaded mesoporous core-shell silica spheres | MDN- and XG-2-type myeloma cancer cells lines (IL-6 dependent) |
↑cellular uptake and accumulation, ↑apoptosis | [169] |
| 35 | TQ loaded (PLGA)-NPs | MDA-MB-231 | ↑EE, ↑cancer cells toxicity | [170] |
| 36 | TQ loaded silver NPs | MDA-MB-231 | ↑cancer cells radiosensitivity | [171] |
Table 4. Surface-modified TQ nanocarrier in the management of cancer (↓: decrease, ↑: increase).
2.2.1. Passive Targeting Approach in Cancer Drug Delivery
Passive Targeting Utilizes the Tumor Microenvironment for Drug Delivery
Tumor vasculature is different from normal cell vasculature. Blood vessels of cancer tissue have comparatively larger fenestration with the poor lymphatic drainage system, which results in enhanced retention and permeation of the nano-sized particulate matter [185]. Based on the delivery site, the size and surface of the NPs can be modulated. NPs’ size and surface architecture modulation also avoid reticuloendothelial system (RES) uptake and make it circulate for a long period of time. This could be explored in passive drug delivery. Various strategies depicting passive targeting of TQ via nanoparticles are reported in Figure 4.
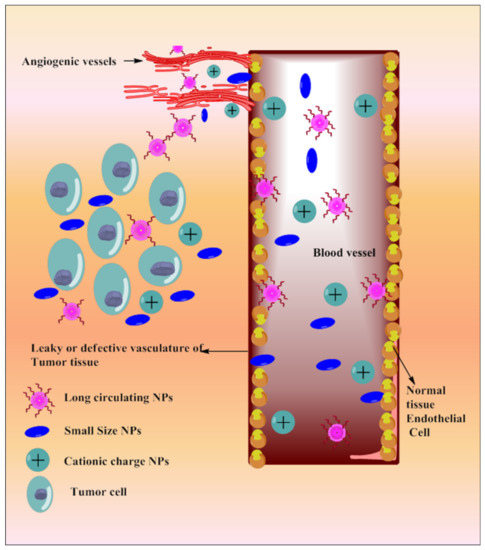
Figure 4. Systemic diagram depicting diverse approaches intended for passive targeting of TQ via nanoparticles.
Passive Targeting through Long-Circulating Nanocarriers
Chitosan-grafted lipid nanocapsules [151] and PEGylated liposomes [181] were reported for the co-delivery of TQ and docetaxel (DTX) against drug-resistant breast cancer. Chitosan grafting improved cellular uptake and escaped endosomal effect; PEGylation increased circulation time of the dual payload [186], resulting in increased cytotoxicity against triple-negative breast cancer (TNBC) cells (MDA-MB-231 and MCF-7). A long-circulating PEGylated vitamin E lipidic nanocapsule loaded with TQ and DTX was also investigated against resistant breast cancer cells (MCF-7 and MDA-MB-231) [177]. PEGylation in vitamin E lipidic nanocapsules inhibits p-glycoprotein efflux, re-sensitizes the resistant TNBC cells and provides enhanced antimetastatic effects with reduced multiple side effects. Co-encapsulation of TQ with DTX improved loading efficiency into PEGylated liposomes and vitamin E lipidic nanocapsules as well as the chemosensitivity of DTX against breast cancer cells (MCF7 and MDA-MB-231).
PLGA-PEG-Pluronic TQ NPs were designed for sustained delivery of TQ into tamoxifen-resistant breast cancer cells (UACC 732, MCF-7) [149]. TQ-NPs reduce the dose and synergize tamoxifen chemoprevention potential with selective tumor cell toxicity. PEGylated LMW chitosan nanocapsules selectively deliver TQ into cancer cells (MCF 7 cells) [184] as chitosan (with pKa 6–6.5) solubilizes in the inter, as well as intracellular acidic microenvironment of cancer cells, thereby delivering TQ in a targeted manner.
Passive Targeting through Surface Charge and Size of NPs
Nanocarriers overcome TQ pharmacokinetics issues and deliver it at the specific site with enhanced efficacy. A co-liposphere of Cabazitaxel (CBZ) and TQ was made of vitamin E-TPGS tricaprin, and egg phosphatidylcholine improved cellular internalization, which potentiates dose-dependent apoptosis as well as anticancer efficacy against MDA-MB-231 and MCF-7 cell lines [150]. The poly-L-lysine (PLL) and polyethylene glycol surface-decorated nanocontainers (NC-PLL) complex of diethylaminoethyl dextran/xanthan gum enhanced intracellular accumulation of TQ [179]. The positive surface charge of the NC-PLL significantly favored nanocontainer binding on the negatively charged cell membrane as compared to nonmodified nanocontainers, resulting in negatively charged NC-PEG. NC-PLL dominated in terms of cytotoxic efficacy, as investigated in MCF-7, likely due to enhanced accumulation in cancer cells.
Mesoporous silica NPs (TQ-MSNPs) improved TQ aqueous solubility and photostability as well as reduced the therapeutic dose (8-fold), which delayed cell migration and enhanced cytotoxic and apoptotic potential, as evaluated in the MCF-7 and HeLa cell lines [153]. The core-shell NPs of mesoporous silica delivered TQ to glioma cells selectively, which triggered cytochrome c, increased caspase-3 activation, and cell cycle arrest at the G2/M phase [138]. Chitosan-coated PLGA NPs containing TQ enhanced cytotoxic potential when compared with surface-decorated TQ-poly(lactic co-glycolic acid) NPs and TQ alone; this was investigated through the MDA-MB-231 and MCF-7 cell lines [172]. The antimetastatic potential of TQ was enhanced by chitosan nanoparticles against HepG2 cell lines through longer duration inhibitory actions when compared with free TQ [141]. TQ-NLC-NPs accumulated in cancer cells and inhibited their proliferation through time and dose-dependent modulation in the cellular morphology, as investigated in HepG2 cancer cells [154]. The polymeric NPs of methoxy poly(ethylene glycol)-b-poly(-caprolactone) improved the systemic bioavailability of TQ (1.3-fold) with slower elimination rates, which provides greater antiproliferative efficacy against varieties of pure cell cultures of human carcinoma (PANC-1, MCF-7, and Caco-2) [45][142]. The nanoarchitecture of polymeric shells increased TQ solubility, intestinal absorption, and bioavailability rates, resulting in higher cancer cell selectivity compared to free TQ. A soy phytosomal formulation of TQ with a dual release pattern (initial burst followed by prolonged release) revealed excellent anticancer activity against a lung cancer cell line (A539) [143]. The sustained release of TQ from phytosome accumulates TQ in the G2-M and pre-G1 phases of cancer cells, which initiate dose-dependent apoptosis and cell necrosis activities via caspase-3 activation. A Soluplus®-Solutol® HS15 micelles formulation enhanced the anti-migratory efficacy of TQ (1.5–10 µM) through improving aqueous solubility (10 times) and encapsulation efficacy, as investigated in SH-SY5Y human neuroblastoma cells [140]. The synergistic potential of TQ loaded in cockle-shell-derived aragonite CaCl3-NPs was reported with doxorubicin to reduce cellular migration in mammary gland carcinoma stem cells (MDA MB 231) [147]. A cubosomal formulation of TQ improved cellular accumulation, which leads to increased apoptotic activity migration in mammary gland carcinoma cell lines (MDA-MB-231 and MCF-7) [148]. Chitosan-coated TQ-PLGA-NPs accumulated in melanoma cancer cells (A375) by taking advantage of the EPR effect and positive surface charge of chitosan, which facilitate binding with the negatively charged cell membrane and induce cellular retention as well as time-dependent cytotoxicity [178]. TQ loading into niosomes improved cellular internalizations with controlled release of TQ, which markedly inhibits the migration of pro-inflammatory markers in mammary gland carcinoma with respect to pure TQ [10]
6. Conclusions and Prospects
TQ is a molecule that has multifaceted modes of action, including anti-arthritic and antineoplastic activities through modulating inflammatory and apoptotic pathways. However, its biological instability, rapid metabolism, poor water solubility, narrow bioavailability, inadequate cellular availability, and lack of targeting halt its transition from research to clinical application. Extensive literature analysis revealed that nanotechnology upgraded drug delivery patterns in cancer and arthritic disease through significant improvement in pharmacokinetics and target-oriented active molecule delivery while decreasing their off-target side effects. To maintain the biological stability of TQ during formulation design or delivery alone, site-specific availability is among the major challenges to utilizing its maximum therapeutic potential in arthritis and cancer management.
The role of TQ individually and its diverse types of nanoformulations for targeted delivery to tumorigenic cells and synovial tissues, with longer circulating time and higher synovial accumulation, improved anti-inflammatory and anticancer potential. The nanoformulation delivery of TQ results in significantly enhanced targeting payload and promising upgrades to its anti-inflammatory and anticancer efficacy.
Nanoparticles are emerging carrier systems for the delivery of a wide range of therapeutic molecules. NPs are extremely attractive due to their important properties (size surface area and charge). Their use, as a drug carrier system or in theranostic applications including personalized medicine, might pave the way for a future strategy of prevention and counteraction of multiple diseases.
In this review, we vitally analyzed and reported the possible mechanistic approach of thymoquinone, such as the downregulation of various cytokines, inflammatory factors, and apoptotic pathways for the management of rheumatoid arthritis and cancer. Moreover, their toxicity reduction potential was also reported. An extensive review of their patent and clinical trials worldwide was also reported.
With the deep dive that we undertook in this review, it was revealed that formulations can transform the applicability of the nano carrier-based formulation of thymoquinone; however, these studies can be dynamic. Significant dots in research have been recognized that need to be connected: various pre-clinical and human trials are taking place worldwide to ascertain the applicability of thymoquinone in humans; there are a lack of comparative findings on various nanoformulations to optimize the best regimen for TQ delivery against rheumatoid arthritis and cancer; the non-availability of toxicity/safety data for thymoquinone-loaded NPs and human studies specifically exploring the pharmaceutical importance of nanoparticulate systems on arthritic and cancer milieu.