Modern developments in cellulosic materials for oil spill removal are briefly showcased in this entry. Different types of lignocellulosic textures and different modification techniques and preparation methods are explained. Materials were classified into 3D-materials such as hydrophobic and oleophobic sponges and aerogels, or 2D-materials such as membranes, fabrics, films, and meshes. Majorly, 3D-materials showed clear correlation between porosity and density, and their absorption behavior. Moreover, it was shown that nanocellulosic precursors are not exclusively suitable to attain considerable absorption performance. This finding can lead to developments in cost- and energy-efficient production processes of future cellulosic oil spillage removal solutions.
- Nanocellulose
- Oil Spill
- Adsorption
- Lignocellulose
- valorisation
1. INTRODUCTION
More than 35 billion gallons of petroleum oil are transported on ships per year, spills and leaks exceed 18 million gallons per annum globally[1][2]. Oil hinders underwater photosynthetic activity. Which forms an obstacle in algal growth, leading to disturbed aquatic food chain. Coral reefs can be contaminated by oil components and eventually die. Microorganisms get trapped to oil spill and larger creatures get intoxicated when assimilating or inhaling oil components. At high concentrations, living creatures die; at much lower contamination levels, their physiologies will become flawed[3].
Lignocelluloses, which constitute any plant tissue, are composites of three natural polymers: cellulose, hemicelluloses, and lignin[4].Lignocellulosic production exceeds 1.3*10^10 metric ton per annum[5]. Which poses monolithic amount of materials (& wastes) to be utilized.
2. PROCESSING OF CELLULOSE & NANOCELLULOSE
Cellulose and its derivatives are among the perfect candidates to constitute aerogel textures. High content of hydroxyl groups in cellulose chains increases the likelihood of linking with other chains to form a stable 3D gel texture. Chemical crosslinkers could be beneficial for improved mechanical stability, shape recovery, and insolubility [6]. In the following lines, we will throw light on aerogel fabrication methods.
2.1 Sol-Gel Preparation
The sol-gel reaction is the most decisive step in creating a porous 3D web structure in an aerogel. In a sol, colloidal cellulose particles with sizes ranging from 1 to 1000 nm are dispersed in a liquid. A gel consists of a solid, spongy 3D network whose clusters are filled with another liquid [7].
Cellulose is not soluble in water and shows amphiphilic nature, but can be dissolved in various solvents and be regenerated afterwards. Solvents include alkali/water/urea, ionic liquids (IL), e.g., 1-allyl-3-methylimidazolium chloride (AmimCl) [8][9].
2.2. Stabilization of the Gel
2.2.1 Chemical Crosslinkers
Chemical crosslinkers are used to ensure higher mechanical stability, better shape recovery, and less solubility of aerogels. They are usually mixed with the cellulose sol and activated via heat treatment after the aerogel formation. The hydroxyl group of the cellulose is a target for crosslinking (typical examples for chemical crosslinkers see figure 1.
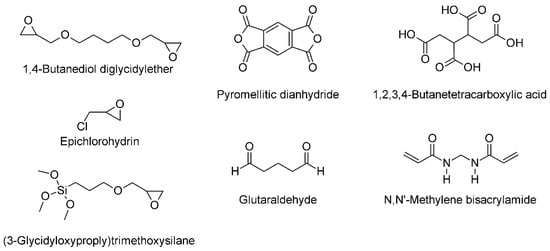
Figure 1. Typical molecules for cellulose crosslinking
2.2.2. Polymeric Matrix Stabilizers
Polymers can be used as matrix stabilizers to increase stiffness and hydrophobicity of formed aerogels. Unlike chemical crosslinkers, polymers do not form covalent bonds with the cellulosic matrix, but adsorbed and interact by weaker intermolecular bonds. wood fines coated with a poly(methyl methacrylate) (PMMA)-solution in tetrahydrofuran (THF) [10]or polyethyleneimine (PEI) on GPTMS stabilized aerogel[11]are examples of polymer coatings from solutions.
2.3. Drying Processes for Aerogel Formation
2.3.1. Supercritical CO
2
Drying
Since CO2 has a suitable critical point (304 K, 7.4 MPa) in addition to low cost and high safety, it is a kind of fluid that is commonly used for drying cellulose aerogels. Supercritical drying is done by diffusing supercritical carbon dioxide (scCO2) into the gel pores substituting solvent molecules then the gel texture will be dominated by supercritical fluid [12].
2.3.2. Vacuum Freeze Drying
The gel is first cooled below the freezing point of the gel liquid then the liquid is eliminated by sublimation (transformation of solid to gas directly, without passing through the liquid phase). Temperature and cooling rate play a drastic role in the crystallization and growth behavior of the newly formed structure, including pore size and distribution [13].
3. BEHAVIOR OFLIGNOCELLULOSIC MATERIALS FOR OIL SPILL CLEANUP
3.1. Three-Dimensional (3D) Materials: Aerogels and Foams
3D materials are defined as materials, which obtain their oil spillage cleanup properties via their volume dimension. They are typically aerogels, foams, or spongy materials and can perform either as absorbers or membranes/filters.
3.1.1. Separation Mechanism
Hydrophobic 3D materials are typically absorber -like, which means that the oil is entirely absorbed by the porous material and has to be recovered after cleanup. Alternatively, some hydrophobic 3D materials are also used as filters whereby the oil phase passes through the porous material and is so separated. By contrast, hydrophilic 3D materials, which are used as separating membranes, retain the oil phase and let the aqueous phase pass. The comparison of different separation mechanisms is depicted in Figure 2.
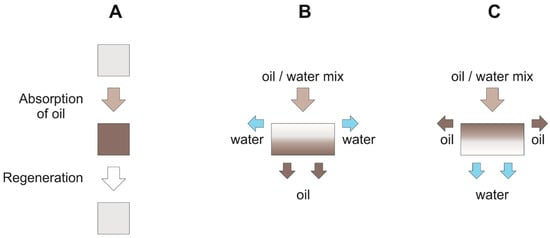
Figure 2. Separation mechanism of 3D materials: (A) absorber type; (B) hydrophobic filters; (C) hydrophilic filters
3.1.2. Recovery and Reusability
Reusability and recovery of 3D materials is vital for enormous applications, as those aspects affect cost and durability. There are three main methods for extracting the absorbed oil after certain use cycles which are mechanical, distillation and extraction. Distillation has a major advantage over other types as it keeps shape consistency after the recovery process. Also, the extraction process is more convenient for preventing hydrophobic layer failure which usually gets damaged by mechanical recovery. The main advantage of the mechanical recovery method is the cheap and easy application. It should be mentioned that the oleophobic 3D materials, which can be used as a filter, show the highest remaining capacity after recovery (95–100%). Some materials also have magnetic properties to collect them more easily from the sea water [14].
3.2. Two-Dimensional (2D) Materials: Membranes, Fabrics and Meshes
Two-dimensional materials have a significant extension in two dimensions and therefore have the shape of membranes, fabric or meshes. Membranes are recently used more frequently in different fields, such as seawater desalination, gas separation, water purification, and so on [15]. The membrane technology is attracting considerable attention with regards to oil removal from wastewater due to its advantages such as high effective oil droplets removal, low energy consumption and required medium temperature.
Membranes of the coated biomass fibers prefabricated by either hydrophilic or oleophilic compounds become impermeable to the counterpart liquids, giving rise to oil–water separation efficiency in a broad spectrum of mixtures.
Materials based on cellulose can be not only chemically modified for oil/water separation applications [16], but also by different methods such as coatings [17], electrospinning [18], & phase inversion [19]. These techniques and materials provide a vital platform to overcome various separation challenges.
3.2.1. Separation Mechanism
According to their shape, the 2D materials can be used as flow-through separators. For establishing a technical separation process, it is beneficial that the minor phase is retained, and the excess phase passes through the 2D material as presented in figure 3.
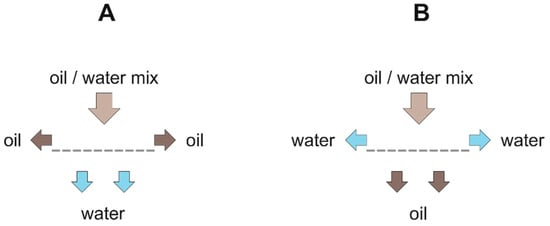
Figure 3. Separation mechanism of 2D materials: (A) hydrophilic/oleophobic filters; (B) hydrophobic/oleophilic filters.
3.2.2. Material Performance of Membranes
The membranes derived from environmentally friendly materials, especially from cotton or kapok fibers, show oil/water separation efficiencies of above 99.98%, fluxes ranging from 4000 to 22,200 L m−2 h−1, and robust performance for regeneration for the filtration of toluene/water mixture and repeated uses.
Dual superlyophobic membranes are obtained based on fast and simple methodologies with low cost [17]. Recently, nano-fibrous membranes with special wettability, excellent antifouling property, and reusability have attracted increasing interest for oil/water emulsions separation were modified through coating polydopamine and polyethyleneimine on the membrane surface in an aqueous system by an electrospinning technique.
Different membrane materials were utilized in separating water from water-in-oil emulsions like GO@CNF, CNC/Chitin-nanocrystals (ChiNC)/chitosan (CH), and polypyrrole modified cellulose membranes. Some comertial materials like Cigarette filter paper were modified through a dip-coating with dodecanethiol-modified polypyrrole particles for superhydrophobic oil/water separation [20].
To elucidate a possible correlation between material properties and separation efficiency, we investigated the impact of thickness (Figure 4A) and under-water contact angle (Figure 4B) of the membranes on the volume flux. However, for the 2D materials no obvious relationship could be statistically deduced.
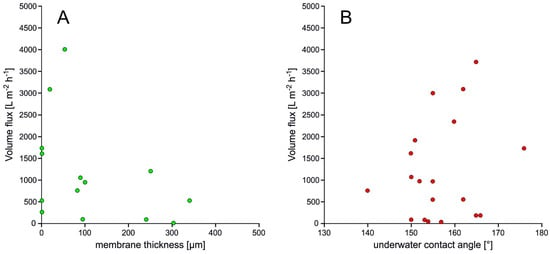
Figure 4. Volume flux of membranes depending from membrane thickness (A) and UWCA (B)
.
3.2.3. Material Performance of Fabrics
A superhydrophobic surface designed by less-expensive, biodegradable and environmentally friendly route for oil/water separation with fabrics has drawn increasing interest across the scientific community [21]. However, the applications of hydrophobic surfaces are still hampered by lengthy preparation procedures and high-cost manufacture.
Mercapto silanes followed by click coupling with methacryl-heptaisobutyl polyhedral oligomeric silsesquioxane (MAPOSS) and polyhedral oligomeric silsesquioxane (POSS) are key surface modifiers for Superhydrophobic modified cotton fabric with ultra-high absorptive capacity, which can absorb oil over 12.5 times of its own weight with strong stabilities against harsh environments [22].
Also cellulose-coated cotton fabric with improved thermal stability, mechanical performance, low cost and high separation performance applications was prepared recently, which was functionalized with super hydrophobicity by immobilization and the in-situ reduction method, in which Cu2+ was uniformly immobilized on cotton fabric in [Cu(NH3)4]2+ solution, and then reduced by sodium borohydride (NaBH4) and modified by n-octadecathiol[23].
3.3 Performance of 3D materials
The material performance for 3D materials can be expressed either as maximum absorption
capacity regarding oil or non-polar solvents or separation efficiencies, expressed as the ratio of removal of oil from water. The average volumetric absorption capacity was compared to
material-specific dimensions of the absorber material, such as density (δ), porosity (ϕ) as
presented in Figure 5 A–D where it is clear that the absorption capacity is in direct proportional with the porosity of the absorber material. Besides, the absorption capacity comes close to zero below 95% porosity and above densities 50 mg cm −3 density, which can be considered as the technical limit for those materials.
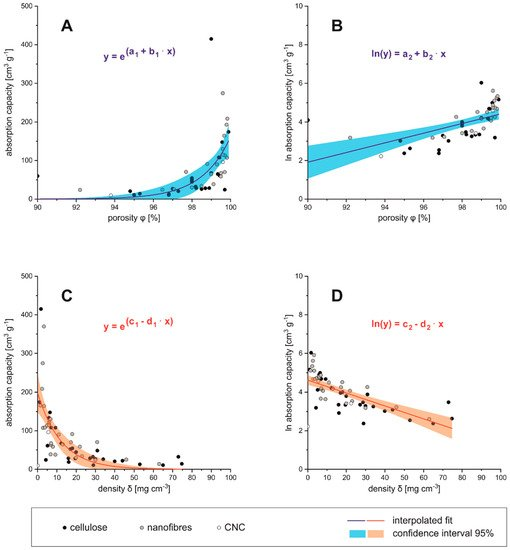
Figure 5 Density (A,B) and porosity (C,D) in relation to absorbance capacity
Regarding the cellulosic raw materials for aerogel preparation one could estimate that the dimensions of precursors (nano- or micrometer sized) would influence the porosity and, respectively, the density of the obtained aerogel. It can be seen from Figure 5 A–D that although low density/high porosity aerogels are produced from a nano- and micro sized fibrous precursor (CNF/MFC), this is not exclusively limited to it, since low densities and high porosities are also found in aerogels produced from bulk cellulose and BNC and vice versa. Therefore, it has to be assumed that density and porosity of cellulose based aerogels is not mainly depending on the raw materials, but likely on the manufacturing process.
4. CONCLUSION & RECOMMENDATIONS
A multitude of cellulose- and nanocellulose-based materials have been explored, which underwent a wide range of chemical modifications to enhance their hydrophobicity, including silanization with a variety of different silanes, grafting of polymers and hydrophobic molecules, and incorporation of inorganic nanoparticles and surface modifiers. Physical modification for hydrophobization has been used as well including carbonization via pyrolysis.
The used materials were mostly 3D such as hydrophobic and oleophobic sponges and aerogels or 2D such as membranes, fabrics, films, and meshes. The preparation conditions and techniques used together were the determinants of the materials properties; hence their performance. Trends in production are the introduction of hierarchical porosity for fast adsorption and desorption processes while maintaining high surface area, increased mechanical stability for efficient recovery of adsorbed oil, and several life cycles of the absorber materials, and development of cost-efficient and reproducible processes while using less-toxic and environmental-friendly modifiers. Unlike the 2D, the 3D materials, there was a clear correlation between the material properties and their performance.
Overall, cellulosic materials have shown promising capabilities to clean up oil spillage, although more thorough investigations should be performed regarding the impact of the material preparation conditions and techniques on its properties and performance, especially for 2D materials.
References
- Adebajo, M., Frost, L., Kloprogge, J., Carmody, O., & Kokot, S.; Porous materials for oil spill cleanup: A review of synthesis and absorbing properties. Journal of Porous Materials 2003, 10, 159-170, https://doi.org/10.1023/A:1027484117065.
- Radetic M., Ilic, V., Radojevic, D., Miladinovic, R., Jocic, D., & Jovancic, P.; Efficiency of recycled wool-based nonwoven material for the removal of oils from water. Chemosphere 2008, 70, 525-530, https://doi.org/10.1016/j.chemosphere.2007.07.005.
- Peterson, C.H.; Rice, S.D.; Short, J.W.; Esler, D.; Bodkin, J.L.; Ballachey, B.E.; Irons, D.B.; Long-term ecosystem response to the Exxon Valdez oil spill. Science 2003, 302, 2082–2086, DOI: 10.1126/science.1084282.
- Mehanny, S.; Ibrahim, H.; Darwish, L.; Farag, M.; El-Habbak, A.-H.M.; El-Kashif, E. Effect of Environmental Conditions on Date Palm Fiber Composites; Springer: Singapore, 2020; pp. 287–320.
- Kumar, R.; Singh, S.; Singh, O.V.; Bioconversion of lignocellulosic biomass: Biochemical and molecular perspectives. J. Ind. Microbiol. Biotechnol. 2008, 35, 377–391, https://doi.org/10.1007/s10295-008-0327-8.
- Nguyen, B.N.; Cudjoe, E.; Douglas, A.; Scheiman, D.; McCorkle, L.; Meador, M.A.B.; Rowan, S.J.; Polyimide cellulose nanocrystal composite aerogels. Macromolecules 2016, 49, 1692–1703, https://doi.org/10.1021/acs.macromol.5b01573.
- Gurav, J.L.; Jung, I.-K.; Park, H.-H.; Kang, E.S.; Nadargi, D.Y.; Silica aerogel: Synthesis and applications. Nanomater 2010, 2010, 409310 , https://doi.org/10.1155/2010/409310.
- Zhang, H.; Li, Y.; Xu, Y.; Lu, Z.; Chen, L.; Huang, L.; Fan, M; Versatile fabrication of a superhydrophobic and ultralight cellulose-based aerogel for oil spillage clean-up. Phys. Chem. Chem. Phys 2016, 18, 28297–28306, DOI: 10.1039/C6CP04932J.
- Jin, C.; Han, S.; Li, J.; Sun, Q.; Fabrication of cellulose-based aerogels from waste newspaper without any pretreatment and their use for absorbents. Carbohydr. Polym 2015, 123, 150–156, https://doi.org/10.1016/j.carbpol.2015.01.056.
- Colson, J.; Amer, H.; Liebner, F.; Gindl-Altmutter, W; Oil-absorbing porous cellulosic material from sized wood pulp fines. Holzforschung 2019, 73, 83–92, https://doi.org/10.1515/hf-2018-0093.
- Li, Y.; Zhu, L.; Grishkewich, N.; Tam, K.C.; Yuan, J.; Mao, Z.; Sui, X; CO2-Responsive Cellulose Nanofibers Aerogels for Switchable Oil-Water Separation. ACS Appl. Mater. Interfaces 2019, 11, 9367–9373, https://doi.org/10.1021/acsami.8b22159.
- Chin, S.F.; Romainor, A.N.B.; Pang, S.C.; Fabrication of hydrophobic and magnetic cellulose aerogel with high oil absorption capacity. Mater. Lett. 2014, 115, 241–243, https://doi.org/10.1016/j.matlet.2013.10.061.
- Long, L.-Y.; Weng, Y.-X.; Wang, Y.-Z.; Cellulose aerogels: Synthesis, applications, and prospects. Polymers 2018, 10, 623, https://doi.org/10.3390/polym10060623.
- Lu, Y.; Wang, Y.; Liu, L.; Yuan, W.; Environmental-friendly and magnetic/silanized ethyl cellulose sponges as effective and recyclable oil-absorption materials. Carbohydr. Polym 2017, 173, 422–430, https://doi.org/10.1016/j.carbpol.2017.06.009.
- Ali, N.; Bilal, M.; Khan, A.; Ali, F.; Iqbal, H.M.N.; Design, engineering and analytical perspectives of membrane materials with smart surfaces for efficient oil/water separation. TrAC Trends Anal. 2020, 127, 115902, https://doi.org/10.1016/j.trac.2020.115902.
- Li, S.; Yang, S.; Zhu, X.; Jiang, X.; Kong, X.Z.; Easy preparation of superoleophobic membranes based on cellulose filter paper and their use for water–oil separation. Cellulose 2019, 26, 6813–6823, https://doi.org/10.1007/s10570-019-02552-4.
- Mai, V.C.; Das, P.; Zhou, J.; Lim, T.T.; Duan, H; Mussel-Inspired Dual-Superlyophobic Biomass Membranes for Selective Oil/Water Separation. Adv. Mater. Interfaces 2020, 7, 1901756, https://doi.org/10.1002/admi.201901756.
- Wang, J.; Wang, L.; Superhydrophilic and underwater superoleophobic nanofibrous membrane for separation of oil/water emulsions. J. Mater. Res 2020, 35, 1504–1513, https://doi.org/10.1557/jmr.2020.137.
- Ang, M.B.M.Y.; Macni, C.R.M.; Caparanga, A.R.; Huang, S.H.; Tsai, H.A.; Lee, K.R.; Lai, J.Y.; Mitigating the fouling of mixed-matrix cellulose acetate membranes for oil–water separation through modification with polydopamine particles. Chem. Eng. Res. Des. 2020, 159, 195–204, https://doi.org/10.1016/j.cherd.2020.04.015.
- Zhang, J.; Xu, H.; Guo, J.; Chen, T.; Liu, H.; Superhydrophobic polypyrrole-coated cigarette filters for effective oil/water separation. Appl. Sci. 2020, 10, 1985, https://doi.org/10.3390/app10061985.
- Cheng, Q.Y.; Zhao, X.L.; Li, Y.D.; Weng, Y.X.; Zeng, J.B; Robust and nanoparticle-free superhydrophobic cotton fabric fabricated from all biological resources for oil/water separation. Int. J. Biol. Macromol. 2019, 140, 1175–1182, https://doi.org/10.1016/j.ijbiomac.2019.08.216.
- Song, Q.; Wang, H.; Han, S.; Wang, J.; Zhang, B.; Zhang, Y; Halloysite nanotubes functionalized cotton fabric for oil/water separation.. Prog. Org. Coat 2020, 148, 105839, https://doi.org/10.1016/j.porgcoat.2020.10583.
- Zhang, Y.R.; Chen, J.T.; Hao, B.; Wang, R.; Ma, P.C; Preparation of cellulose-coated cotton fabric and its application for the separation of emulsified oil in water. Carbohydr. Polym. 2020, 240, 116318, https://doi.org/10.1016/j.carbpol.2020.116318.
