Torulaspora delbrueckii has attracted interest in recent years, especially due to its biotechnological potential, arising from its flavor- and aroma-enhancing properties when used in wine, beer or bread dough fermentation, as well as from its remarkable resistance to osmotic and freezing stresses.
- Torulaspora delbrueckii
- non-Saccharomyces
- wine
- bread
- biotechnology
- genomics
1. Introduction
2. Occurrence and General Characteristics
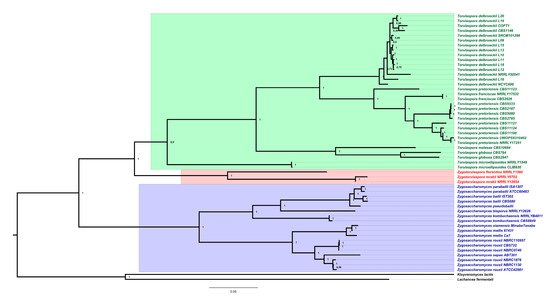
3. Genomics and Taxonomy
4. Metabolism
|
Product |
Torulaspora delbrueckii |
Saccharomyces cerevisiae |
Notes |
References |
|||
|---|---|---|---|---|---|---|---|
|
Acetic acid |
0.27–0.56 g/L |
1.0–1.17 g/L |
Key signature in volatile acidity of wines |
||||
|
Malic acid |
Consumption between 10.5–25% |
Whether degradation or production is desirable depends on the must characteristics. |
[23] |
||||
] |
Citric acid |
2.18–2.36 g/L |
2.23 g/L |
Citrus-like taste |
|||
|
Honey |
Honey sugar | [ | ] |
||||
Rapidly ferment sugar |
Large-scale productions only in combination with S. cerevisiae |
[7] |
Succinic acid |
0.84–1.11 g/L |
|||
|
Olive oil |
Black olives |
Maximum of 0.65 g/L |
Minor acid in the overall wine acidity, although the combination with one |
Easy hydrolyzation of olive oil |
molecule of ethanol creates the ester mono-ethyl succinate, responsible for a mild, fruity aroma |
Growth inhibition at concentrations higher than 0.5% ( |
[41] |
|
- |
Maximum of 1.13 g/L |
[42] |
|||||
|
Mannoproteins |
T. delbrueckii produces 25% more than S. cerevisiae |
Released during fermentation or ageing processes |
[23] |
||||
|
Polysaccharides |
T. delbrueckii releases 50% more than S. cerevisiae |
Increases the quality of mouthfeel properties |
[43] |
||||
|
Glycerol |
1–10.5 g/L |
Maximum of 9.1 g/L |
Smoothness and viscosity features |
||||
|
Ethanol |
40.6–72.68 g/L |
103–121 g/L |
|||||
5. Biotechnological Importance of T. delbrueckii
5.1. Bread Industry
5.2. Production of Fermented Beverages
w | ||||
/ | ||||
v | ||||
) of oleuropein | ||||
[ | ] |
|||
|
Coffee |
Coffee cherries |
Improve coffee’s sensorial quality |
Pronounced astringency depending on the coffee variety |
|
|
Bio-protection |
– |
Reduction in the use of chemical preservatives to control food spoilage |
– |
|
Beverages Applications |
Used Substrate |
Advantages |
Disadvantages |
References |
|---|---|---|---|---|
|
Beer |
Wort |
High tolerance to hop compounds; good flavor-forming properties |
Low sugar utilization |
|
|
Mezcal |
Agave juice † |
Rich in volatile compounds; acceptable in sensory tests |
Low performance |
|
|
Tequila |
Agave juice * |
Positive influence on the final sensory profile |
– |
[64] |
|
Cider |
Apple juice † |
Great production of ethyl decanoate and ethyl hexanoate |
Low performance; negligible amounts of acetate esters |
|
|
Mead |
Honey sugar |
Good fermentation ability; Good sensory features |
Grassy flavor |
[7] |
|
Soy alcoholic beverage |
Soy whey |
Enrich aroma profiles: high levels of ethyl decanoate and ethyl hexanoate; metabolize hexanal; |
– |
[67] |
* Specifically from Agave tequilana; † sterile.
5.3. Other Food Applications
The reported versatility of T. delbrueckii makes it a remarkable asset to be explored, not only for bread and fermented beverages purposes, but also in other diverse food products (Table 3). One example is the production of chocolate in which yeasts play a key role in flavour development, as the quality of chocolate is reduced if the cocoa fermentation process is conducted without these microorganisms [68]. This importance is reinforced by Visitin et al. [69] by showing the involvement of T. delbrueckii in the fermentation of cocoa beans (Theobroma cacao [68]) to produce chocolate, despite not yet being standard in this industry. Authors showed that through a combination with S. cerevisiae, modifications on the analytical profile of the chocolate are obtained. Moreover, differences in the samples obtained from S. cerevisiae and T. delbrueckii inoculated chocolate had a significant impact on the consumers’ perception of the final product, mentioned by some as fruitier. Therefore, the use of this unconventional yeast resulted in a positive contribution to the development of the chocolate’s final aroma. In addition, T. delbrueckii can also be explored in the cheese industry, benefiting from its tolerance to low temperatures, low pH, high salt concentrations and low water activity [70]. Andrade et al. [71] produced cheese from fermented milk, with the aim of evaluating the impact of T. delbrueckii (in mixed or pure inocula) on cheese production, detecting a slow consumption of lactose which can be translated into a reduced β-galactosidase activity, as stated by the authors.|
Food Applications |
Used Substrate |
Advantages |
Disadvantages |
References |
|---|---|---|---|---|
|
Chocolate |
Cocoa beans |
Good flavor quality of cocoa and, therefore, the chocolate |
Expedite in mixed fermentations with S. cerevisiae |
[69] |
|
Cheese |
Cheese |
Varied aromatic properties |
Unable to inhibit pathogenic bacteria; low β-glucosidase activity |
References
- Drumonde-Neves, J.; Fernandes, T.; Lima, T.; Pais, C.; Franco-Duarte, R. Learning from 80 years of studies: A comprehensive catalogue of non-Saccharomyces yeasts associated with viticulture and winemaking. FEMS Yeast Res. 2021, 21, foab017.
- Kosel, J.; Čadež, N.; Schuller, D.; Carreto, L.; Franco-Duarte, R.; Raspor, P. The influence of Dekkera bruxellensis on the transcriptome of Saccharomyces cerevisiae and on the aromatic profile of synthetic wine must. FEMS Yeast Res. 2017, 17, 1–11.
- Azzolini, M.; Tosi, E.; Lorenzini, M.; Finato, F.; Zapparoli, G. Contribution to the aroma of white wines by controlled Torulaspora delbrueckii cultures in association with Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2015, 31, 277–293.
- Benito, S. The impact of Torulaspora delbrueckii yeast in winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 3081–3094.
- Benito, Á.; Calderón, F.; Benito, S. The influence of non-Saccharomyces species on wine fermentation quality parameters. Fermentation 2019, 5, 54.
- Albertin, W.; Chasseriaud, L.; Comte, G.; Panfili, A.; Delcamp, A.; Salin, F.; Marullo, P.; Bely, M. Winemaking and bioprocesses strongly shaped the genetic diversity of the ubiquitous yeast Torulaspora delbrueckii. PLoS ONE 2014, 9, e94246.
- Barry, J.P.; Metz, M.S.; Hughey, J.; Quirk, A.; Bochman, M.L. Two Novel Strains of Torulaspora delbrueckii isolated from the honey bee microbiome and their use in honey fermentation. Fermentation 2018, 4, 22.
- Nguyen, N.H.; Suh, S.O.; Blackwell, M. Five novel Candida species in insect-associated yeast clades isolated from Neuroptera and other insects. Mycologia 2007, 99, 842–858.
- Capriotti, A. Torulaspora nilssoni nov. spec. Arch. Für Mikrobiol. 1957, 28, 247–254.
- Byzov, B.A.; Thanh, V.N.; Babjeva, I.P. Yeasts associated with soil invertebrates. Biol. Fertil. Soils 1993, 16, 183–187.
- Limtong, S.; Koowadjanakul, N. Yeasts from phylloplane and their capability to produce indole-3-acetic acid. World J. Microbiol. Biotechnol. 2012, 28, 3323–3335.
- Yurkov, A.M.; Chernov, I.Y. Geographical races of certain species of ascomycetous yeasts in the Moscow and Novosibirsk regions. Microbiology 2005, 74, 597–601.
- Silva-Bedoya, L.M.; Ramirez-Castrillon, M.; Osorio-Cadavid, E. Yeast diversity associated to sediments and water from two Colombian artificial lakes. Braz. J. Microbiol. 2014, 45, 135–142.
- Bely, M.; Stoeckle, P.; Masneuf-Pomarède, I.; Dubourdieu, D. Impact of mixed Torulaspora delbrueckii–Saccharomyces cerevisiae on high-sugar fermentation. Int. J. Food Microbiol. 2008, 122, 312–320.
- Renault, P.; Coulon, J.; de Revel, G.; Barbe, J.C.; Bely, M. Increase of fruity aroma during mixed T. delbrueckii/S. cerevisiae wine fermentation is linked to specific esters enhancement. Int. J. Food Microbiol. 2015, 207, 40–48.
- Kaygusuz, I.; Mulazimoglu, L.; Cerikcioglu, N.; Toprak, A.; Oktay, A.; Korten, V. An unusual native tricuspid valve endocarditis caused by Candida colliculosa. Clin. Microbiol. Infect. 2003, 9, 319–322.
- De Vuyst, L.; Harth, H.; Van Kerrebroeck, S.; Leroy, F. Yeast diversity of sourdoughs and associated metabolic properties and functionalities. Int. J. Food Microbiol. 2016, 239, 26–34.
- Hendriks, L.; Goris, A.; Van De Peer, Y.; Neefs, J.M.; Vancanneyt, M.; Kersters, K.; Berny, J.F.; Hennebert, G.L.; De Wachter, R. Phylogenetic Relationships among Ascomycetes and Ascomycete-like Yeasts as Deduced from Small Ribosomal Subunit RNA Sequences. Syst. Appl. Microbiol. 1992, 15, 98–104.
- Wahyono, A.; Lee, S.B.; Kang, W.W.; Park, H.D. Improving bread quality using co-cultures of Saccharomyces cerevisiae, Torulaspora delbrueckii JK08, and Pichia anomalia JK04. Ital. J. Food Sci. 2016, 28, 298–313.
- Ramírez, M.; Velázquez, R. The yeast Torulaspora delbrueckii: An interesting but difficult-to-use tool for winemaking. Fermentation 2018, 4, 94.
- Saluja, P.; Yelchuri, R.K.; Sohal, S.K.; Bhagat, G.; Prasad, G.S. Torulasporaindica a novel yeast species isolated from coal mine soils. Antonie Van Leeuwenhoek 2012, 101, 733–742.
- Ramírez, M.; Ambrona, J. Construction of sterile ime1Δ-transgenic Saccharomyces cerevisiae wine yeasts unable to disseminate in nature. Appl. Environ. Microbiol. 2008, 74, 2129–2134.
- Cherry, J.M.; Hong, E.L.; Amundsen, C.; Balakrishnan, R.; Binkley, G.; Chan, E.T.; Christie, K.R.; Costanzo, M.C.; Dwight, S.S.; Engel, S.R.; et al. Saccharomyces Genome Database: The genomics resource of budding yeast. Nucleic Acids Res. 2012, 40, D700–D705.
- Gordon, J.L.; Armisen, D.; Proux-We´ra, E.; ÓhEígeartaigh, S.S.; Byrne, K.P.; Wolfe, K.H. Evolutionary Erosion of Yeast Sex Chromosomes by Mating-Type Switching Accidents. Proc. Natl. Acad. Sci. USA 2011, 108, 20024–20029.
- Santiago, C.; Rito, T.; Vieira, D.; Fernandes, T.; Pais, C.; Sousa, M.J.; Soares, P.; Franco-Duarte, R. Improvement of Torulaspora delbrueckii genome annotation: Towards the exploitation of genomic features of a biotechnologically relevant yeast. J. Fungi 2021, 7, 287.
- Shen, X.X.; Opulente, D.A.; Kominek, J.; Zhou, X.; Steenwyk, J.L.; Buh, K.V.; Haase, M.A.; Wisecaver, J.H.; Wang, M.; Doering, D.T.; et al. Tempo and mode of genome evolution in the budding yeast subphylum. Cell 2018, 175, 1533–1545.
- Belda, I.; Navascués, E.; Marquina, D.; Santos, A.; Calderon, F.; Benito, S. Dynamic analysis of physiological properties of Torulaspora delbrueckii in wine fermentations and its incidence on wine quality. Appl. Microbiol. Biotechnol. 2015, 99, 1911–1922.
- Loira, I.; Vejarano, R.; Bañuelos, M.A.; Morata, A.; Tesfaye, W.; Uthurry, C.; Villa, A.; Cintora, I.; Suárez-Lepe, J.A. Influence of sequential fermentation with Torulaspora delbrueckii and Saccharomyces cerevisiae on wine quality. LWT–Food Sci. Technol. 2014, 59, 915–922.
- Almeida, M.J.; Pais, C. Leavening ability and freeze tolerance of yeasts isolated from traditional corn and rye bread doughs. Appl. Environ. Microbiol. 1996, 62, 4401–4404.
- Pacheco, A.; Santos, J.; Chaves, S.; Almeida, J.; Leão, C.; Sousa, M.J. The Emerging Role of the Yeast Torulaspora delbrueckii in Bread and Wine Production: Using Genetic Manipulation to Study Molecular Basis of Physiological Responses. Struct. Funct. Food Eng. 2012, 339–370.
- Santos, J.; Sousa, M.J.; Cardoso, H.; Inacio, J.; Silva, S.; Spencer-Martins, I.; Leão, C. Ethanol tolerance of sugar transport, and the rectification of stuck wine fermentations. Microbiology 2008, 154, 422–430.
- Alves-Araújo, C.; Pacheco, A.; Almeida, M.J.; Spencer-Martins, I.; Leão, C.; Sousa, M.J. Sugar utilization patterns and respiro-fermentative metabolism in the baker’s yeast Torulaspora delbrueckii. Microbiology 2007, 153, 898–904.
- Velázquez, R.; Zamora, E.; Álvarez, M.L.; Ramírez, M. Using Torulaspora delbrueckii killer yeasts in the elaboration of base wine and traditional sparkling wine. Int. J. Food Microbiol. 2019, 289, 134–144.
- Holm Hansen, E.; Nissen, P.; Sommer, P.; Nielsen, J.C.; Arneborg, N. The effect of oxygen on the survival of non-Saccharomyces yeasts during mixed culture fermentations of grape juice with Saccharomyces cerevisiae. J. Appl. Microbiol. 2001, 91, 541–547.
- Ciani, M.; Picciotti, G. The growth kinetics and fermentation behaviour of some non-Saccharomyces yeasts associated with winemaking. Biotechnol. Lett. 1995, 17, 1247–1250.
- Ciani, M.; Maccarelli, F. Oenological properties of non-Saccharomyces yeasts associated with winemaking. World J. Microbiol. Biotechnol. 1997, 14, 199–203.
- King, A.; Dickinson, J.R. Biotransformation of monoterpene alcohols by Saccharomyces cerevisiae, Torulaspora delbrueckii and Kluyveromyces lactis. Yeast 2000, 16, 499–506.
- Chen, K.; Escott, C.; Loira, I.; Del Fresno, J.M.; Morata, A.; Tesfaye, W.; Calderon, F.; Suárez-Lepe, J.A.; Han, S.; Benito, S. Use of non-Saccharomyces yeasts and oenological tannin in red winemaking: Influence on colour, aroma and sensorial properties of young wines. Food Microbiol. 2018, 69, 51–63.
- Mecca, D.; Benito, S.; Beisert, B.; Brezina, S.; Fritsch, S.; Semmler, H.; Rauhut, D. Influence of nutrient supplementation on Torulaspora delbrueckii wine fermentation aroma. Fermentation 2020, 6, 35.
- Liu, S.; Laaksonen, O.; Kortesniemi, M.; Kalpio, M.; Yang, B. Chemical composition of bilberry wine fermented with non-Saccharomyces yeasts (Torulaspora delbrueckii and Schizosaccharomyces pombe) and Saccharomyces cerevisiae in pure, sequential and mixed fermentations. Food Chem. 2018, 266, 262–274.
- Puertas, B.; Jiménez, M.J.; Cantos-Villar, E.; Cantorial, J.M.; Rodríguez, M.E. Use of Torulaspora delbrueckii and Saccharomyces cerevisiae in semi-industrial sequential inoculation to improve quality of Palomino and Chardonnay wines in warm climates. J. Appl. Microbiol. 2016, 122, 733–746.
- Franco-Duarte, R.; Bessa, D.; Gonçalves, F.; Martins, R.; Silva-Ferreira, A.C.; Schuller, D.; Sampaio, P.; Pais, C. Genomic and transcriptomic analysis of Saccharomyces cerevisiae isolates with focus in succinic acid production. FEMS Yeast Res. 2017, 17, 1–12.
- Domizio, P.; Liu, Y.; Bisson, L.F.; Barile, D. Use of non-Saccharomyces wine yeasts as novel sources of mannoproteins in wine. Food Microbiol. 2014, 43, 5–15.
- Escribano, R.; González-Arenzana, L.; Portu, J.; Garijo, P.; López-Alfaro, I.; López, R.; Santamaria, P.; Gutiérrez, A.R. Wine aromatic compound production and fermentative behaviour within different non-Saccharomyces species and clones. J. Appl. Microbiol. 2018, 124, 1521–1531.
- Ivit, N.N.; Longo, R.; Kemp, B. The Effect of Non-Saccharomyces and Saccharomyces Non-cerevisiae Yeasts on Ethanol and Glycerol Levels in Wine. Fermentation 2020, 6, 77.
- Franco-Duarte, R.; Umek, L.; Mendes, I.; Castro, C.C.; Fonseca, N.; Martins, R.; Silva-Ferreira, A.C.; Sampaio, P.; Pais, C.; Schuller, D. New integrative computational approaches unveil the Saccharomyces cerevisiae pheno-metabolomic fermentative profile and allow strain selection for winemaking. Food Chem. 2016, 211, 509–520.
- Alves-Araújo, C.; Almeida, M.J.; Sousa, M.J.; Leão, C. Freeze tolerance of the yeast Torulaspora delbrueckii: Cellular and biochemical basis. FEMS Microbiol. Lett. 2004, 240, 7–14.
- Li, Z.; Li, H.; Bian, K. Microbiological characterization of traditional dough fermentation starter (Jiaozi) for steamed bread making by culture-dependent and culture-independent methods. Int. J. Food Microbiol. 2016, 234, 9–14.
- Hernandez-Lopez, M.J.; Prieto, J.A.; Randez-Gil, F. Osmotolerance and leavening ability in sweet and frozen sweet dough. Comparative analysis between Torulaspora delbrueckii and Saccharomyces cerevisiae baker’s yeast strains. Antonie Van Leeuwenhoek 2003, 84, 125–134.
- Spencer, J.F.T.; Spencer, D.M. Taxonomy: The names of the yeasts. In Yeasts in Natural and Artificial Habitats; Springer: Berlin/ Heidelberg, Germany, 1997; pp. 11–32.
- Renault, P.; Miot-Sertier, C.; Marullo, P.; Hernández-Orte, P.; Lagarrigue, L.; Lonvaud-Funel, A.; Bely, M. Genetic characterization and phenotypic variability in Torulaspora delbrueckii species: Potential applications in the wine industry. Int. J. Food Microbiol. 2009, 134, 201–210.
- Lu, Y.; Chua, J.Y.; Voon, M.K.W.; Huang, D.; Lee, P.R.; Liu, S.Q. Effects of Different Inoculation Regimes of Torulaspora delbrueckii and Oenococcus oeni on Fermentation Kinetics and Chemical Constituents of Durian Wine. South Afr. J. Enol. Vitic. 2017, 38, 273–285.
- Romano, P.; Ciani, M.; Fleet, G.H. Yeasts in the Production of Wine; Springer: New York, NY, USA, 2019; pp. 81–115.
- Wei, J.; Zhang, Y.; Yuan, Y.; Dai, L.; Yue, T. Characteristic fruit wine production via reciprocal selection of juice and non-Saccharomyces species. Food Microbiol. 2019, 79, 66–74.
- Ebeler, S.E. Analytical Chemistry: Unlocking the Secrets of Wine Flavor. Food Rev. Int. 2001, 17, 45–64.
- Lambrechts, M.G.; Pretorius, I.S. Yeast and its Importance to Wine Aroma—A Review. South Afr. J. Enol. Vitic. 2000, 21, 97–129.
- Belda, I.; Ruiz, J.; Esteban-Fernández, A.; Navascués, E.; Marquina, D.; Santos, A.; Moreno-Arribas, M. Microbial contribution to wine aroma and its intended use for wine quality improvement. Molecules 2017, 22, 189.
- Michel, M.; Kopecká, J.; Meier-Dörnberg, T.; Zarnkow, M.; Jacob, F.; Hutzler, M. Screening for new brewing yeasts in the non- Saccharomyces sector with Torulaspora delbrueckii as model. Yeast 2016, 33, 129–144.
- King, A.J.; Dickinson, J.R. Biotransformation of hop aroma terpenoids by ale and lager yeasts. FEMS Yeast Res. 2003, 3, 53–62.
- Canonico, L.; Agarbati, A.; Comitini, F.; Ciani, M. Torulaspora delbrueckii in the brewing process: A new approach to enhance bioflavour and to reduce ethanol content. Food Microbiol. 2016, 56, 45–51.
- Gibson, B.; Dahabieh, M.; Krogerus, K.; Jouhten, P.; Magalhães, F.; Pereira, R.; Siewers, V.; Vidgren, V. Adaptive laboratory evolution of ale and lager yeasts for improved brewing efficiency and beer quality. Annu. Rev. Food Sci. Technol. 2020, 11, 23–44.
- Arrizon, J.; Morel, S.; Gschaedler, A.; Monsan, P. Fructanase and fructosyltransferase activity of non-Saccharomyces yeasts isolated from fermenting musts of Mezcal. Bioresour. Technol. 2012, 110, 560–565.
- la Torre-González, D.; Javier, F.; Narváez-Zapata, J.A.; Taillandier, P.; Larralde-Corona, C.P. Mezcal as a novel source of mixed yeasts inocula for wine fermentation. Processes 2020, 8, 1296.
- Lachance, M.A. Yeast communities in a natural tequila fermentation. Antonie Van Leeuwenhoek 1995, 68, 151–160.
- Wei, J.; Wang, S.; Zhang, Y.; Yuan, Y.; Yue, T. Characterization and screening of non-Saccharomyces yeasts used to produce fragrant cider. LWT 2019, 107, 191–198.
- Lorenzini, M.; Simonato, B.; Slaghenaufi, D.; Ugliano, M.; Zapparoli, G. Assessment of yeasts for apple juice fermentation and production of cider volatile compounds. LWT 2019, 99, 1–18.
- Chua, J.Y.; Lu, Y.; Liu, S.Q. Evaluation of five commercial non-Saccharomyces yeasts in fermentation of soy (tofu) whey into an alcoholic beverage. Food Microbiol. 2018, 76, 533–542.
- Ho, V.T.T.; Zhao, J.; Fleet, G. Yeasts are essential for cocoa bean fermentation. Int. J. Food Microbiol. 2014, 174, 72–87.
- Visintin, S.; Ramos, L.; Batista, N.; Dolci, P.; Schwan, F.; Cocolin, L. Impact of Saccharomyces cerevisiae and Torulaspora delbrueckii starter cultures on cocoa beans fermentation. Int. J. Food Microbiol. 2017, 257, 31–40.
- Blaisonneau, J.; Sor, F.; Cheret, G.; Yarrow, D.; Fukuhara, H. A Circular Plasmid from the Yeast Torulaspora delbrueckii. Plasmid 1997, 38, 202–209.
- Andrade, R.P.; Oliveira, D.R.; Lopes, A.C.A.; de Abreu, L.R.; Duarte, W.F. Survival of Kluyveromyces lactis and Torulaspora delbrueckii to simulated gastrointestinal conditions and their use as single and mixed inoculum for cheese production. Food Res. Int. 2019, 125, 1–12.
- Ferreira, A.D.; Viljoen, B.C. Yeasts as adjunct starters in matured Cheddar cheese. Int. J. Food Microbiol. 2003, 86, 131–140.
- Psani, M.; Kotzekidou, P. Technological characteristics of yeast strains and their potential as starter adjuncts in Greek-style black olive fermentation. World J. Microbiol. Biotechnol. 2006, 22, 1329–1336.
- da Mota, M.C.B.; Batista, N.N.; Rabelo, M.H.S.; Ribeiro, D.E.; Borém, F.M.; Schwan, R.F. Influence of fermentation conditions on the sensorial quality of coffee inoculated with yeast. Food Res. Int. 2020, 136, 109482.
- Bressani, A.P.P.; Martinez, S.J.; Sarmento, A.B.I.; Borém, F.M.; Schwan, R.F. Influence of yeast inoculation on the quality of fermented coffee (Coffea arabica var. Mundo Novo) processed by natural and pulped natural processes. Int. J. Food Microbiol. 2021, 343, 109107.
- Simonin, S.; Alexandre, H.; Nikolantonaki, M.; Coelho, C.; Tourdot-Maréchal, R. Inoculation of Torulaspora delbrueckii as a bio-protection agent in winemaking. Food Res. Int. 2018, 107, 451–461.
- Al-Qaysi, S.A.S.; Abdullah, N.M.; Jaffer, M.R.; Abbas, Z.A. Biological Control of Phytopathogenic Fungi by Kluyveromyces marxianus and Torulaspora delbrueckii Isolated from Iraqi Date Vinegar. J. Pure Appl. Microbiol. 2021, 15, 300–311.
