Nanoparticles (NPs) have an outstanding position in pharmaceutical, biological, and medical disciplines. Polymeric NPs based on chitosan (CS) can act as excellent drug carriers because of some intrinsic beneficial properties including biocompatibility, biodegradability, non-toxicity, bioactivity, easy preparation, and targeting specificity. Drug transport and release from CS-based particulate systems depend on the extent of cross-linking, morphology, size, and density of the particulate system, as well as physicochemical properties of the drug. All these aspects have to be considered when developing new CS-based NPs as potential drug delivery systems. This review is summarizing and discussing recent advances in CS-based NPs being developed and examined for drug delivery including the following sections: (i) CS and its derivatives, basic characteristics of CS NPs, (ii) preparation procedures used for CS NPs, (iii) CS-based-nanocomposites with organic polymers and inorganic material, and (iv) implementations of CS NPs and nanocomposites in drug delivery.
- chitosan
- modified chitosan
- enhanced properties
- nanoparticles
- drug delivery
- new formulations
- targeting
- controlled release
- nanocomposites
- preparation schemes
1. Introduction
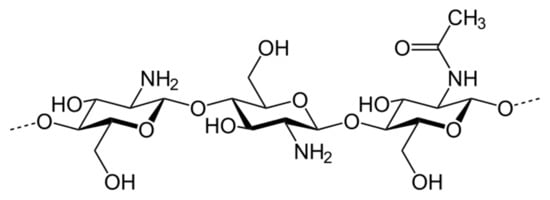
2. Enhancement of Chitosan Properties by Its Modification
Although CS possesses many functional properties, it has several limitations such as high hydrophilicity, low ductility, a high degree of swelling and it is thermally less stable [12]. A major limiting factor in its utilization is poor solubility. CS is insoluble at physiological pH (pH 7.4) and ineffective as an absorption enhancer which interferes with its biomedical application. Improving the solubility of CS is a crucial factor for judicious use of the multitude of applications. Chemical derivatives of CS can overcome limitations of unmodified CS and have received increasing interest over the past decade due to their advocated chemical, biological, and functional advantages over unmodified CS concerning their solubility, gelling properties, design of hydrophobic derivatives with amphiphilic character, and capacity to harness self-assembling nanostructures and chemical conjugates with an assortment of bioactive and therapeutic molecules. Other noteworthy benefits include improved biocompatibility and enhanced properties for complexing biomolecules (e.g., DNA, RNA). Varieties of CS derivatives have been developed throughout these years depending upon the requirements for various applications in biomedical areas [9]. The most used derivatives in the current pharmaceutical industry are made by quaternization, acylation, thiolation, and carboxymethylation [12]. Due to a variety of substituents of different nature, various derivatization procedures, and different activity and/or use of the products, it can be quite difficult to define criteria for specific groups of CS derivatives unambiguously. Although some aspects can overlap in particular groups, a specification of the basic categories should simplify orientation in produced CS derivatives. Negm et al. [19] divided CS modifications into the following categories: (i) substituted CS derivatives such as (I) thiolated CS, (II) phosphorylated CS, and (III) phtaloylated CS, (ii) cross-linked CS derivatives, such as (I) CS-glutaraldehyde cross-linked polymers, (II) EDTA CS polymer, and (III) CS-epichlorohydrin crosslinked polymers, (iii) carboxylic acid CS derivatives such as (I) CS carboxyalkylate derivatives, (II) CS methacrylate derivatives and (III) CS benzoylate derivatives, (iv) ionic CS derivatives such as (I) cationic CS derivatives and (II) sulphated CS derivatives and (v) bounded CS to specific molecules such as cyclodextrin linked CS. Bakshi et al. [13] presented the scheme highlighting (i) chemical modifications on -NH2 of glucosamine or on -OH groups of the polymer (such as (I) alkylation, acylation, hydroalkylation, carboxyalkylation, azidation, thiolation, (II) sugar derivatives, CS dendrimer hybrid, cyclodextrin linked, crown ether bonding, (III) metal ion chelates, grafting, cross-linking) and (ii) enzymatic modifications (using tyrosinase or chlorogenic acid). In their scheme following types of CS derivatives were included: hydroxyalkyl, acyl, N-alkylated, amphiphilic, thiolated, succinyl, cyclodextrin linked, quaternary ammonium derivative, enzymatic graft, crown ether bound, sialo dendrimer hybrid, PEGylated, galactosylated derivatives. In this review, we divided CS derivatives into six groups, according to the character of substituent/derivative, namely hydrophobic, amphiphilic, ionic derivatives, derivatives with specific substituents (sugar bound CS derivatives, CS derivatives with cyclic structures, CS derivatives with thiol groups, thiosemicarbazone linked CS derivatives), CS copolymers, and cross-linked CS derivatives. Wang et al. [26] summarized basic synthetic strategies and properties of CS derivatives in their review. They discussed particular CS structural modifications with respect to their biological activities and applications. In the following sections, particular groups of derivatives with modified properties, as they are listed in Table 1, are discussed in terms of their implementations into NP and nanocomposite systems.| Chitosan Derivative Groups/Derivatives |
Formula | |||
|---|---|---|---|---|
| Hydrophobic derivatives | ||||
| Alkylated chitosan | 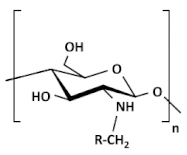 |
|||
| Acylated chitosan | 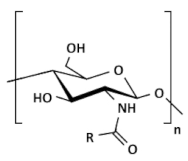 | (A) N-acylated chitosan |
||
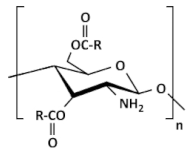 | (B) O-acylated chitosan |
|||
| N-phtaloylated chitosan | 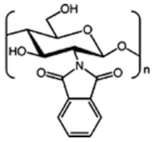 |
|||
| Benzoylated chitosan | 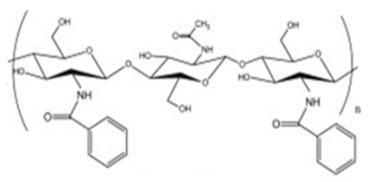 |
|||
| Methacrylated chitosan | 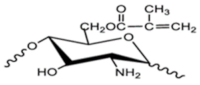 |
|||
| Amphiphilic derivatives | ||||
| Cholic and deoxycholic acid-modified chitosan | 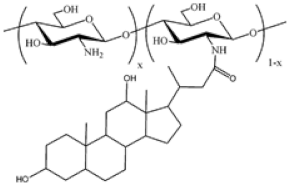 | (A) Deoxycholic acid | 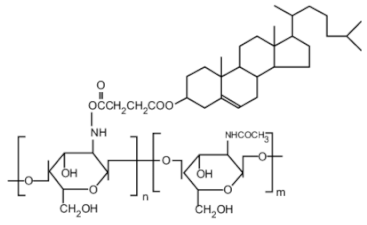 | (B) Cholic acid |
| Ionic derivatives | ||||
| Quarternary ammonium chitosan derivatives | 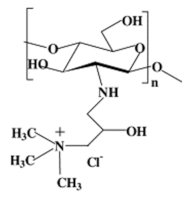 |
|||
| Sulfated chitosan derivatives | 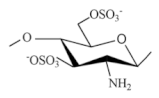 |
|||
| Succinylated chitosan | 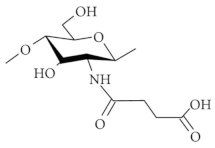 |
|||
| Sulfonated chitosan | 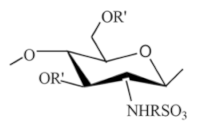 |
|||
| Phosphorylated chitosan | 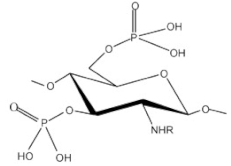 |
|||
| Carboxyalkylated chitosan (carboxymethylchitosan) | 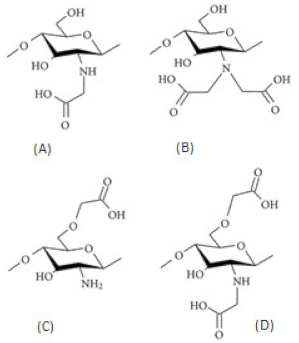 | (A) N-CMC, (B) N,N-CMC, (C) O-CMC, and (D) N,O-CMC (showing the modification at the D-glucosamine unit) |
||
| Chitosan copolymers | ||||
| PEGylated chitosan | 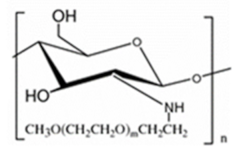 |
|||
| PEG-methacrylated chitosan | 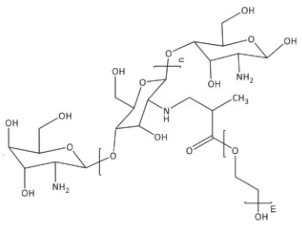 |
|||
| Derivatives with specific substituents | ||||
| Sugar bound chitosan derivatives | 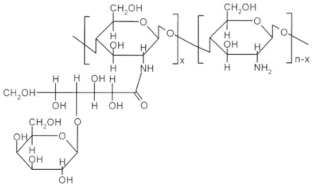 | Galactosylated chitosan |
||
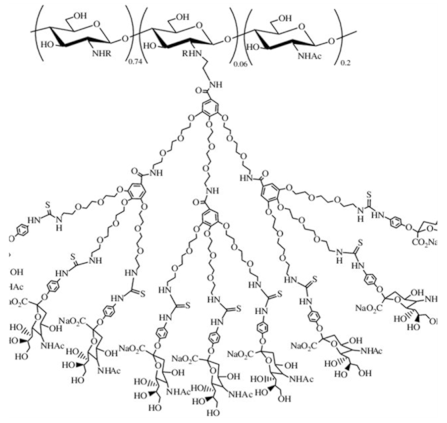 | Sialo dendrimer hybrid chitosan |
|||
| Chitosan derivatives with cyclic structure |
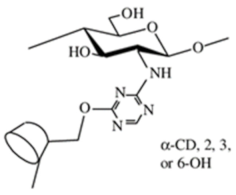 | Crown ether-linked chitosan |
||
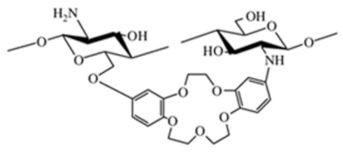 | Cyclodextrin-linked chitosan |
|||
| Chitosan derivatives with thiol groups | 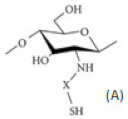 | (A) Thiolated chitosan with –SH group |
||
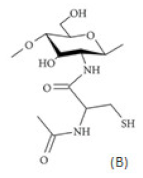 | (B) Thiolated chitosan with cysteine: chitosan-N-acetyl-cysteine |
|||
| Glycol chitosan | 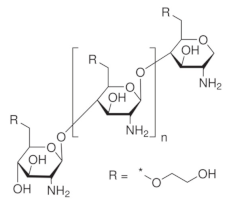 |
|||
| Thiosemicarbazone linked chitosan derivatives | 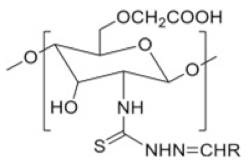 |
|||
| Crosslinked chitosan derivatives | ||||
| Chitosan-glutaraldehyde crosslinked polymer | 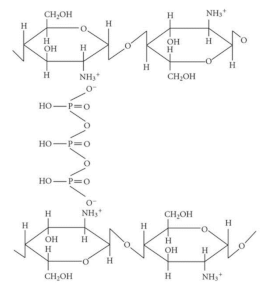 |
|||
| Chitosan-TPP crosslinked polymer | 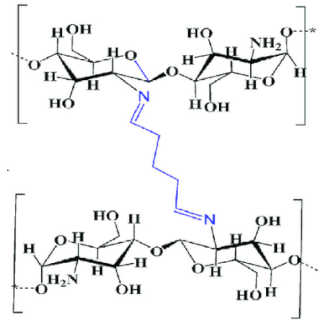 |
|||
| Chitosan-EDTA crosslinked polymer | 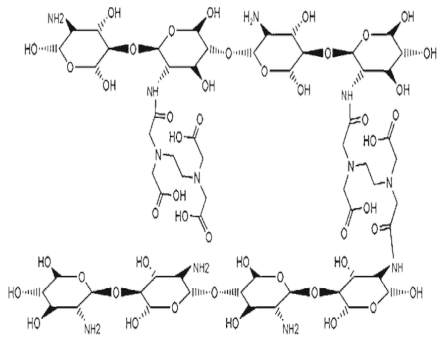 |
3. Enhancement in Drug Delivery by Chitosan Nanoparticles
There are several advantages of CS-based drug delivery systems (DDSs), mentioned in Section 3.4., therefore this area of CS use is extensively studied. The applications of NPs in the pharmaceutical industry are increasing day by day. In recent years, CS has been proven as a highly potent material in designing nanoparticle-based formulations for drug delivery, as reported by many review papers. Ali et al. [18] discussed the different types of CS nanocomposites and their use in biomedical applications. Garg et al. [27] and [28] Agnihotri et al. described methods for the preparation of CS NPs and their use in drug delivery. In addition, El Shoueir et al. [29] dealt with preparation methods of CS NPs and in addition, with physicochemical properties of CS. Rizwan et al. [12] studied the effect of CS properties on drug release applications. The following subsections are focused on a brief view of basic characteristics of NPs (Section 3.1), the most important/frequently used procedures for the preparation of CS NPs (Section 3.2), types and properties of CS-based nanocomposites (Section 3.3), and recent applications of CS NPs in drug delivery (Section 3.4).3.1. Basic Aspects of (Chitosan) Nanoparticle Systems
- (i).
-
CS NSs are a matrix system, where the drug may be absorbed in the surface or encapsulated within the CS particle. As an example, Liu et al. [40] constructed the photothermal sensitive carboxymethyl CS nanospheres (CMC NSs) carrier by introducing controllable heat-sensitive groups into CMC molecules. The carrier owned high drug loading and improved the stability of coated-drug DOX. The NSs generated photothermal response through NIR irradiation to improve the drug release amount and to achieve the combined treatment effect of photodynamic therapy and chemotherapy. In vitro photothermal tests proved that the NSs had excellent light stability and photothermal conversion performance. The cytotoxicity test results showed that the NSs had no obvious toxicity, but the drug-loaded nanospheres could effectively inhibit the growth of HepG-2 cells via photo-response to release DOX and Indocyanine green for achieving photothermal-chemotherapy under NIR irradiation.
- (ii).
-
CS NCs are vesicular systems in which the drug is generally confined to a cavity consisting of an oily core covered by a CS shell. As an example, Castro et al. [41] evaluated the physicochemical and biological properties of docetaxel (DCX) loaded chitosan nanocapsules (DCX-CS NCs) functionalized with the chimeric monoclonal antibody ChiTn mAb (highly specific antigen for carcinomas) (DCX-CS/PEG-ChiTn mAb NCs) as a potential improvement treatment for cancer therapy. The NCs, formed as a polymeric shell around an oily core, allowed a 99.9% encapsulation efficiency of DCX with a monodispersity particle size in the range of 200 nm and a high positive surface charge that provided substantial stability to the nanosystems. Uptake studies and viability assay conducted in A549 human lung cancer cell line in vitro demonstrated that ChiTn mAb enhanced NPs internalization and cell viability reduction.
- (iii).
-
CS NFs can be used in various fields mainly due to the presence of -NH2 and -OH groups, along with their specific structure. Their nanofibrous structure offers enormous possibilities for chemical modifications that create new properties applicable, particularly in the biomedical field. CS NFs can be prepared by electrospinning of CS into ultrafine fibers of nano size. Owing to the large specific surface area, NFs can deliver drugs, peptides, and vaccine antigens. The release of the drug may be immediate, delayed, or modified depending on the type of interactions between the polymer and the drug. Usually, an immediate release is noticed when a composition of a water-soluble substance and a water-soluble polymer is used. The prolonged release can be achieved by integrating the drug into other nanocarriers, such as NPs, liposomes, dendrimers, then loaded into NFs or use hydrophobic polymers [18][42]. As an example, Amiri et al. [43] reported the development of a local antibiotic delivery system using chitosan/polyethylene oxide (CS/PEO) NFs for delivery of teicoplanin. Uniform and bead-less NFs were prepared via electrospinning of a CS/PEO solution containing teicoplanin. The NFs were able to release teicoplanin for up to 12 days. Antibacterial test in agar diffusion and time-kill study on Staphylococcus aureus also demonstrated that loading teicoplanin in CS/PEO NFs enhanced the antibacterial activity up to 1.5- to 2-fold. An in vivo study on a rat full-thickness wound model confirmed the safety and efficacy of applying the teicoplanin-loaded NFs and significant improvement in wound closure were observed especially with the NFs containing 4% teicoplanin.
3.2. Preparation Procedures for Chitosan Nanoparticles
Processes of nanoparticulation can be divided into different categories: (i) physicochemical processes in which the NPs are precipitated by using preformed polymers, with yield induced by emulsification-solvent evaporation, diffusion, or reverse salting-out; (ii) by in situ chemical synthesis of macromolecules which lead to polymerizations or interfacial polycondensation reactions; and (iii) mechanical processes using high energy devices such as high-pressure homogenizers, ultrasonic devices, or wet high energy milling. Several methods have been reported for the preparation of CS NPs [18]. The main methods for producing CS NPs and nanocapsules (NCs) are ionic gelation, emulsification and crosslinking, complexation with polyelectrolytes, self-assembly, and drying processes [29]. The following subsections are describing the most important methods for the preparation of CS NPs, discussing also recent improvements in production schemes of conventional as well as novel CS NPs (such as optimized working parameters and conditions, new crosslinking agents, proper combinations of preparation schemes, etc.).3.2.1. Covalent Cross-Linking
Covalent cross-linking refers to the coupling of two or more molecules together via covalent chemical bonds (in ionic cross-linking it was physical binding through ionic interactions). In the case of CS NPs, covalent bonds are formed between CS or its derivatives and a functional cross-linking agent [27]. Crosslinkers usually possess a plurality of functional groups such as organic dibasic acid or polyhydric alcohol responsible for covalent cross-linking. Generally, the bridge bonds between the different polymer chains allow nanomaterials to form a three-dimensional structure. Mostly used crosslinkers are glutaraldehyde, genipin, polyethylene glycol, or monofunctional agents [11].For the first time, Savin et al. [44] prepared polymeric nanocarriers based on the chitosan grafted-poly(ethylene glycol) methacrylate derivative. The technique selected for the preparation of the micro-nanoparticles (MNPs) was a double crosslinking (ionic and covalent) process in the reverse emulsion which provided the mechanical stability of the polymeric nanocarrier. The covalent cross-linking process was carried out by adding in the glutaraldehyde, the ionic crosslinking agent was TPP or Na2SO4.3.2.2. Self-Assembly
Self-assembly is described as the association of certain molecules, macromolecules, or composite materials with themselves to form 3D networks or other structures with new distinguishing properties. The self-assembling process can take place at the molecular or supramolecular level. It can occur by self-association or by an association with other structures through interactions such as hydrogen bond, van der Waals forces, and ionic or hydrophobic interactions. It can also be caused by an inclusion/complexation mechanism, like the iodine inclusion complex with starch [45].Self-assembled NPs can be formed in the aqueous media of amphiphilic polymers. Hydrophobic interactions between amphiphilic polymer components tend to minimize interfacial energy and allow nanoparticle formation [29]. Although CS is not an amphiphilic bio-polymer, the self-organizing NPs can be obtained by a structural modification of CS, particularly the introduction of hydrophobic moieties into the CS molecules by grafting, to modify its hydrophobic–hydrophilic balance. Such balance is promoting self-assembly in an aqueous or polar medium. The grafting agent can be a hydrophobic moiety, such as cholesterol, cholic, and deoxycholic acid, 5β-cholanic acid, phthaloyl, polyester, fatty acids, etc. [46]. Hydrophobic molecules are usually immobilized on CS by N-acylation, N-alkylation, O-alkylation, and Schiff-base reaction [11]. CS self-assembled NPs are particularly useful for encapsulating hydrophilic as well as lipophilic drugs [45].Ionic Cross-Linking (Ionic Gelation)
Ionic cross-linking (or also found in the literature as ionic gelation) is one of the most widely used methods for the preparation of CS NPs. It has been extensively used for loading biopharmaceuticals. A major advantage of this method of physical CS crosslinking is related to its mild conditions attained. This method avoids the use of organic solvents, high temperatures, or vigorous agitation. Therefore, it can retain bioactive molecules such as proteins, DNA, etc. [29][47].CS as a cationic polysaccharide can gel with negatively charged compounds or specific polyvalent polyanionic molecules to form NPs. This combination leads to the formation of electrostatic interactions between opposite charges of the components. At acidic pH, there is a spontaneous formation of particles in the submicron size. As there is a formation of gels due to ionic linkage, this method is also known as the ionic-gelation method [11][27][29].Two main groups have been used as anionic cross-linkers: (i) anionic low-MW molecules, like cyclodextrin derivatives or tripolyphosphate (TPP), and (ii) anionic macromolecules, such as poly-y-glutamic acid, dextran sulfate, hyaluronic acid, and sodium alginate [11][47].TPP is the earliest and the most used cross-linking agent developed for ionic crosslinking with CS. Scheme illustrating preparation of CS NPs by ionic crosslinking with TPP is in Figure 3. When using TPP as a cross-linking agent, the NPs are prepared at room temperature by dissolving CS in a diluted solution of glacial acetic acid and adjusting pH to the expected value by the addition of NaOH. The TPP solution is added dropwise to the CS solution with constant stirring until it forms an opaque suspension, which indicates the formation of particles with an average diameter controlled by the molar ratio of CS/TPP. Factors influencing the mechanism of CS NPs formation by this method are dependent on parameters like the degree of acetylation and MW of CS, intrinsic viscosity, concentration, and the molar ratio of -NH3+/TPP. Other factors may also influence the properties of the NPs, such as operating temperature, stirring speed, and flow rate of TPP addition. They can affect particle size and polydispersity, as they appear to significantly reduce the amount of NP aggregation. Smaller particles can be isolated from larger particles by filtration and ultracentrifugation [29].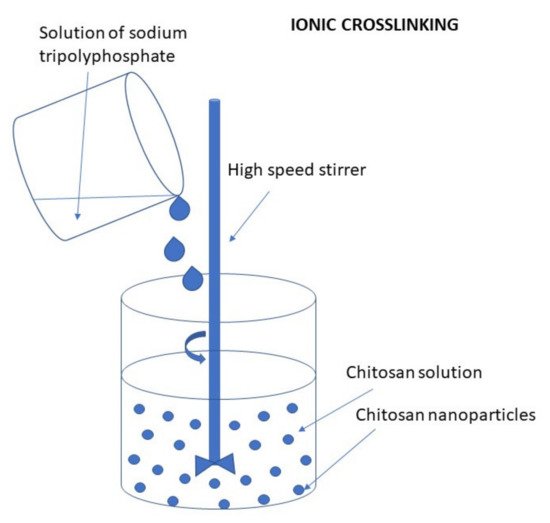 Figure 3. Scheme illustrating preparation of CS NPs by ionic crosslinking with TPP.Sang et al. [48] used the ionotropic gelation method to prepare CS NPs using polyphosphate crosslinking agents, namely TPP, phytic acid (PA), and sodium hexametaphosphate (SHMP). The encapsulation efficiency of myricetin (MYR) in the CS NPs crosslinked by PA and SHMP (67.3 ± 0.4% and 62.2 ± 0.2%, respectively) was significantly higher than that in the CS NPs crosslinked by TPP (47.7 ± 0.1%) (p < 0.05); their drug release rate (43.7 ± 5.1% and 44.0 ± 3.7%, respectively) was also significantly slower than that of MYR-CS NPs crosslinked by TPP (103.4 ± 4.0%) (p < 0.05). Furthermore, a strong mucoadhesiveness of the CS NPs crosslinked by PA was shown by a fast increase in the turbidity value and a sharp decrease in the zeta potential in the mucin solution test.Pan et al. [49] studied the relationship between the degree of crosslinking and the properties of CS NPs. They established a potassium polyvinyl sulfate (PVSK) titration method for the determination of free amino group in CS, which showed that there was a window effect in the crosslinking degree and particle size. They examined three kinds of molecular weight CS cross-linked by different content of TPP; the NPs with a moderate crosslinking degree were smaller. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) data showed the moderate degree of cross-linking NPs had strong antibacterial properties. IR analysis revealed that the interactions between CS NPs with a different crosslinking degree and TPP were different. X-ray diffraction analysis showed that the cross-linked CS NPs were amorphous. In conclusion, the crosslinking degree of the TPP cross-linked CS NPs was related to the particle size and antibacterial properties.Colloidal chitosan/tripolyphosphate (CS/TPP) particles prepared by ionic gelation have attracted significant attention as potential delivery vehicles for drugs, genes, and vaccines. Yet, there have been several fundamental studies that showed these particles disintegrate at physiological pH (pH 7.2–7.4) and ionic strength levels. Echeverri-Cuartas et al. [50] explored the possibility of improving a TPP-crosslinked CS NP stability through a chemical modification of CS. Specifically, CS samples with either 76% or 92% degrees of deacetylation (DD) were grafted with either polyethylene glycol (PEG), or folic acid (F). Within the degrees of substitution (~1% for PEG, and 3% and 6% for F), neither PEG nor F qualitatively improved CS NP stability at physiological conditions (pH 7.2). Another way to improve this stability was explored by Abdelgawad et al. [51]. They used hexametaphosphate (HMP) instead of TPP as a cross-linking agent. HMP is a hexavalent molecule in the neutral and slightly basic medium which offers more binding sites readily available for interaction with CS. It is thought that increasing the availability of the binding sites in the HMP molecule would result in stronger ionic complexation with CS cationic moieties. Consequently, such stronger binding should improve particles’ stability and lead to average size reduction. A comparative study between CS/TPP and CS/HMP NPs under different complexation conditions was conducted to investigate the effect of HMP on NPs formation. Although the drug loading efficiency of BSA, 96.3%, was higher than when using TPP, 91.87%, the TPP cross-linked particles showed superior stability upon storage. Cai and Lapitsky [52] proved that the particles could be stabilized by their bioactive payloads. They studied the enhancement of the CS/TPP particle stability at physiological ionic strengths, using 150 mM NaCl (pH 5.5) and PBS (pH 6.0) as the dissolution media when associating the CS/TPP particles with model anionic proteins (α-lactalbumin, α-LA, and bovine serum albumin, BSA) and polynucleotides (DNA). Light scattering and UV–VIS spectroscopy revealed that anionic protein uptake had no impact on particle stability. It was likely due to the relatively weak protein/particle binding at near-physiological ionic strengths, which caused the protein to be rapidly released. Conversely, a DNA uptake increased the CS fractions persisting in a complexed/particulate form in model dissolution media, with the DNA remaining largely complexed to the CS at all investigated conditions. The results suggested that, while most bioactive payloads do not interact with CS strongly enough to stabilize the CS/TPP particles, these particles can be stabilized to dissolution through the incorporation of polyanions.The literature on the release performance of CS/TPP micro- and nanoparticles is filled with conflicting results, with some reporting nearly instantaneous release, while others showing the release to be sustained for up to multiple days. To resolve these opposing findings, Cai et al. [53] examined several experimental artifacts that may arise during such in vitro experiments and showed that conflicting findings on release from CS/TPP particles can arise from: (1) incomplete particle separation from the release media upon centrifugation; (2) irreversible particle coagulation; and (3) failure to maintain sink conditions. They provided guidelines for obtaining more reliable release profiles for CS/TPP micro- and nanoparticles and other/related colloidal carriers. To minimize problems with an incomplete particle separation, high centrifugal forces, and long centrifugation times (which exceed those used typically) are needed. A glycerol bed can help to prevent particle coagulation; however, this method no longer appears to work for CS/TPP micro- and nanoparticles after the first 3–4 centrifugation/redispersion cycles (at least under the centrifugation conditions needed for their full separation with the setup used by the authors). For particles with fast release rates (such as the colloidal CS/TPP particles explored in their work), frequent sampling/solvent replacement must also be used to capture the release kinetics and maintain sink conditions. Due to the long centrifugation times required to fully sediment the particles, however, this ultracentrifugation-assisted solvent re-placement method cannot accurately determine the release profiles. As an alternative, sink conditions can likely be achieved by diluting the dispersions in large volumes of release media and collecting small dispersion portions (after removing the particles with a filter) to quantify the release. The authors demonstrated that it is essential to use release media with physiologically relevant ionic strength levels, as a release from CS/TPP particles can be highly salt-sensitive.
Figure 3. Scheme illustrating preparation of CS NPs by ionic crosslinking with TPP.Sang et al. [48] used the ionotropic gelation method to prepare CS NPs using polyphosphate crosslinking agents, namely TPP, phytic acid (PA), and sodium hexametaphosphate (SHMP). The encapsulation efficiency of myricetin (MYR) in the CS NPs crosslinked by PA and SHMP (67.3 ± 0.4% and 62.2 ± 0.2%, respectively) was significantly higher than that in the CS NPs crosslinked by TPP (47.7 ± 0.1%) (p < 0.05); their drug release rate (43.7 ± 5.1% and 44.0 ± 3.7%, respectively) was also significantly slower than that of MYR-CS NPs crosslinked by TPP (103.4 ± 4.0%) (p < 0.05). Furthermore, a strong mucoadhesiveness of the CS NPs crosslinked by PA was shown by a fast increase in the turbidity value and a sharp decrease in the zeta potential in the mucin solution test.Pan et al. [49] studied the relationship between the degree of crosslinking and the properties of CS NPs. They established a potassium polyvinyl sulfate (PVSK) titration method for the determination of free amino group in CS, which showed that there was a window effect in the crosslinking degree and particle size. They examined three kinds of molecular weight CS cross-linked by different content of TPP; the NPs with a moderate crosslinking degree were smaller. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) data showed the moderate degree of cross-linking NPs had strong antibacterial properties. IR analysis revealed that the interactions between CS NPs with a different crosslinking degree and TPP were different. X-ray diffraction analysis showed that the cross-linked CS NPs were amorphous. In conclusion, the crosslinking degree of the TPP cross-linked CS NPs was related to the particle size and antibacterial properties.Colloidal chitosan/tripolyphosphate (CS/TPP) particles prepared by ionic gelation have attracted significant attention as potential delivery vehicles for drugs, genes, and vaccines. Yet, there have been several fundamental studies that showed these particles disintegrate at physiological pH (pH 7.2–7.4) and ionic strength levels. Echeverri-Cuartas et al. [50] explored the possibility of improving a TPP-crosslinked CS NP stability through a chemical modification of CS. Specifically, CS samples with either 76% or 92% degrees of deacetylation (DD) were grafted with either polyethylene glycol (PEG), or folic acid (F). Within the degrees of substitution (~1% for PEG, and 3% and 6% for F), neither PEG nor F qualitatively improved CS NP stability at physiological conditions (pH 7.2). Another way to improve this stability was explored by Abdelgawad et al. [51]. They used hexametaphosphate (HMP) instead of TPP as a cross-linking agent. HMP is a hexavalent molecule in the neutral and slightly basic medium which offers more binding sites readily available for interaction with CS. It is thought that increasing the availability of the binding sites in the HMP molecule would result in stronger ionic complexation with CS cationic moieties. Consequently, such stronger binding should improve particles’ stability and lead to average size reduction. A comparative study between CS/TPP and CS/HMP NPs under different complexation conditions was conducted to investigate the effect of HMP on NPs formation. Although the drug loading efficiency of BSA, 96.3%, was higher than when using TPP, 91.87%, the TPP cross-linked particles showed superior stability upon storage. Cai and Lapitsky [52] proved that the particles could be stabilized by their bioactive payloads. They studied the enhancement of the CS/TPP particle stability at physiological ionic strengths, using 150 mM NaCl (pH 5.5) and PBS (pH 6.0) as the dissolution media when associating the CS/TPP particles with model anionic proteins (α-lactalbumin, α-LA, and bovine serum albumin, BSA) and polynucleotides (DNA). Light scattering and UV–VIS spectroscopy revealed that anionic protein uptake had no impact on particle stability. It was likely due to the relatively weak protein/particle binding at near-physiological ionic strengths, which caused the protein to be rapidly released. Conversely, a DNA uptake increased the CS fractions persisting in a complexed/particulate form in model dissolution media, with the DNA remaining largely complexed to the CS at all investigated conditions. The results suggested that, while most bioactive payloads do not interact with CS strongly enough to stabilize the CS/TPP particles, these particles can be stabilized to dissolution through the incorporation of polyanions.The literature on the release performance of CS/TPP micro- and nanoparticles is filled with conflicting results, with some reporting nearly instantaneous release, while others showing the release to be sustained for up to multiple days. To resolve these opposing findings, Cai et al. [53] examined several experimental artifacts that may arise during such in vitro experiments and showed that conflicting findings on release from CS/TPP particles can arise from: (1) incomplete particle separation from the release media upon centrifugation; (2) irreversible particle coagulation; and (3) failure to maintain sink conditions. They provided guidelines for obtaining more reliable release profiles for CS/TPP micro- and nanoparticles and other/related colloidal carriers. To minimize problems with an incomplete particle separation, high centrifugal forces, and long centrifugation times (which exceed those used typically) are needed. A glycerol bed can help to prevent particle coagulation; however, this method no longer appears to work for CS/TPP micro- and nanoparticles after the first 3–4 centrifugation/redispersion cycles (at least under the centrifugation conditions needed for their full separation with the setup used by the authors). For particles with fast release rates (such as the colloidal CS/TPP particles explored in their work), frequent sampling/solvent replacement must also be used to capture the release kinetics and maintain sink conditions. Due to the long centrifugation times required to fully sediment the particles, however, this ultracentrifugation-assisted solvent re-placement method cannot accurately determine the release profiles. As an alternative, sink conditions can likely be achieved by diluting the dispersions in large volumes of release media and collecting small dispersion portions (after removing the particles with a filter) to quantify the release. The authors demonstrated that it is essential to use release media with physiologically relevant ionic strength levels, as a release from CS/TPP particles can be highly salt-sensitive.Polyelectrolyte Complex (PEC)
A suitable way to generate self-assembled CS NPs is through the formation of polyelectrolyte complexes with polyanions. Polyelectrolyte complexes (PECs) are formed when the solutions of two polyelectrolytes carrying complementary charges (i.e., a polycation and a polyanion or their corresponding salts) are mixed. In this case, PEC formation is mainly caused by the strong Coulomb interactions between the oppositely charged polyelectrolytes. The formation of complexes brings about at least a partial charge neutralization of polymers. In addition, inter-macromolecular interactions such as Van der Waals forces, hydrogen bonding, and hydrophobic interactions are involved in the formation of PEC structures as well [54].The PECs formation generally involves two or three steps. The scheme of the different steps of the polyelectrolyte complexes formation is shown in Figure 4. The first step is instantaneous and leads to the formation of a random primary complex with significant distortions of the configuration of polymer chains. Then, the secondary complex is formed by rearrangement of existing linkages within intracomplexes. It involves the formation of new bonds, e.g., electrostatic bonds, hydrogen bonds, hydrophobic interactions, etc. Finally, under certain conditions, primary and secondary complexes can aggregate (probably by hydrophobic interactions) and lead to various stable structures: entangled aggregates, fibrils, ordered networks, etc. Preparation of CS PECs is a safe green process in water, with no organic solvents nor chemical cross-linkers or surfactants used. It is a simple fabrication process where spontaneously mixing the oppositely charged polyelectrolyte solutions leads to interpolymer ionic condensation [55].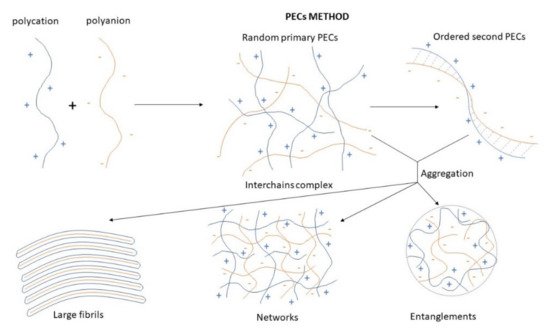 Figure 4. Scheme of the different steps of the polyelectrolyte complexes formation.The stability of PECs depends on several factors including the degree of ionization within each polyelectrolyte with an opposite charge, the density of the charges presents in the structure of polyelectrolyte, concentration, and proportion of polyelectrolytes in the mixture, a sequence of additions in the mixture and the type and location of ionic groups. In polymer chains, the stability is also correlated with the MW of the polyelectrolytes, the flexibility of the polymer chain as well as the temperature, ionic resistance, and pH of the reaction medium. The structure of CS-based nanoPECs is highly impacted by the presence of salt. A high concentration of salt may disrupt the integrity of the nanoPECs and dissociate the polyelectrolytes. When the positively charged surface of CS interacts with anionic biopolymers such as sodium alginate, carboxymethylcellulose, chondroitin sulfate, dextran sulfate, poly(acrylic acid), pectin, carrageenans, heparin, and other polyions, various CS-based PECs can be formed [55].Several conditions, such as pH (particularly important in weak polyelectrolytes), ionic strength, and the mixing rate, should be adjusted to the particular CS-polyanion pair system selected since these variables will also influence the size and charge of CS NPs. Different preparation methods will result in diverse kinds of CS NPs, which can be classified as nanoaggregates, nanocapsules, or nanospheres. The particular procedure selected can be largely determined by the water solubility of the active agent that will be encapsulated and the polyanion used [55].By chitosan’s favorable properties, especially the mucoadhesive nature and absorption enhancement capability, as well as the safe and green manufacturing process of colloidal PECs, CS-based nanoPECs are particularly appropriate for the delivery of sensitive biological molecules such as vaccines and proteins like insulin via the mucosal route. Cationic CS-based PECs can also promote the internalization of the loaded cargos into cells and subsequently escape the lysosome via the proton sponge effect, therefore exhibit high therapeutic potential, e.g., in anti-tumor applications [54].
Figure 4. Scheme of the different steps of the polyelectrolyte complexes formation.The stability of PECs depends on several factors including the degree of ionization within each polyelectrolyte with an opposite charge, the density of the charges presents in the structure of polyelectrolyte, concentration, and proportion of polyelectrolytes in the mixture, a sequence of additions in the mixture and the type and location of ionic groups. In polymer chains, the stability is also correlated with the MW of the polyelectrolytes, the flexibility of the polymer chain as well as the temperature, ionic resistance, and pH of the reaction medium. The structure of CS-based nanoPECs is highly impacted by the presence of salt. A high concentration of salt may disrupt the integrity of the nanoPECs and dissociate the polyelectrolytes. When the positively charged surface of CS interacts with anionic biopolymers such as sodium alginate, carboxymethylcellulose, chondroitin sulfate, dextran sulfate, poly(acrylic acid), pectin, carrageenans, heparin, and other polyions, various CS-based PECs can be formed [55].Several conditions, such as pH (particularly important in weak polyelectrolytes), ionic strength, and the mixing rate, should be adjusted to the particular CS-polyanion pair system selected since these variables will also influence the size and charge of CS NPs. Different preparation methods will result in diverse kinds of CS NPs, which can be classified as nanoaggregates, nanocapsules, or nanospheres. The particular procedure selected can be largely determined by the water solubility of the active agent that will be encapsulated and the polyanion used [55].By chitosan’s favorable properties, especially the mucoadhesive nature and absorption enhancement capability, as well as the safe and green manufacturing process of colloidal PECs, CS-based nanoPECs are particularly appropriate for the delivery of sensitive biological molecules such as vaccines and proteins like insulin via the mucosal route. Cationic CS-based PECs can also promote the internalization of the loaded cargos into cells and subsequently escape the lysosome via the proton sponge effect, therefore exhibit high therapeutic potential, e.g., in anti-tumor applications [54].3.2.3. Emulsion Technique
In general, the particles formed in the emulsion system have structures of nanocapsules, high drug loading efficiency, and good bioavailability [56]. NPs can be obtained using the nanoemulsion technique in various ways. Some of them are low power emulsification methods but emulsification processes usually require high mechanical energy to obtain small droplet sizes. The most common emulsification method used for the preparation of CS NPs is the water-in-oil emulsion, where an aqueous CS solution is emulsified in an oil phase. Surfactants are applied for stabilizing the formed particles [9][57].Briefly, in the first step, a dispersion of CS in a glacial acetic acid solution is prepared as an aqueous phase. Then, a drop of a hydrophobic solvent, such as cyclohexane, cyclohexanol, soybean oil, or another solvent with similar properties is added to the CS solution, all mixing continuously. In the final step a surfactant, for example, Triton X-100 or Tween is being added to the resulting mixture under constant stirring until the mixture becomes translucent. This indicates the formation of emulsion-containing NPs [58].To skip a step in the process, the surfactant can be pre-mixed in one of the solutions. Shahab et al. [59] prepared CS-coated polycaprolactone NPs by a single-step emulsification technique with a modification compared to the previously established method. The organic phase was prepared by dissolving polycaprolactone and dorzolamide in dichloromethane. Separately, the aqueous phase was prepared by solubilizing CS in 1% acetic acid solution (pH 5.0) with polyvinyl alcohol (PVA). The organic phase was added dropwise to the aqueous phase with constant stirring for 6 h to evaporate the organic solvent. The prepared NP dispersion was sonicated, and the created NPs were further lyophilized using mannitol as cryoprotectant.Emulsion Droplet Coalescence (Emulsion Crosslinking and Precipitation)
There are various modifications in the preparation of an emulsion. For example, the addition of gelling agents such as chlorides, TPP, or polycations can be used. An emulsion droplet coalescence method is based on the principles of emulsion crosslinking and precipitation methods. Preparation of CS NPs by emulsion droplet coalescence method is schematically illustrated in Figure 5. In the first step of the procedure, two emulsions are prepared; one containing an aqueous solution of CS and liquid paraffin along with a drug and the second one containing an aqueous alkaline (e.g., NaOH) solution of CS in liquid paraffin oil. Both the emulsions are mixed under a high-speed stirring, resulting in random collisions of droplets of each emulsion. Coalescence of CS droplets with NaOH droplets takes place and thereby precipitation of CS in small solid particles [60].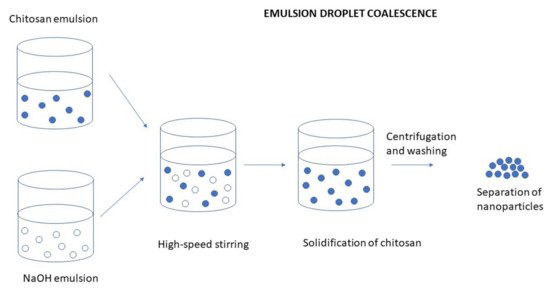 Figure 5. Preparation of CS NPs by emulsion droplet coalescence method.To intensify the emulsion crosslinking process for the synthesis of CS NPs, Zhang et al. [61] developed a controlled hydrodynamic cavitation technique. Their work demonstrated the feasibility of hydrodynamic cavitation as an energy-efficient approach for intensifying the emulsion crosslinking synthesis of CS NPs compared with ultrasonic horn and conventional drop-by-drop process. The novel approach can greatly reduce the particle size and distribution of the synthesized CS NPs. Although the emulsification method facilitates particle size control, strong crosslinking agents are usually used in this process and complete removal of unreacted crosslinking agents can be an issue [62].Riegger et al. [63] investigated the impact of glutaraldehyde concentration and molecular weight (MW) of six commercially available, highly deacetylated CS on the nanoparticle formation by emulsion crosslinking technique. Increasing MW of CS resulted in larger particle sizes ranging from 109.9 nm for the lowest MW up to 200.3 nm for the highest MW. An adsorption capacity of up to 351.8 mg g−1 diclofenac for low MW CS NPs was observed and all CS NPs showed superior adsorptions when compared to an untreated CS. Hence, the results suggested the use of the prepared CS NPs as promising adsorbers for diclofenac and carbamazepine.
Figure 5. Preparation of CS NPs by emulsion droplet coalescence method.To intensify the emulsion crosslinking process for the synthesis of CS NPs, Zhang et al. [61] developed a controlled hydrodynamic cavitation technique. Their work demonstrated the feasibility of hydrodynamic cavitation as an energy-efficient approach for intensifying the emulsion crosslinking synthesis of CS NPs compared with ultrasonic horn and conventional drop-by-drop process. The novel approach can greatly reduce the particle size and distribution of the synthesized CS NPs. Although the emulsification method facilitates particle size control, strong crosslinking agents are usually used in this process and complete removal of unreacted crosslinking agents can be an issue [62].Riegger et al. [63] investigated the impact of glutaraldehyde concentration and molecular weight (MW) of six commercially available, highly deacetylated CS on the nanoparticle formation by emulsion crosslinking technique. Increasing MW of CS resulted in larger particle sizes ranging from 109.9 nm for the lowest MW up to 200.3 nm for the highest MW. An adsorption capacity of up to 351.8 mg g−1 diclofenac for low MW CS NPs was observed and all CS NPs showed superior adsorptions when compared to an untreated CS. Hence, the results suggested the use of the prepared CS NPs as promising adsorbers for diclofenac and carbamazepine.Emulsification Solvent Diffusion
The process of emulsion solvent diffusion is based on the partial miscibility of an organic solvent as an oil phase with water. Preparation of CS NPs by emulsion solvent diffusion method is schematically illustrated in Figure 6. A drug is dissolved in an organic solvent and is added upon injection to an aqueous phase containing CS with a stabilizing agent such as lecithin or poloxamer. As a result, the simple water-in-oil emulsion is formed upon high-pressure homogenization. The emulsion is then diluted with a large amount of water to overcome organic solvent miscibility in water. Finally, NPs are formed through CS precipitation due to reduced CS solubility as acetone diffuses into the aqueous phase. This leads to the formation of small particles. As the concentration of water-miscible solvent increases, a decrease in the size of particles can be achieved. The isolation of NPs is made using the centrifugation process. This method is suitable for hydrophobic as well as hydrophilic drugs. In the case of hydrophilic drugs, a multiple water/oil/water emulsion (e.g., double emulsion) needs to be formed with the drug dissolved in the internal aqueous phase. The major drawbacks of this method include harsh processing conditions (e.g., the use of organic solvents) and the high shear forces used during NP preparation [47][64][65].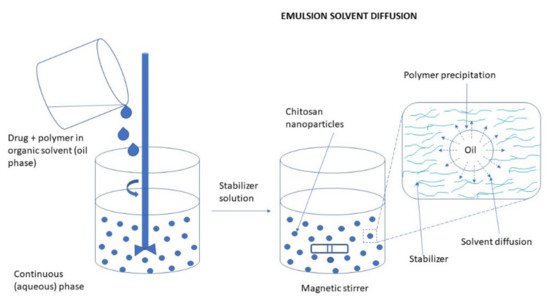 Figure 6. Preparation of CS NPs by emulsion solvent diffusion method.Often, this method is used for the preparation of PLGA NPs with CS as a coating material. Liu et al. [56] prepared dual drug-loaded NPs by the double emulsification solvent evaporation method. Salmon calcitonin (sCT) was dissolved in water as an internal aqueous phase. Puerarin (PR) and PLGA were dissolved in acetone, and then Tween-20 was added to the mixture to prepare an organic phase. PVA solution and CS solution were mixed as an external aqueous phase. Subsequently, the internal aqueous phase was added to the organic phase and W/O emulsion was obtained by sonication. W/O/W emulsion was formed by adding the W/O emulsion into the external aqueous phase. Finally, vacuum evaporation was conducted to remove the organic solvent, and sCT-PR-CS/PLGA NPs were recovered. The NPs solution was placed in a vacuum dryer over 12 h after rotating evaporation to remove residual acetone.
Figure 6. Preparation of CS NPs by emulsion solvent diffusion method.Often, this method is used for the preparation of PLGA NPs with CS as a coating material. Liu et al. [56] prepared dual drug-loaded NPs by the double emulsification solvent evaporation method. Salmon calcitonin (sCT) was dissolved in water as an internal aqueous phase. Puerarin (PR) and PLGA were dissolved in acetone, and then Tween-20 was added to the mixture to prepare an organic phase. PVA solution and CS solution were mixed as an external aqueous phase. Subsequently, the internal aqueous phase was added to the organic phase and W/O emulsion was obtained by sonication. W/O/W emulsion was formed by adding the W/O emulsion into the external aqueous phase. Finally, vacuum evaporation was conducted to remove the organic solvent, and sCT-PR-CS/PLGA NPs were recovered. The NPs solution was placed in a vacuum dryer over 12 h after rotating evaporation to remove residual acetone.Emulsification Solvent Evaporation
This is one of the most popular methods for the encapsulation of a drug within the water-insoluble polymer. It consists of preparing an emulsion, with a different external phase depending on the nature of the polymer and the drug used for the encapsulation and evaporating the solvent with the subsequent formation of the nanospheres [64]. The preparation of CS NPs by the emulsion solvent evaporation method is depicted in Figure 7.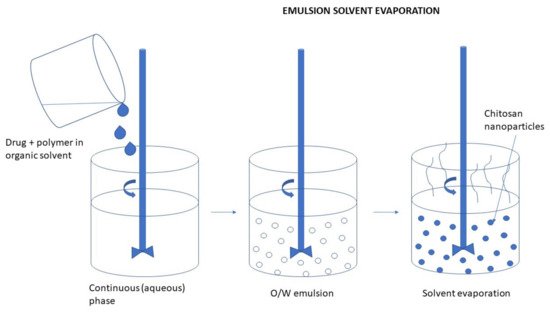 Figure 7. Preparation of CS NPs by the emulsion solvent evaporation method.Essa et al. [66] prepared CS NPs using a single-emulsion oil in water (O/W) solvent evaporation approach. CS was agitated in glacial acetic acid to produce the aqueous phase. The organic phase was prepared by dissolving PLGA in either acetone, chloroform, or dichloromethane and added dropwise under stirring to the aqueous phase. The emulsion was left to agitate under magnetic stirring for 8 h to allow complete evaporation of the organic solvent. Thereafter the formulation was centrifuged and washed with distilled water to remove any unentrapped drug molecule. The resultant pellets were redispersed in distilled water and then sonicated again to produce free-flowing NPs that were frozen at −80 °C and then lyophilized for 24 h.
Figure 7. Preparation of CS NPs by the emulsion solvent evaporation method.Essa et al. [66] prepared CS NPs using a single-emulsion oil in water (O/W) solvent evaporation approach. CS was agitated in glacial acetic acid to produce the aqueous phase. The organic phase was prepared by dissolving PLGA in either acetone, chloroform, or dichloromethane and added dropwise under stirring to the aqueous phase. The emulsion was left to agitate under magnetic stirring for 8 h to allow complete evaporation of the organic solvent. Thereafter the formulation was centrifuged and washed with distilled water to remove any unentrapped drug molecule. The resultant pellets were redispersed in distilled water and then sonicated again to produce free-flowing NPs that were frozen at −80 °C and then lyophilized for 24 h.3.2.4. Reverse Micellar Method
This method is based on the formation of the NPs in an aqueous core of reverse micellar droplets, followed by cross-linking with glutaraldehyde. It is used to prepare ultrafine polymer NPs with a narrow size range. The preparation of CS NPs by the reverse micellar method is shown in Figure 8.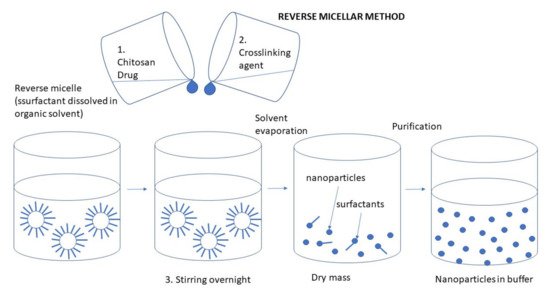 Figure 8. Preparation of CS NPs by the reverse micellar method.The formation of reverse micelles is carried out by adding a surfactant to an organic solvent. An aqueous solution of CS with drug and a glutaraldehyde solution is then added to that the organic micellar solution under constant stirring to avoid turbidity. Water is then added to maintain the mixture in an optically transparent microemulsion phase. The amount of water is increased to obtain NPs of larger size [67]. To attain complete cross-linkage for CS, it is advised to maintain the stirring overnight. The organic solvent is evaporated and the created NPs can be harvested by precipitation with a suitable salt. The solution is then centrifuged, and the aqueous supernatant is dialyzed for about 1 h and lyophilized to dry powder [9][65].The advantages of this method are associated with the ability to produce a small particle size with a narrow size of the distribution. On the other hand, the disadvantages include the laborious and time-consuming process and the presence of organic solvent and surfactant [65].Orellano et al. [68] studied the effect of the micellar interface on the synthesis of CS NPs. Both benzyl-n-hexadecyltrimethylammonium chloride (BHDC) and 1,4-bis-2-ethylhexylsulfosuccinate (AOT) reverse micelles were assessed since there were found remarkable differences between their interfacial water entrapped structures. The CS NPs obtained under different conditions were assessed in terms of their ability to solubilize curcumin, whose numerous therapeutic properties are somewhat countered by its poor solubility in water. The results showed that the crosslinking reaction took place in the micellar interface and was more effective in the AOT reverse micelles. This difference in effectiveness can be attributed to the different positions that CS acquired in each of the two reverse micelles tested. Finally, the NPs notably enhanced the water solubility of curcumin, and particle size was the main determining factor for encapsulation efficiency.
Figure 8. Preparation of CS NPs by the reverse micellar method.The formation of reverse micelles is carried out by adding a surfactant to an organic solvent. An aqueous solution of CS with drug and a glutaraldehyde solution is then added to that the organic micellar solution under constant stirring to avoid turbidity. Water is then added to maintain the mixture in an optically transparent microemulsion phase. The amount of water is increased to obtain NPs of larger size [67]. To attain complete cross-linkage for CS, it is advised to maintain the stirring overnight. The organic solvent is evaporated and the created NPs can be harvested by precipitation with a suitable salt. The solution is then centrifuged, and the aqueous supernatant is dialyzed for about 1 h and lyophilized to dry powder [9][65].The advantages of this method are associated with the ability to produce a small particle size with a narrow size of the distribution. On the other hand, the disadvantages include the laborious and time-consuming process and the presence of organic solvent and surfactant [65].Orellano et al. [68] studied the effect of the micellar interface on the synthesis of CS NPs. Both benzyl-n-hexadecyltrimethylammonium chloride (BHDC) and 1,4-bis-2-ethylhexylsulfosuccinate (AOT) reverse micelles were assessed since there were found remarkable differences between their interfacial water entrapped structures. The CS NPs obtained under different conditions were assessed in terms of their ability to solubilize curcumin, whose numerous therapeutic properties are somewhat countered by its poor solubility in water. The results showed that the crosslinking reaction took place in the micellar interface and was more effective in the AOT reverse micelles. This difference in effectiveness can be attributed to the different positions that CS acquired in each of the two reverse micelles tested. Finally, the NPs notably enhanced the water solubility of curcumin, and particle size was the main determining factor for encapsulation efficiency.3.2.5. Drying Methods
During drying processes water or solvents are separated through evaporation from liquids, solids, or semi-solids and the resulting vapor is collected by vacuum. Hot air, a microwave oven, spray drying, freezing, supercritical drying, and natural drying with air are common drying methods. The techniques of spray drying and supercritical drying are often used in the production of CS NPs because they are fast, simple, continuous, reproducible, and adapt well to the active material. They are scaled down in one phase without modification. These techniques are important and can achieve particles of various sizes and high stability [69].Spray Drying
The spray drying technique is the most common drying method used to prepare NPs based on CS. It is based on the use of a flux of hot air to dry spray drops. This method requires the preparation of an aqueous CS solution in which the drug is dispersed. The addition of natural cross-linking agents improves the biocompatibility and performance of drugs. A TPP cross-linking agent is usually used to overcome the problem of poor solubility of non-cross-linked CS in aqueous media. The use of an adequate excipient reduces the risk of thermal degradation during the spray drying process. For example, polysorbate 20 can be used as a protective agent of proteins from denaturation due to high shear rates during the atomization step [47].The preparation of CS NPs by spray drying method is schematically illustrated in Figure 9. The solution of polymer with crosslinker is sprayed into a drying chamber through a nozzle and it is atomized in a stream of hot air. This results in the formation of small droplets from which the solvent evaporates and form free-flowing particles. The resulting particles have a smooth surface, aspherical shape, and reduced size distribution, and they also exhibit good drug stability with high efficiency when the drug is turned on. Different factors such as the size of the nozzle, spray flow rate, inlet air temperature, atomization pressure, the extent of crosslinking, etc. influence the particle size [9][70].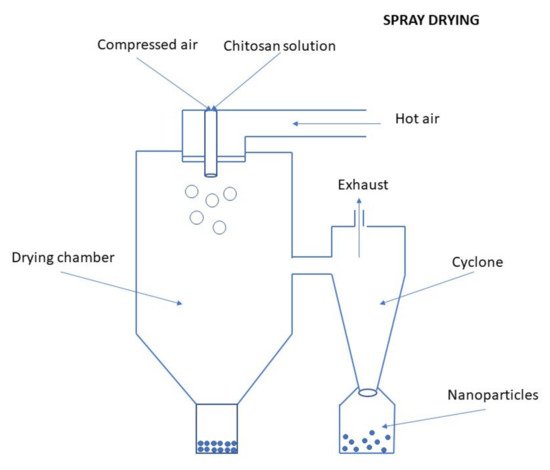 Figure 9. Preparation of CS NPs by the spray-drying method.The spray drying technique provides a convenient, one-step, and protein-friendly method for protein-loaded CS MPs or NPs [69].Ozturk et al. [71] prepared CS NPs containing dexketoprofen by spray-drying method and tested them for anti-inflammatory activity.
Figure 9. Preparation of CS NPs by the spray-drying method.The spray drying technique provides a convenient, one-step, and protein-friendly method for protein-loaded CS MPs or NPs [69].Ozturk et al. [71] prepared CS NPs containing dexketoprofen by spray-drying method and tested them for anti-inflammatory activity.Supercritical Fluid Drying
Usually, a supercritical fluid process with carbon dioxide (CO2) is used to prepare diverse pharmaceutical applications at lower pressure and temperature. An illustrative scheme of the preparation of CS NPs by the supercritical fluid drying method is depicted in Figure 10. The process is nontoxic, nonflammable, and ensures the minimal decomposition of drugs like proteins. It provides also the possibility to prepare MPs especially those oriented for inhalation [47].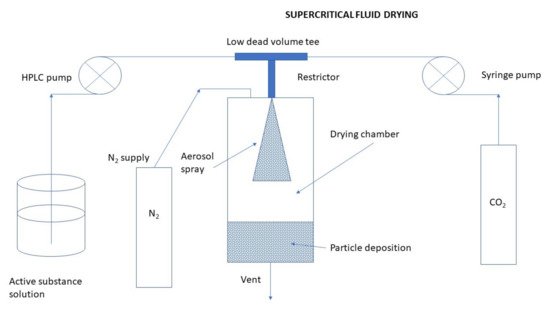 Figure 10. Preparation of CS NPs by the supercritical fluid drying method.Peng et al. [72] prepared DOX-loaded CS NPs using supercritical fluid assisted atomization introduced by a hydrodynamic cavitation mixer (SAA-HCM) from an aqueous solution. The influences of solution concentration, CO2/solution ratio, mixer pressure, CS/DOX ratio, and CS molecular weight on the particle morphologies and sizes were investigated in detail. FT-IR results showed that the structure of DOX was not changed after the SAA-HCM process. The in vitro drug release behavior conducted in the media with pH of 4.5, 6.5, and 7.4, respectively, was found to be strongly pH-responsive. The in vitro cytotoxicity profiles revealed the activity of DOX was well maintained after being loaded into the CS NPs. The SAA-HCM process was demonstrated to be a promising technique for the one-step production of polymer/drug composite NPs suitable for cancer drug delivery from aqueous solutions.In another work by Peng et al. [73] prepared nano-in-microparticles composed of CS NPs and mannitol, using a modified supercritical CO2 assisted atomization (SAA-HCM) technique. Drug-free CS-based NPs were prepared using an ionic gelation method with TPP. CO2 from a cylinder was turned into liquid flowing through a cooling bath and sent to the mixer via a high-pressure pump. Meanwhile, the liquid nanosuspension, which was made up of the resuspended NPs in the D-mannitol solution at different weight ratios, was loaded into the mixer by a high-pressure pump. After mixing, a nozzle (200 μm) was used to atomize the mixture into the precipitator. The nano-in-microparticles were formed after evaporation of the solvent from the nanosuspension droplets by the drying of hot nitrogen. Finally, the particles were collected at the bottom of the precipitator using a cyclone separator for further analysis.
Figure 10. Preparation of CS NPs by the supercritical fluid drying method.Peng et al. [72] prepared DOX-loaded CS NPs using supercritical fluid assisted atomization introduced by a hydrodynamic cavitation mixer (SAA-HCM) from an aqueous solution. The influences of solution concentration, CO2/solution ratio, mixer pressure, CS/DOX ratio, and CS molecular weight on the particle morphologies and sizes were investigated in detail. FT-IR results showed that the structure of DOX was not changed after the SAA-HCM process. The in vitro drug release behavior conducted in the media with pH of 4.5, 6.5, and 7.4, respectively, was found to be strongly pH-responsive. The in vitro cytotoxicity profiles revealed the activity of DOX was well maintained after being loaded into the CS NPs. The SAA-HCM process was demonstrated to be a promising technique for the one-step production of polymer/drug composite NPs suitable for cancer drug delivery from aqueous solutions.In another work by Peng et al. [73] prepared nano-in-microparticles composed of CS NPs and mannitol, using a modified supercritical CO2 assisted atomization (SAA-HCM) technique. Drug-free CS-based NPs were prepared using an ionic gelation method with TPP. CO2 from a cylinder was turned into liquid flowing through a cooling bath and sent to the mixer via a high-pressure pump. Meanwhile, the liquid nanosuspension, which was made up of the resuspended NPs in the D-mannitol solution at different weight ratios, was loaded into the mixer by a high-pressure pump. After mixing, a nozzle (200 μm) was used to atomize the mixture into the precipitator. The nano-in-microparticles were formed after evaporation of the solvent from the nanosuspension droplets by the drying of hot nitrogen. Finally, the particles were collected at the bottom of the precipitator using a cyclone separator for further analysis.Electrospraying Technique
Electrospraying is an electrohydrodynamic process used in the formation of NPs. The preparation of CS NPs by the electrospraying method is schematically illustrated in Figure 11. A high voltage electric field is applied into a polymeric solution flowing out of the nozzle to break it down to very fine nano-sized droplets possessing the same charge which assists their dispersion and prevents possible coagulation [74]. By altering specific variables such as applied electric voltage, solution flow rate, the distance between needle tip and collector, and the type of collector, the particle size, and morphology can be tuned [75].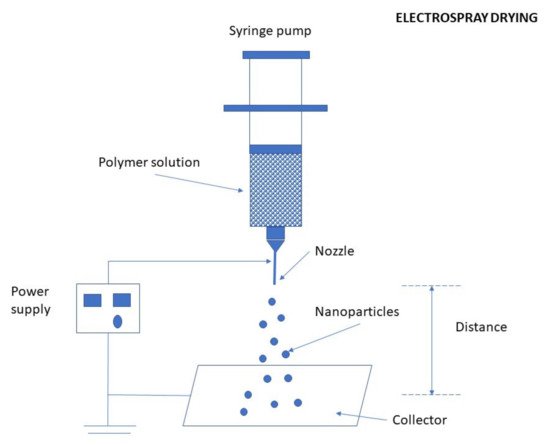 Figure 11. Preparation of CS NPs by the electrospraying method.Electrospraying has several advantages and very few limitations. It is a simple and one-step technique of a low cost. The entire spraying procedure can be performed at ambient temperature and pressure conditions, making a great benefit for the fabrication of carriers for the delivery of sensitive (high MW) bio-molecules/actives or living cells. Additionally, the probable absence of an external medium or solvent, that may cause migration or dissolution of the hydrophilic carrier, may greatly benefit in achieving specific use. The absence of coalescence in the case of electrospray droplets, due to the electrostatic charge repulsion, will result in uniform spread across large surface areas. Hence, monodispersed NPs can be easily obtained by this technique. The main disadvantage, however, is associated with relatively low product yields [65][76].CS NPs have been recently prepared using an electrospraying technique by Wang et al. [77]. They prepared sample solutions for electrospraying by dispersing CS in an aqueous solution of glacial acetic acid. Then, different quantities of tea polyphenols (TP) were slowly added to the CS solution to obtain different mass ratios. An aqueous solution of TPP was prepared and continuously pushed by a syringe pump at different flow rates, using a homemade electrospinning/electrospraying apparatus. The NPs were collected by centrifugation and lyophilized overnight to remove any solvent and water residues.
Figure 11. Preparation of CS NPs by the electrospraying method.Electrospraying has several advantages and very few limitations. It is a simple and one-step technique of a low cost. The entire spraying procedure can be performed at ambient temperature and pressure conditions, making a great benefit for the fabrication of carriers for the delivery of sensitive (high MW) bio-molecules/actives or living cells. Additionally, the probable absence of an external medium or solvent, that may cause migration or dissolution of the hydrophilic carrier, may greatly benefit in achieving specific use. The absence of coalescence in the case of electrospray droplets, due to the electrostatic charge repulsion, will result in uniform spread across large surface areas. Hence, monodispersed NPs can be easily obtained by this technique. The main disadvantage, however, is associated with relatively low product yields [65][76].CS NPs have been recently prepared using an electrospraying technique by Wang et al. [77]. They prepared sample solutions for electrospraying by dispersing CS in an aqueous solution of glacial acetic acid. Then, different quantities of tea polyphenols (TP) were slowly added to the CS solution to obtain different mass ratios. An aqueous solution of TPP was prepared and continuously pushed by a syringe pump at different flow rates, using a homemade electrospinning/electrospraying apparatus. The NPs were collected by centrifugation and lyophilized overnight to remove any solvent and water residues.3.2.6. Precipitation/Coacervation
This method is based on the utilization of characteristic physicochemical properties of CS, i.e., its insolubility in alkaline pH medium and thereby forming a precipitate. Preparation of CS NPs by precipitation/coacervation method is schematically illustrated in Figure 12. In this method, a CS solution is sprayed into an alkali solution such as NaOH or ethylenediamine, using a compressed air nozzle forming coacervate droplets [27][47]. Afterward, the separation and purification of the particles are performed by either filtration or centrifugation, followed by successive washing with hot and cold water. Another (alternative) method uses a sodium sulfate solution which is added dropwise to an acidic solution of CS containing surfactant while undergoing stirring and continuous sonication for 30 min [78].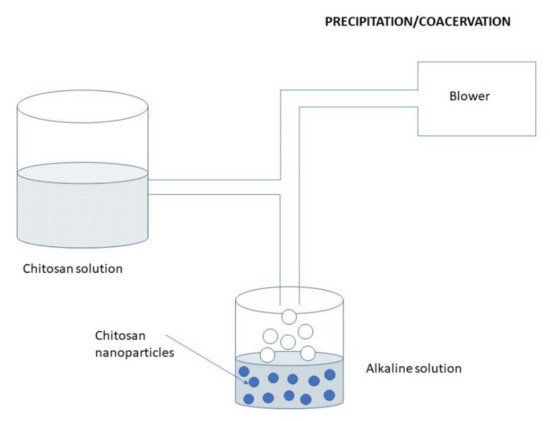 Figure 12. Preparation of CS NPs by the precipitation/coacervation method.
Figure 12. Preparation of CS NPs by the precipitation/coacervation method.3.2.7. Microfluidic Method
Conventional techniques including precipitation, spray drying, homogenization usually suffers from limitations, particularly due to the lack of control over the fabrication processes. Specifically, the fabricated CS-based materials have high batch-to-batch variations in physicochemical properties, such as the particle size, size distribution, and surface charge. Moreover, the structures of the fabricated CS-based materials cannot be elaborately tuned according to demand. Consequently, the accurate relationships between physicochemical properties and performance of the fabricated CS-based materials cannot be systematically investigated, which further hampers their applications. Besides these drawbacks, the conventional methods are highly complex and therefore difficult in scale-up production [79].Recently, microfluidics has been demonstrated as one of the most promising platforms to fabricate CS-based NPs with monodisperse size distribution and accurately controlled morphology and microstructures. The preparation of CS NPs by the microfluidic method is depicted in Figure 13. Microfluidic technology has precise fluid control, various shapes of a microchannel, and a multi-channel programmed mixing process, which provides a new opportunity for the synthesis of various types of CS with particle morphology, uniform particle size distribution, and batch quality repetition control. In other words, it is possible to achieve a series of preparation and processing that are difficult to complete with conventional methods, including higher particle size uniformity and precise control of the structural assembly. Fluids manipulated by microfluidic technology in these narrow channels have many unique properties, one of the most important is laminar flow [80][81]. In general, flows of common liquids in typical microfluidic channels (<100 μm) are characterized by the laminar state. Specifically, the equivalent diameter of microfluidic tubes is usually micron-sized or even nano-sized, and laminar flow can be achieved. Due to the steady streamlines of the laminar flow, fluids in different microfluidic devices (divided into three basic types, namely T-type, Y-type, and coaxial flowing) can be stretched or fractured to form monodispersed droplets or a liquid jet. Furthermore, the use of multiple microfluidic tubes in series can also provide the possibility to prepare particle shells and fiber sheaths [82].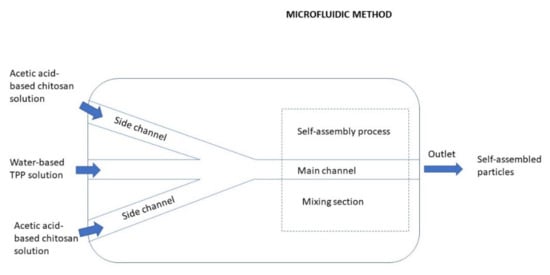 Figure 13. Preparation of CS NPs by the microfluidic method.In the process of preparing CS NPs by a microfluidic technology, the characteristics of CS itself are particularly important. For example, the concentration of CS, molecular weight and deacetylation degree of CS, and type of derivatives of CS can affect the physical properties of the CS solution like density, viscosity, and surface tension [83]. Under the same flow rate and pressure, the thickness of the CS liquid layer and the size of the droplets in the microfluidic channel may also be affected [79].Lari et al. [84] synthesized a novel crosslinked carboxymethyl chitosan NPs (CMC NPs) containing metformin hydrochloride (MET) using microfluidics and evaluated their performance for diabetes therapy. The microfluidic fabricated NPs exhibited slower and gradual release of the drug in comparison to a bulk method. The animal study on the rats indicated that the produced NPs were able to prevent weight loss, decrease blood glucose levels, and regenerate the pancreatic islets. The histopathological analysis showed that the MET-loaded CMC NPs had a better effect on the diabetes treatment compared with free MET. Therefore, the NPs synthesized by the microfluidic method seems to be a potent and effective therapeutic tool for diabetes therapy.Faharani et al. [85] attempted to set up a new approach to preventing fluid impassability as a result of microchannel blockage by introducing an acidic TPP solution. Thanks to this approach, the life span of the microfluidic chip also was increased, and no microchannel blockage occurred during the production process. Berberine as a model anticancer drug was successfully encapsulated in the chitosan/TPP NPs in a reproducible manner with optimized physical properties. Compared to other methods for delivering berberine via CS NPs prepared by bulk methods, the microfluidic produced berberine-CS/TPP NPs exhibited an enhanced drug loading content, narrow PDI (polydispersity index), spherical morphology, and controlled drug release profile.
Figure 13. Preparation of CS NPs by the microfluidic method.In the process of preparing CS NPs by a microfluidic technology, the characteristics of CS itself are particularly important. For example, the concentration of CS, molecular weight and deacetylation degree of CS, and type of derivatives of CS can affect the physical properties of the CS solution like density, viscosity, and surface tension [83]. Under the same flow rate and pressure, the thickness of the CS liquid layer and the size of the droplets in the microfluidic channel may also be affected [79].Lari et al. [84] synthesized a novel crosslinked carboxymethyl chitosan NPs (CMC NPs) containing metformin hydrochloride (MET) using microfluidics and evaluated their performance for diabetes therapy. The microfluidic fabricated NPs exhibited slower and gradual release of the drug in comparison to a bulk method. The animal study on the rats indicated that the produced NPs were able to prevent weight loss, decrease blood glucose levels, and regenerate the pancreatic islets. The histopathological analysis showed that the MET-loaded CMC NPs had a better effect on the diabetes treatment compared with free MET. Therefore, the NPs synthesized by the microfluidic method seems to be a potent and effective therapeutic tool for diabetes therapy.Faharani et al. [85] attempted to set up a new approach to preventing fluid impassability as a result of microchannel blockage by introducing an acidic TPP solution. Thanks to this approach, the life span of the microfluidic chip also was increased, and no microchannel blockage occurred during the production process. Berberine as a model anticancer drug was successfully encapsulated in the chitosan/TPP NPs in a reproducible manner with optimized physical properties. Compared to other methods for delivering berberine via CS NPs prepared by bulk methods, the microfluidic produced berberine-CS/TPP NPs exhibited an enhanced drug loading content, narrow PDI (polydispersity index), spherical morphology, and controlled drug release profile.3.3. Chitosan Based-Nanocomposites, Types, Their Properties, and Utilization
Improvement of physicochemical properties of CS can also enhance the utility of CS NPs. CS NPs have different properties which can further be enhanced for more efficient drug delivery. For improving the intestinal solubility of CS, N-trimethyl CS chloride has been produced. To increase the mucoadhesiveness of CS, NPs with thiolated CS were formulated. pH sensitivity can be achieved by grafting carboxylated CS with poly (methyl methacrylate). For specific applications, physical modifications can be performed in CS by blending, i.e., physically mixing two or more polymers. For example, blending CS with polyvinyl alcohol improves the mechanical and barrier properties of CS [27]. Thus, CS-based nanocomposites, combining native or derivatized CS with other organic polymers (Section 3.3.1) or inorganic substances (Section 3.3.2), can significantly enhance drug delivery performance in various specific situations. One of the most important/advanced areas of their use is represented by stimuli-responsive (CS-based nanocomposite) materials for drug delivery (Section 3.3.3). Stimuli-responsive CS-based materials do not represent exclusively nanocomposites but also simpler NPs formulated from some CS derivatives suitable for this purpose. Therefore, also such specific CS NPs are presented here along with CS nanocomposites via recent application examples.3.3.1. Chitosan-Polymer Nanocomposites
A blend of natural or synthetic polymers represents an innovative class of materials and has paid much attention, especially for biomedical applications. Although natural polymers have good biocompatibility and biodegradability for such applications, they can have often low solubility, mechanical and thermal stability limitations. Thus, the blending of natural and synthetic polymers results in new materials exhibiting combinations of properties about both polymers, that could not be obtained by individual polymers. In drug delivery systems, the blending of polymers provides stable, controlled release increases their encapsulation and loading capacity. Furthermore, the incorporation of nanofillers to the blend matrix additionally controls the release profile of the drug by acting as a diffusion barrier [18][44]. In the literature, CS is often combined with synthetic polymers such as polyethylene glycol (PEG), polyvinylalcohol (PVA), and natural polymers such as alginate, dextran, curdlan, carrageenan, caseinate, and pectin.Synthetic Polymers
PEGylated CS derivatives enhance the solubility of CS. In addition, they have lower cytotoxicity, higher ductility, and high stability in body fluids, which enable them to be applied in highly efficient drug delivery, gene delivery, stimuli-responsive hydrogel formation, and nanofiber formation [86]. A nanoparticle formulation with a core formed by CS polymer, surface-functionalized with PEG, and a cell-targeting peptide (CP15) is illustrated in Figure 14 (see Figure 14. A nanoparticle formulation with a core formed by CS polymer, surface-functionalized with PEG, and a cell-targeting peptide (CP15). The formulation encases a biotin-tagged siRNA [87] in our recent paper V. Mikusova, P. Mikus, Int. J. Mol. Sci. 2021, 22, 9652).CS/PVA blends have greater thermal stability, unique morphology, and reduced solubility in acidic solutions [88]. Menazea et al. [89] prepared PVA/CS and PVA/CS doped with selenium NPs at different laser ablation times and studied the structural, optical, and antibacterial properties of the new composite blend. The results showed that doping of selenium NPs to PVA/CS increased the antibacterial activity in comparison to the pure PVA/CS blend.Natural Polymers
To increase the specificity of CS as a drug carrier, it has been used along with another biopolymer (possessing specific biological properties) as a drug delivery device [18].A chitosan-alginate (CS/ALG) polyionic complex is formed during ionic gelation via the ionic interactions between the amine group of CS and the carboxylic group of ALG. Since these interactions reduce the porosity of the complex it protects the encapsulated drug and slows the release more effectively than either CS or ALG alone. The high solubility of CS in low pH is reduced by the poor solubility of the ALG network at low pH, while ALG is stabilized at high pH by CS which is less soluble at high pH [90].The other polymers to combine with CS, reported recently in the literature, are dextran [91], curdlan, carrageenan, caseinate [92], and pectin [93]. Curdlan possesses the ability to form high-set and low-set thermo-reversible gels at two distinct temperatures. Pectin is mucoadhesive at alkaline pH of the colon, passes intact through the upper gastrointestinal tract, and is degraded by colonic microflora so it is often used as a carrier for colon drug delivery. However, if used alone, pectin swells at alkaline conditions which may lead to premature release of a drug payload. When used in conjunction with other polymers such as CS, more stable matrices are formed for this drug delivery.The preparation of chitosan and albumin-coated insulin-loaded alginate/dextran NPs by Lopes et al. [94] is illustrated in Figure 15 (see Figure 15. Schematic representation of the emulsification/internal gelation technique used to prepare the CS and albumin coated insulin-loaded alginate/dextran NPs [94] in our recent paper V. Mikusova, P. Mikus, Int. J. Mol. Sci. 2021, 22, 9652). The formation of the egg-box structure (calcium/alginate gel) was induced by the pH decrease in the water/oil (W/O) nanoemulsion, which enabled the controlled release of calcium. Insulin entrapment in the NPs matrix was reinforced by the presence of dextran sulfate. The dual coating was applied by polyelectrolyte complexation by dropwise addition of CS and albumin, sequentially.3.3.2. Chitosan-Inorganic Material Nanocomposite
Many nontoxic inorganic NPs are developed for drug delivery because of their excellent and well-controlled physical and chemical properties as the porosity of mesoporous silica, photosensitive fever, and luminescence of gold particles, magnetic responsiveness of iron oxide, the luminescence of carbon quantum dots. However, some disadvantages limit their applications in the biomedical field. Inorganic NPs may tend to aggregate under physiological conditions for their poor stability, they have potential side effects for non-targeting, and there are difficulties in their modification [11]. Therefore, various organic-inorganic hybrid nanocomposites have been emerging as a new and interesting tool for biomedical applications. Such NPs are mainly composed of an inorganic NP core and a multifunctional surface coating. CS is a good candidate for decorating the inorganic NPs because of its excellent properties aforementioned. The resulting hybrid materials exhibit remarkably improved properties as compared to their parent material [18].Montmorillonite Clay (Mnt)
Mnt is a soft phyllosilicate group of minerals with a 2:1 layered structure consisted of silicate tetrahedral layers with alumina octahedral sheets sandwiched between them. The crystal lattice imperfection and isomorphous substitution create negative charge distribution with the plane counter-balanced by the adsorption of electropositive alkaline earth metal ions in the interlayer space. This is responsible for the activity and exchange reactions with organic compounds. Mnt has a large surface area which shows good cation exchange capacity, adsorption capacity, adhesive, and drug-carrying ability [18][95].
