Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Alexandra Pépin and Version 2 by Peter Tang.
Free sugars overconsumption is associated with an increased prevalence of risk factors for metabolic diseases such as the alteration of the blood lipid levels. Natural fruit juices have a free sugar composition quite similar to that of sugar-sweetened beverages. Despite the similarity of fruits juices to sugar-sweetened beverages in terms of free sugars content, it remains unclear whether they lead to the same metabolic consequences if consumed in equal dose.
- free sugars
- fruit juices
- fructose
- high-fructose corn syrup
- sugar-sweetened beverages
- dyslipidemia
1. Introduction
The ingestion of free sugars may favor the overconsumption of energy, thus promoting the development of risk factors associated with metabolic diseases such as hypertriglyceridemia, hypercholesterolemia, and insulin resistance [1][2][3][1,2,3]. Moreover, the current literature strongly suggests that ingestion of sugar-sweetened beverages increases the cardiometabolic risk and risk factors more than isocaloric amounts of complex carbohydrates [4]. Free sugars are defined as any types of simple sugars (monosaccharides or disaccharides) that have been added to beverages or food products during their transformation or preparation by food industries or by the consumer per se, plus sugars naturally present in fruit juices, fruit juice concentrates, honey, and various syrups [5]. Sugars that occur in the natural structure of entire fruits and vegetables as well as those from milk (lactose) are not categorized as free sugars [5][6][5,6]. Added sugars include sugars and syrups that are added during the preparation or the transformation of food and beverages. Therefore, natural fruit juices do not contain added sugars, but on the basis of the above definition, they are a significant source of free sugars.
While it is mainly accepted that the overconsumption of sugar-sweetened beverages may lead to adverse effects on health [3][4][3,4], the evidence pertaining to the consumption of fruit juices is a matter of debate. This is reflected in the inconsistency between dietary guidelines that relate to the consumption of natural fruit juice. The World Health Organization (WHO) recommends reducing the intake of free sugars to less than 10% (and, ideally, less than 5%) of total daily energy intake, thus including sugars naturally present in fruit juices in the category of sugars whose consumption should be reduced. The 2015–2020 Edition of the Dietary Guidelines for Americans, a resource for health professionals and policymakers for the design and implementation of nutrition programs in the United States, recommends consuming less than 10% of calories per day from added sugars, thus not including sugars naturally present in fruit juices in the category of sugars whose consumption should be reduced [7]. However, the American Academy of Pediatrics recommends limiting the consumption of fruit juice for children between the age of 1 and 3 years to 4 oz (120 mL)/day, for those from 4 to 6 years to 4–6 oz (120–180 mL)/day, and for those from 7 to 18 years to 8 oz (240 mL)/day [8][9][8,9].
2. Added Sugars: Sucrose and High-Fructose Corn Syrup
Simple sugars have been a part of the human diet for millennia. They were provided mainly by fruits and honey until white sugar (sucrose) became a common consumer product in the 19th century [10]. Nowadays, the worldwide consumption of sucrose is widespread, to the extent that it has tripled over the past 50 years [11]. In the United States, 77% of all calories purchased from 2005 to 2009 contained sweeteners, of which corn syrup, cane sugar, High-Fructose Corn Syrup (HFCS), and fruit juice concentrate were listed as the most commonly used [12]. Sucrose is naturally occurring in sugar cane and sugar beet and therefore extracted and purified directly from sugar cane or beet sap. In contrast, HFCS, which replaces sucrose in 40% of processed foods and beverages, is not naturally occurring [13].3. How Does Fructose Can Alter Lipemia?
3.1. Glucose Metabolism
Concerns about the ratio of fructose to glucose in beverages relate to the well-established differences between glucose and fructose metabolism. Glucose enters the enterocytes mostly by secondary active transport via sodium–glucose transporters (SGLT1) located in the apical membrane of the enterocytes. SGLT1 transporters have a high-affinity for glucose, but a low transportation capacity. Thus, under high concentrations of glucose in the lumen of the intestine, glucose also enters the enterocytes by facilitated diffusion via low-affinity, but high-capacity glucose transporters (GLUT2) [14][26]. GLUT2 transporters are expressed to a lesser extent in the apical membrane of the enterocytes but can be rapidly translocated from the basolateral membrane to the apical membrane to enhance glucose uptake under high concentrations of intestinal glucose [14][26]. Then, glucose exits the enterocytes to enter the bloodstream by facilitated diffusion via GLUT2 transporters located in the basolateral membrane (Figure 1). Glucose is then transported to the liver by the portal vein. Hepatic glucose metabolism is regulated by insulin and hepatic energy needs. This allows much of ingested glucose arriving via the portal vein to bypass the energy-replete liver and to rapidly reach the systemic circulation [15][27]. The first step of glycolysis, the responsible pathway for glucose metabolism, is the phosphorylation of glucose on its 6th carbon by the enzyme glucokinase (hexokinase). This step is then followed by an isomerization reaction resulting in fructose 6-phosphate (F6P). The major limiting step of glycolysis is the phosphorylation of F6P to fructose 1,6-biphosphate (catalyzed by phosphofructokinase), which will allow the molecule to be cleaved in two three-carbon units that can later be used to generate ATP [16][28].
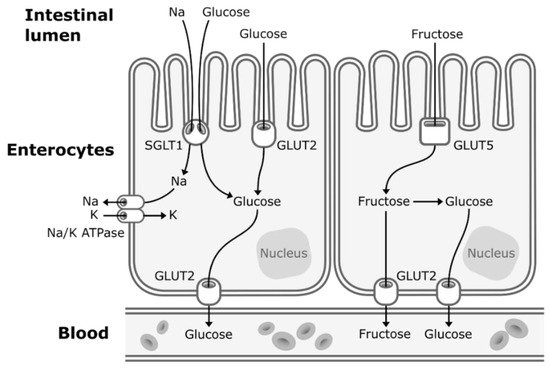

Figure 1. Absorption of fructose and glucose in the enterocytes. Glucose enters the enterocytes mostly by secondary active transport via sodium-glucose transporters (SGLT1) located in the apical membrane of the enterocytes. Under high concentrations of glucose in the lumen of the intestine, glucose also enters the enterocytes by facilitated diffusion via glucose transporters (GLUT2). Fructose enters the enterocytes through a specific fructose transporter (GLUT5). Then, both glucose and fructose exit the enterocytes to enter the systematic circulation by facilitated diffusion via GLUT2 transporters located in the basolateral membrane of the enterocytes. A small part of dietary fructose will be converted and released in the bloodstream by the enterocytes as glucose.
3.2. Fructose Metabolism
When fructose is ingested, it enters enterocytes through a specific fructose transporter (GLUT5), which is independent of sodium-glucose linked transporters and does not require ATP hydrolysis as opposed to SGLT1 [1]. Fructose will then enter the systematic circulation in a similar way to glucose, that is by facilitated diffusion via GLUT2 transporters (Figure 1). A small part of dietary fructose will be converted and released in the bloodstream by the enterocytes as glucose [1][17][1,29], lactate [1][17][1,29], and fatty acids in chylomicrons [18][19][20][21][22][30,31,32,33,34] (Figure 2). Yet, the role of the enterocytes in determining the metabolic fate of fructose has not been clearly established.
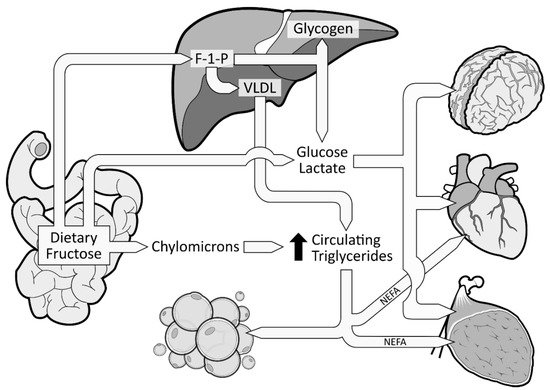

Figure 2. Metabolic fate of dietary fructose. Dietary fructose is ingested and released by the enterocytes mostly as fructose but also converted and released as glucose, lactate, and fatty acids (in chylomicrons). Fructose spills over to the liver where it is phosphorylated as Fructose 1-Phosphate (F 1-P). The largest part of F 1-P will be metabolized and converted by the hepatocytes as glucose, which can be stored as glycogen or released in the bloodstream [23][35]. Hepatocytes can also convert F 1-P into lactate and fatty acids. Fatty acids accumulate into the liver, consequently favoring the production and secretion of very low-density lipoproteins (VLDL), which leads to increased levels of circulating triglycerides and dyslipidemia.
The excessive amount of fructose will spillover to the liver where it is nearly all cleared from the portal blood after its first pass. Fructose will be rapidly phosphorylated (catalyzed by fructokinase C, a key enzyme in the metabolism of fructose [24][25][26][36,37,38]) on its first carbon, resulting in fructose 1-phosphate (F 1-P) instead of F6P [16][28] (Figure 2). F 1-P has the capacity to bypass the first limiting step of glycolysis [16][28] without being regulated by insulin nor inhibited by ATP production. F 1-P will mostly be metabolized by aldolase B into glyceraldehyde 3-P (G 3-P). Subsequently, G 3-P can be: (1) converted into pyruvate (resulting in acetyl-CoA production); (2) converted and released as lactate; or (3) converted to glucose (gluconeogenesis) [1]. The largest part of G 3-P will be converted to glucose, which can be stored as glycogen or released as glucose 6-phosphate in the bloodstream [23][35]. In fact, in the 1990s, isotope tracing with intravenously infused 13C-labeled fructose in humans showed that ~50% of a fructose load was converted and recirculated as 13C-labeled glucose [27][39]. When released in the systematic circulation, glucose and lactate can be utilized as an energy substrate by the brain, heart, and muscle tissue [23][35] (Figure 2). As mentioned previously, a large part of F 1-P will be metabolized in G 3-P. Nonetheless, the excessive supply of fructose spilled to the liver has also been shown to simultaneously inhibit lipid oxidation [28][40] and to enhance hepatic de novo lipogenesis (DNL) [29][30][41,42]. DNL has the capacity to convert fructose, more precisely F 1-P, into fatty acids [31][43], thus consequently increasing the intrahepatic lipid supply. Elevated levels of intrahepatic lipids content favor very low-density lipoproteins (VLDL) production and secretion [32][44], which also leads to increased levels of postprandial triglycerides and dyslipidemia [1][30][1,42] (Figure 2). Increased levels of intrahepatic lipids are associated with hepatic insulin resistance [33][34][45,46]. Of note, a systematic review indicated that fructose consumption in an energy-matched exchange for other carbohydrates (mostly glucose) induces hepatic insulin resistance [35][47]. This suggests that the promotion of hepatic insulin resistance by fructose could not be attributed only to the excess of energy intake under hypercaloric diet conditions. Knowing that DNL is more strongly activated in the insulin-resistant liver [36][48], fructose consumption has the potential to generate a vicious cycle that would further increase the intrahepatic lipid supply, thus amplifying VLDL-triglyceride production and secretion [2]. Continued exposure to triglycerides promotes muscle lipid accumulation [37][49], which may also promote whole-body insulin resistance [38][39][50,51].
4. Sugar-Sweetened Beverages and Their Implication in Metabolic Health
4.1. Free Sugars Consumed from Sugar-Sweetened Beverages Induce Metabolic Perturbations
Epidemiological studies have shown that the consumption of sugar-sweetened beverages is associated with increased energy intake, long-term weight gain, and prevalence of metabolic and cardiovascular diseases [3][40][41][3,52,53]. Experimental evidence strongly suggests the fructose component of HFCS and sucrose promotes metabolic perturbations such as dyslipidemias [29][30][37][42][43][44][45][46][47][48][49][50][51][52][41,42,49,54,55,56,57,58,59,60,61,62,63,64] and insulin resistance [23][30][45][53][35,42,57,65]. The consumption of free sugars at the level that is currently consumed by Americans may adversely alter lipemia. It has indeed been shown that sucrose, when consumed at 13% of estimated daily energy requirements (Ereq) (80 g/day) as a beverage along with a usual ad libitum diet for three weeks, led to increased low-density lipoprotein cholesterol (LDL-C) and decreased hepatic insulin sensitivity in healthy young men compared to the consumption of glucose [53][65]. It should be noted that daily energy requirements refer to the calorie intake needed to balance energy expenditure in order to maintain a healthy individual’s body weight stable. These results are in line with recent findings showing that a 12-week intervention where 13% of diet energy as fructose was served in the habitual diet of 71 men with abdominal obesity led to enhanced DNL and increased body weight, liver fat content, and postprandial triglyceride levels [44][56]. Young men and women consuming beverages containing 0, 10, 17.5, and 25% of Ereq as HFCS along with ad libitum diets exhibited a dose-dependent increase of LDL-C, apolipoprotein B (the protein backbone of VLDL), apoCIII, uric acid, and postprandial triglycerides [54][66]. Importantly, even the group consuming the 10% dose exhibited significantly increased concentration of LDL-C, apolipoprotein B, and postprandial triglycerides compared with their baseline concentration [54][66]. In a six-month dietary intervention study, subjects consuming one liter of sucrose-sweetened beverages/day exhibited increased triglycerides, cholesterol, and liver fat [37][49]. A recent meta-analysis revealed that the dose and overall caloric intake of free sugars have the strongest deteriorating effect on blood lipids as compared to interventions where isocaloric substitution of free sugars with complex carbohydrates is provided [55][67]. However, most of these studies are limited in that free sugars were consumed along with the subjects’ own usual ad libitum diets during most of the intervention, thus the total amount of free sugars consumed daily are unknown. This prevents attributing adverse effects to precise doses of free sugars. Another limitation to the above studies is that many of the subjects exhibited increases in body weight. This makes it difficult to separate the direct effects of fructose from those indirectly mediated by increased adiposity.
There are several studies that compared sugar (sucrose, HFCS, and/or fructose) consumption from sugar-sweetened beverages with isocaloric substitutions for complex carbohydrates [29][45][41,57] or glucose [47][53][59,65], or from low-sugar diets [42][43][49][54][56][57][54,55,61,66,68,69] in healthy individuals on health outcomes (Figure 3). However, to the ouresearchers' knowledge, there are only three studies [29][56][57][41,68,69] in which the effects of sugar consumption from sugar-sweetened beverages at levels less than 30% Ereq were investigated in healthy subjects utilizing a controlled dietary protocol that prevented body weight gain (eucaloric) and diet macronutrient variations between experimental groups. Black et al. (2006) [57][69] conducted a six-week crossover study with healthy men who consumed eucaloric diets containing high (25% Ereq) or low (10% Ereq) amounts of sucrose. The 25% Ereq sucrose diet increased the levels of total cholesterol by 15% and of LDL-C by 24% as compared to the 10% Ereq sucrose diet; the authors suggested this could have been caused by the high-sucrose diet containing more saturated fats [57][69]. More recently, Lewis et al. (2013) [56][68] conducted a randomized six-week crossover study in which individuals with obesity consumed low- (5%) or high- (15%) sucrose diets for six weeks. While no differences in lipid levels were observed, the subjects displayed increased glucose (5.0 ± 0.2 vs. 5.4 ± 0.2 mmol/L, p < 0.01) and insulin responses (59.0 vs. 109.2 mU/L, p < 0.01) during the oral glucose tolerance test (OGTT) when consuming the high-sucrose diet [56][68]. Likewise, Schwarz and colleagues (2015) [29][41] investigated eight healthy men consuming crossover diets containing eucaloric amounts of either fructose or complex carbohydrates (25% of Ereq) for nine days, while maintaining their body weight stable and providing the same macronutrient distributions. The subjects exhibited elevated DNL (average, 18.6 ± 1.4% vs 11.0 ± 1.4%; p = 0.001), liver lipids (median, + 137%, p = 0.016), and postprandial triglycerides (in seven of eight participants: average, 172 ± 29 vs. 140 ± 28 mg/dL; p = 0.002) when consuming the high-fructose diet compared with the complex-carbohydrate diet [29][41]. Overall, these results suggest that sucrose or fructose consumption at levels as low as 18% Ereq, increases the risk factors for metabolic diseases, even when consumed with a diet that does not promote weight gain. However, this conclusion could be confounded by the vulnerability of subjects with obesity [56][68], the different saturated fat content between diets [57][69], and the use of pure fructose instead of HFCS or sucrose [29][41]. Therefore, because of the limited number of studies and the limitations of these studies, it is difficult to establish firm conclusions regarding the weight-independent effects of sugar consumption on health outcomes.
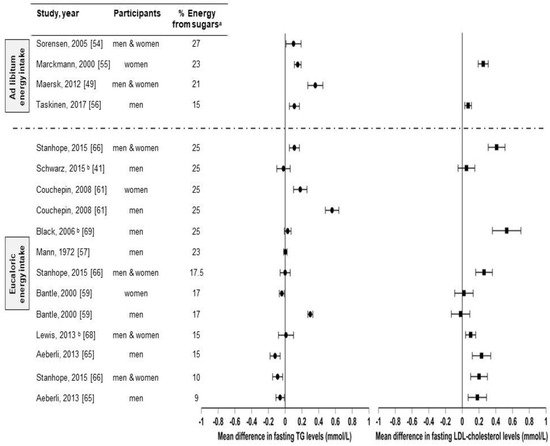

Figure 3. Effects of sugar consumption from sugar-sweetened beverages on fasting blood triglyceride (TG) and fasting LDL-cholesterol levels in healthy individuals with normal weight, overweight, or obesity. Mean difference in fasting blood triglyceride and fasting low-density lipoprotein cholesterol (LDL-C) levels in studies that compared higher with lower sugar intakes from sugar-sweetened beverages in healthy individuals with normal weight, overweight, or obesity. a Refers to the higher sugar intake intervention. The percentage of energy from the lower sugar intake intervention is detailed in Table I. Studies with ad libitum energy intake controlled for a minimal sugar intake but not for total energy intake. Studies with eucaloric energy intake controlled for a minimal sugar intake and for weight maintenance throughout the studies. b Studies that controlled for a minimal sugar intake, for weight maintenance throughout the studies, and for diet macronutrient variations between experimental groups.
5. What about the Effects of Natural Fruit Juices on Blood Lipids?
It is well accepted that fruit intake is protective for human health, but there is no clear consensus about the effects of consuming the juices that are extracted from them [58][70]. The primary component of fruit juices, apart from water, is free sugars in a concentration of about 100–120 g/L depending on the variety and the quality of fruits [59][71]. The fructose content of most natural fruit juices is quite similar to that of beverages sweetened with HFCS-55. For instance, orange juice has an average total fructose concentration (including free fructose and fructose from sucrose) of 51–57 g/L, which represents 52 to 54% of its total sugar content [60][61][25,72]. However, it is important to note that, despite their similarity to sugar-sweetened beverages in terms of fructose composition, fruit juices are also a source of various bioactive compounds such as phytonutrients, whose consumption has been shown to be beneficial to human health and the prevention of chronic diseases [62][73]. The question still needs to be answered: Are fruit juices healthier than sugar-sweetened beverages?
