Mucormycosis, previously known as zygomycosis, is a lethal fungus in which molds called mucormycetes can cause fungal infection. Mucormycosis causes angioinvasive infection among immunocompromised patients, with a mortality rate of 60%. Mucormycosis is the third most prevalent fungal infection in hematology patients, accounting for 8.3–13% of all fungal infections.
- COVID-19
- mucormycosis
- nanoparticles
- pathogenesis
- amphotericin-B
1. Introduction
Mucormycosis, previously known as zygomycosis, is a lethal fungus in which molds called mucormycetes can cause fungal infection [1][2][3][1,2,3]. Mucormycosis causes angioinvasive infection among immunocompromised patients, with a mortality rate of 60% [4]. Mucormycosis is the third most prevalent fungal infection in hematology patients, accounting for 8.3–13% of all fungal infections [5][6][5,6]. Mucorales fungi access the human body mostly by inhalation, percutaneous contact, or ingestion [7]. Mucormycosis generally occurs in patients who are immunocompromised by leukemia, lymphoma, neutropenia, diabetes, burn, trauma, childhood malnutrition, and the like [8][9][8,9]. Diabetes mellitus is the key vulnerability factor related to mucormycosis in India [10], which leads the world in mucormycosis as well as diabetes [11][12][11,12]. Mucormycosis is notoriously difficult to diagnose, with Ingram et al. having found that only 9% of cases were identified in antemortem diagnosis [13].
SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), the virus behind the COVID-19 outbreak, is linked to a variety of bacterial and fungal infections [14]. In India and some other countries, mucormycosis has co-occurred extensively with COVID-19 and is considered to be an epidemic by the Indian government based on reports of the infection mainly affecting hospitalized COVID-19 patients, leading to prolonged morbidity and death. Researchers have found that it chiefly affects immunocompromised patients admitted to the hospital when fungal spores enter a COVID-19–infected person through an airborne vector, affecting the sinuses and lungs, though rarely in persons who have strong immunity. Patients who have been treated for COVID-19 by using steroids and other drugs to cure inflammation are the most vulnerable to mucormycosis [14]. The erroneous administration of corticosteroids (i.e., Prednisone, Hydrocortisone, or Dexamethasone) is a contributing factor to mucormycosis infection in COVID-19 patients. Even though steroids are effective in treating respiratory illnesses including chronic obstructive pulmonary disease and asthma, and other illnesses such as rheumatoid arthritis, the long-term or excessive use of steroids suppresses the body’s immunological system, making the person more susceptible to diseases such as mucormycosis infection [15].
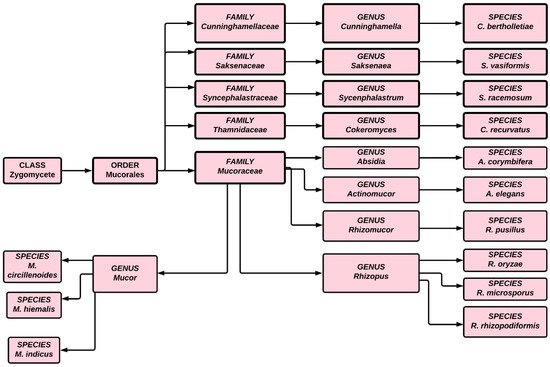
The first case of black fungus was reported during the first wave of the COVID-19 pandemic in India, a couple of weeks after the patient’s discharge from a hospital. During the second wave of COVID-19, infections were reported even while patients were undergoing hospital treatment. Although mucormycosis can be treated by antifungal medication, ultimately surgery is required. Traditionally treatment has involved intravenous infusion of regular saline followed by an infusion of amphotericin, but a lack of clinical trial data has hindered researchers and scientists from choosing specific antifungal agents for treating mucormycosis. Because of the high mortality associated with this infection, effective treatment requires early detection and depends on recovery from predisposing factors. The condition can also be improved through surgical debridement and administration of medication [16][17][18][16,17,18]. In India, 28,252 occurrences of mucormycosis, or black fungus, have been documented in 28 states and union territories, with Maharashtra and Gujarat accounting for the overwhelming majority [19]. Figure 1 illustrates the classification of fungi in the zygomycete order.
2. Manifestation of Mucormycosis
Mucormycosis symptoms vary depending on where the fungus develops in the body [14][20][14,20]. Symptoms of rhinocerebral mucormycosis include black sores on the nasal bridge, fever, one-sided face edema, headache, and nasal congestion, whereas the symptoms of cutaneous mucormycosis are swelling around the wound, pain, and excessive redness. By contrast, the symptoms of pulmonary mucormycosis include breathlessness, chest pain, coughing, and fever. Finally, the symptoms of gastrointestinal mucormycosis include stomach pain, stomach bleeding, and nausea and vomiting.
Because disseminated mucormycosis develops in patients who have been admitted to the hospital for other diseases, determining which symptoms are caused by mucormycosis can be difficult. Eventually such patients may develop mental status changes that may lead to coma. Because some of the symptoms of mucormycosis and COVID-19 are similar, physicians may have difficulty determining whether an individual is infected with a fungus or with COVID-19. Furthermore, certain patients may have COVID-19 along with a fungal infection.
3. Mucormycosis Outbreak
An outbreak occurs when two or more people are infected by the same source or at the same place or time. The sources of outbreaks may be outdoors or may be in a healthcare setting, such as a hospital [21][73]. Most published works describe outbreaks of cutaneous mucormycosis, which has been tied to contaminated dressings and is less fatal than other forms of mucormycosis, with a medical literature review revealing 16% mortality versus 67% for rhinocerebral, 83% for pulmonary, and 100% for disseminated and gastrointestinal mucormycosis [22][74]. Hospital bedding has been identified as a vector for spreading R. delemar to vulnerable patients. In hospital epidemic investigations, DNA-based approaches to fungal species detection have confirmed epidemiological connections. Hospital bedding must be washed, wrapped, distributed, and stored in ways that minimize their exposure to environmental pollutants [23][75].
4. Mucormycosis Diagnosis Limitations in Patients Infected with Microbial Infection
Given the limited treatment choices available, which usually include disfiguring and painful operations, early and accurate diagnosis is, in theory, the most important factor in improving the outcome of mucormycosis. Furthermore, approximately 4 to >90% of suspected mucormycosis cases are not verified until post-mortem investigation [24][25][26][141,142,143]. A combination of variables, the non-specific clinical appearance of mucormycosis, as well as the various limitations of currently available diagnostic techniques, make a definite diagnosis challenging. It is critical to isolate the fungus and identify it to the genus or species level for prognostic, epidemiological, and therapeutic objectives. [25][27][28][142,144,145]. The cultural isolation output ranges from 50–71%, with evidence that it has improved considerably over time [29][24][37,141]. Mucorales recovery from clinical microbiology specimens, on the other hand, is difficult. Mucorales hyphae may be difficult to see on wet mounts and require special chitin-binding stains to be seen using a fluorescence microscope, or they may be too few to see. Furthermore, vigorous homogenization or tissue grinding may obliterate the coenocytic hyphae during tissue processing [25][30][142,146]. Nonculture techniques, such as detecting biochemical or serological indicators, are currently unavailable to aid in diagnosing invasive mucormycosis. Invasive candidiasis is diagnosed using circulating mannan antigen and (1-3)-β- d -glucan, whereas invasive aspergillosis is diagnosed using galactomannan in bronchoalveolar fluid and serum.
