You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Te-Sheng Chang.
Ganoderma lucidum is a medicinal fungus abundant in triterpenoids, its primary bioactive components. Although numerous Ganoderma triterpenoids have already been identified, rare Ganoderma triterpenoid saponins were recently discovered. To expand Ganoderma triterpenoids diversity and create novel Ganoderma saponins, Bacillus glycosyltransferases were used to glycosylate Ganoderma triterpenoids to produce novel Ganoderma saponins, which have high potential in pharmaceutical usages.
- Bacillus
- Ganoderma lucidum
- glycosyltransferase
- saponin
1. Introduction
Ganoderma lucidum is a medicinal fungus that has been used for health improvement and the treatment of various diseases in Asia over thousands of years [1]. In modern studies, both triterpenoids and polysaccharides have been demonstrated to exhibit bioactivity [2]. Triterpenoids contain six isoprene units and usually form tetra- or penta-cyclic structures. Triterpenoids are widely noted to exist in plants and some fungi. Until now, more than 14,000 different triterpenoids have been identified, and many of them have been proven to play important roles in bioactivity [3]. Triterpenoid saponins are important natural products in plants, and some triterpenoid saponins exhibit greater bioactivity than triterpenoid aglycones [4]. Therefore, the glycosylation of triterpenoids is important for the biosynthesis of triterpenoid saponins. G. lucidum also contains large amounts of triterpenoids, and over 300 triterpenoids that form Ganoderma sp. have been identified previously [5,6,7][5][6][7]. All Ganoderma triterpenoids belong to the tetracyclic lanostane type of triterpenoids which contain different types of main skeletons, including ganoderic acids. These Ganoderma triterpenoids were found to possess varying degrees of bioactivity. For example, a Ganoderma triterpenoid, ganoderic acid A (GAA), was recently shown to have bioactive medical applications including the inhibition of bleomycin-induced lung fibrosis in mice [8], the attenuation of lipopolysaccharide-induced lung injury in mice [9], the retardation of renal cyst development in polycystic kidney disease [10], and the protection of neural cells against NO stress injury [11]. Considering the diversity of the Ganoderma triterpenoid group, studying the bioactivity of Ganoderma triterpenoids has become a core focus of biotechnology studies in recent years. Consequently, rare Ganoderma triterpenoid saponins have been identified recently [5,6,7][5][6][7].
In nature, the glycosylation of triterpenoids is initiated by glycosyltransferase (EC 2.4.1.X; GT) [12]. GT uses a nucleotide-activated sugar donor, such as uridine diphosphate glucose (UDP-G), to transfer the sugar moiety to a sugar acceptor. According to the carbohydrate-activating enzyme database there are 117 subfamilies of GTs, with over 850,000 GTs having already been identified [13]. However, only some rare bacterial GTs are able to glycosylate triterpenoids to triterpenoid saponins [14]. In our previous studies, we used GAA as a precursor to screen microbes with the ability to biotransform GAA to GAA saponins. Two bacteria, the Bacillus subtilis (Bs) American Type Culture Collection (ATCC) 6633 strain and the Bacillus thuringiensis (Bt) GA A07 strain, were able to produce GAA saponins. Four Bacillus GTs were further identified, based on their genomes, as the major enzymes able to catalyze the glycosylation of GAA to GAA saponins. BsGT110 [15], BsUGT398, and BsUGT489 [16] were identified using the ATCC 6633 strain, and BtGT_16345 was identified using the GA A07 strain [17]. So far, only seven Bacillus GTs with triterpenoid glycosylation activity have been identified, including the four Bacillus GTs mentioned previously [14]. Phylogenetic analysis suggests that the four Bacillus GTs are genetically distinct from each other (Figure 1) [14]. Although the four Bacillus GTs have been proven to have the ability to produce Ganoderma triterpenoid saponins, previous studies have only focused on GAA glycosylation [15,16,17][15][16][17]. Thus, it is worth investigating the catalytic activity of the four Bacillus GTs toward other triterpenoids.
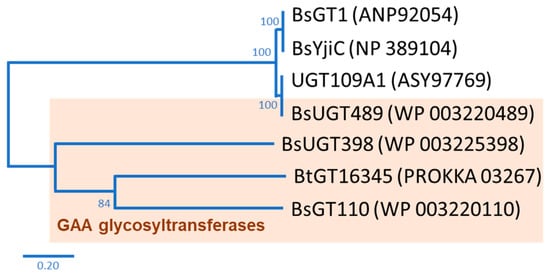
Figure 1. Seven known bacterial glycosyltransferases (GTs) from previous studies. The phylogenetic tree was adopted from Figure 1 in the study by Chang et al. [14].
Therefore, the glycosylation sites of ganoderic acid G (GAG), namely, the C-3, C-7, and C-12 hydroxyl groups and the C-26 carboxyl group, were selected to test the biotransformation of the four Bacillus GTs. The glycosylated saponins produced by the four Bacillus GTs using GAG were purified and their chemical structures were identified. In addition, the aqueous solubility of the GAG saponins was determined and compared with that of GAG. Accordingly, the catalytic specificity of the four Bacillus GTs toward the different functional groups of the ganoderic acids was clarified.
2. Biotransformation
Ganoderic acid is one of most common types of Ganoderma triterpenoids and primarily contains five functional groups that are available for modification, including the C-3, C-7, C-12, and C-15 hydroxyl groups and the C-26 carboxyl group [5,6,7][5][6][7]. In this study, the four Bacillus GTs (Figure 1) were assayed for their glycosylation activity toward GAG containing C-3, C-7, and C-12 hydroxyl groups and the C-26 carboxyl group (Figure 2). After the completion of the biotransformation reaction, the reaction mixture was analyzed using high-performance liquid chromatography (HPLC), the results of which are shown in Figure 3. The results indicate that BsUGT489 and BsGT110 biotransformed GAG to compound (1) and compound (2), with yields of 74% and 35%, respectively. Both BsUGT398 and BtGT_16345 showed little to no activity toward GAG, respectively.
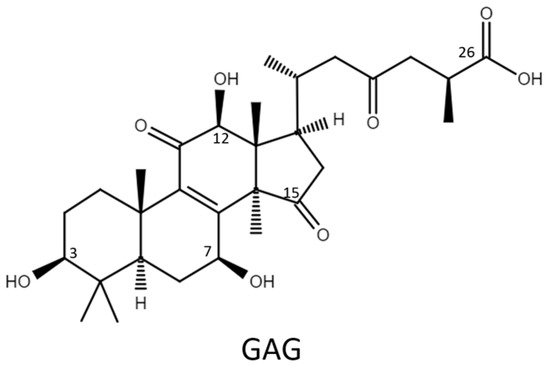
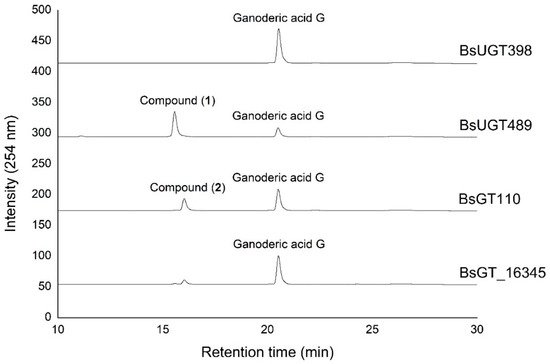

Figure 2. The chemical structure of ganoderic acid G (GAG).

Figure 3. High-performance liquid chromatography (HPLC) results of the biotransformation products of GAG using the four Bacillus GT enzymes. The biotransformation and HPLC conditions are described in the Materials and Methods section.
3. Purification and Identification of Biotransformed Products
The biotransformation reactions of GAG with BsUGT489 and BsGT110 were selected to purify compound (1) and compound (2), respectively. The two biotransformation reactions were scaled up to 20 mL and the products were purified by preparative HPLC. From the 20 mL reaction mixture, 14.4 mg of compound (1) and 7.8 mg of compound (2) were purified. The molecular weights of the purified products were then determined by mass spectrometry. The mass spectrometer showed an [M−H]− ion peak at m/z: 693.5 in the electrospray ionization mass spectrum (ESI-MS), corresponding to the molecular formula C36H54O13. The mass data imply that both compound (1) and compound (2) contain one glucosyl moiety attached to the GAG structure (Figures S1 and S2). To identify the structures in advance, the structures of both products were determined using nuclear magnetic resonance (NMR) spectroscopy. The 1H and 13C NMR, including the distortionless enhancement by polarization transfer (DEPT), heteronuclear multiple bond connectivity (HMBC), heteronuclear single quantum coherence (HSQC), nuclear Overhauser effect spectroscopy (NOESY), and correlation spectroscopy (COSY) spectra were obtained. The NMR spectra of compound (1) exhibited characteristic glucosyl signals, with the anomeric proton signal at δH 3.98 (1H, ddd, J = 8.6, 5.6, 2.1 Hz, H-5′), 4.03 (1H, t, J = 8.6 Hz, H-2′), 4.22 (1H, t, J = 8.6 Hz, H-4′), 4.24 (1H, t, J = 8.6 Hz, H-3′), 4.40 (1H, dd, J = 11.9, 5.6 Hz, H-6′a), 4.57 (1H, dd, J = 11.9, 2.1 Hz, H-6′b), and 4.91 (1H, d, J = 8.6 Hz, H-1′); and the anomeric carbon signal at δC 63.0 (C-6′), 71.8 (C-4′), 75.7 (C-2′), 78.4 (C-5′), 78.7 (C-3′), and 107.0 (C-1′). The large coupling constant (8.6 Hz) of the anomeric proton H-1′ (4.91 ppm) indicated the β-configuration. An ether linkage between the H-1′ of glucose and C-3 (4.91/88.3 ppm) of GAG was proven by the HMBC and NOESY (H-3/H-1′) spectra. The structure of compound (1) was thus confirmed to be GAG-3-o-β-glucoside. The NMR spectroscopic data are shown in Figures S3–S9. The siignals of compound (2) were attributed to a glucose moiety, with δH 4.03 (1H, ddd, J = 8.8, 4.9, 2.8 Hz, H-5′), 4.20 (1H, t, J = 8.8 Hz, H-2′), 4.29 (1H, t, J = 8.8 Hz, H-3′), 4.35 (1H, t, J = 8.8 Hz, H-4′), 4.36 (1H, dd, J = 11.9, 4.9 Hz, H-6′a), 4.46 (1H, dd, J = 11.9, 2.8 Hz, H-6′b), and 6.33 (1H, d, J = 8.8 Hz, H-1′); and δC 62.1 (C-6′), 71.0 (C-4′), 74.2 (C-2′), 78.5 (C-3′), 79.5 (C-5′), and 96.3 (C-1′). The cross peak of H-1′ with C-26 (6.33/175.0 ppm) in the HMBC spectrum demonstrated the structure of compound (2) to be GAG-26-o-β-glucoside. The NMR spectroscopic data are shown in Figures S10–S16. The strtructures of the GAG saponins and the biotransformation process are shown in Figure 4.
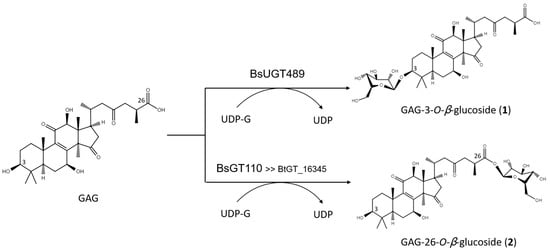

Figure 4.
The biotransformation process of GAG to GAG saponins by the
Bacillus
GTs.
References
- Ahmad, M.F. Ganoderma lucidum: Persuasive biologically active constituents and their health endorsement. Biomed. Pharmacother. 2018, 107, 507–519.
- Xu, J.W.; Zhao, W.; Zhong, J.J. Biotechnological production and application of ganoderic acids. Appl. Microbiol. Biotechnol. 2010, 87, 457–466.
- Sultana, N.; Saify, Z.S. Enzymatic biotransformation of terpenes as bioactive agents. J. Enzym. Inhib. Med. Chem. 2013, 28, 1113–1128.
- Shi, Z.Y.; Zeng, J.Z.; Wong, A.S.T. Chemical structures and pharmacological profiles of ginseng saponins. Molecules 2019, 24, 2443.
- Yang, Y.; Zhang, H.; Zuo, J.; Gong, X.; Yi, F.; Zhu, W.; Li, L. Advances in research on the active constituents and physiological effects of Ganoderma lucidum. Biomed. Dermatol. 2019, 3, 6.
- Liang, C.Y.; Tian, D.N.; Liu, Y.Z.; Li, H.; Zhu, J.L.; Li, M.; Xin, M.H.; Xia, J. Review of the molecular mechanisms of Ganoderma lucidum triterpenoids: Ganoderic acids A, C2, D, F, DM, X and Y. Eur. J. Med. Chem. 2019, 174, 130–141.
- Xia, Q.; Zhang, H.Z.; Sun, X.F.; Zhao, H.J.; Wu, L.F.; Zhu, D.; Yang, G.H.; Shao, Y.Y.; Zhang, X.X.; Mao, X.; et al. A comprehensive review of the structure elucidation and biological activity of triterpenoids from Ganoderma spp. Molecules 2014, 19, 17478–17535.
- Wen, G.; Li, T.; He, H.; Zhou, X.; Zhu, J. Ganoderic Acid A Inhibits Bleomycin-Induced Lung Fibrosis in Mice. Pharmacology 2020, 105, 568–575.
- Wan, B.; Li, Y.; Sun, S.; Yang, Y.; Lv, Y.; Wang, L.; Song, M.; Chen, M.; Wu, C.; Pan, H.; et al. Ganoderic acid A attenuates lipopolysaccharide-induced lung injury in mice. Biosci. Rep. 2019, 39, BSR20190301.
- Meng, J.; Wang, S.Z.; He, J.Z.; Zhu, S.Z.; Huang, B.Y.; Wang, S.Y.; Li, M.; Zhou, H.; Lin, S.Q.; Yang, B.X. Ganoderic acid A is the effective ingredient of Ganoderma triterpenes in retarding renal cyst development in polycystic kidney disease. Acta Pharmacol. Sin. 2020, 41, 782–790.
- Yu, Z.R.; Jia, W.H.; Liu, C.; Wang, H.Q.; Yang, H.G.; He, G.R.; Chen, R.Y.; Du, G.H. Ganoderic acid A protects neural cells against NO stress injury in vitro via stimulating β adrenergic receptors. Acta Pharmacol. Sin. 2020, 41, 516–522.
- Mestrom, L.; Przypis, M.; Kowalczykiewicz, D.; Pollender, A.; Kumpf, A.; Marsden, S.R.; Bento, I.; Jarzebski, A.B.; Szymanska, K.; Chrusciel, A.; et al. Leloir glycosyltransferases in applied biocatalysis: A multidisciplinary approach. Int. J. Mol. Sci. 2019, 20, 5263.
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238.
- Chang, T.S.; Wang, T.Y.; Chiang, C.M.; Lin, Y.J.; Chen, H.L.; Wu, Y.W.; Ting, H.J.; Wu, J.Y. Biotransformation of celastrol to a novel, well-soluble, low-toxic and anti-oxidative celastrol-29-O-beta-glucoside by Bacillus glycosyltransferases. J. Biosci. Bioeng. 2021, 131, 176–182.
- Chang, T.S.; Chiang, C.M.; Kao, Y.H.; Wu, J.Y.; Wu, Y.W.; Wang, T.Y. A new triterpenoid glucoside from a novel acidic glycosylation of ganoderic acid A via recombinant glycosyltransferase of Bacillus subtilis. Molecules 2019, 24, 3457.
- Chang, T.S.; Wu, J.Y.; Wang, T.Y.; Wu, K.Y.; Chiang, C.M. Uridine diphosphate-dependent glycosyltransferases from Bacillus subtilis ATCC 6633 catalyze the 15-O-glycosylation of ganoderic acid A. Int. J. Mol. Sci. 2018, 19, 3469.
- Chang, T.S.; Wang, T.Y.; Hsueh, T.Y.; Lee, Y.W.; Chuang, H.M.; Cai, W.X.; Wu, J.Y.; Chiang, C.M.; Wu, Y.W. A genome-centric approach reveals a novel glycosyltransferase from the GA A07 Strain of Bacillus thuringiensis responsible for catalyzing 15-O-glycosylation of ganoderic acid A. Int. J. Mol. Sci. 2019, 20, 5192.
More
