You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 3 by Vivi Li and Version 2 by Maria João Mendes Cardoso Barroca.
Corema album (L.) D. Don is a dioecious perennial shrub of the Ericaceae family, endemic of the Iberian Peninsula Atlantic coastal dunes. It is a branched bush, that can reach up to 1 m, with a spherical (5–8 mm diameter) and white acidic edible berries (Portuguese white crowberries, Atlantic pearls, beachberries or “camarinhas” in Portuguese).
- white crowberries
- plant extracts
- antibacterial activity
- nutraceutical
- FTIR and Raman spectroscopies
1. Introduction
The Portuguese coastline is rich in many indigenous maritime plants with a high potential to become novel functional food ingredients (or sources of these). Corema album (L.) D. Don is a dioecious perennial shrub of the Ericaceae family, endemic of the Iberian Peninsula Atlantic coastal dunes. It is a branched bush, that can reach up to 1 m, with white acidic edible berries (Portuguese white crowberries or “camarinhas” in Portuguese), 5–8 mm in diameter [1]. The genus Corema was included in the Ericaceae family in 1959 since traditionally it belonged to the Empetraceae family, which comprises two more genus—Empetrum and Ceratiola. The two species of Corema genus, C. conradii Torrey and C. album (L.) D. Don ex Steudel, can be found in Atlantic coastlines—C. conradii in the eastern coast of North America and C. album in the Iberian Peninsula and in the Azores islands (subsp. azoricum Pinto da Silva) [2].
C. album berries are known to be exceptional sources of nutrients and phytochemicals. Their dietary intake is highly recommended since it is associated with the prevention of chronic and degenerative diseases [3]. The nutraceutical potential of berries is due to their phytochemical composition. High levels of phenolic compounds have been identified, particularly phenolic acids (benzoic and caffeic acids being the most predominant), flavonols (especially quercetin 3-O-hexoside and rutin), anthocyanins (delphinidin 3-O-hexoside, cyanidin 3-O-glucoside, and cyanidin 3-O-pentoside), and tannins [4,5,6][4][5][6]. This confers them beneficial biological properties, namely antioxidant, antimicrobial, and anticancer activities, rendering them promising chemopreventive agents against anti-inflammatory and anti-neurodegenerative disorders, as well as cancer [7,8][7][8]. The berries have also been described as a valuable source of fibers, water, and sugars [9].
The human consumption of C. album berries dates back to ancient times, having been used in traditional medicine as antipyretic [10] and antiparasitic agents [11]. Since they are not yet approved as novel food products—having not entered into the regular market—these types of berries are not consumed by the general population [12]. However, they have been sold in Portuguese local markets, in the regions where Corema exists. Even though this plant has gained the attention of the scientific community in the last few years, the number of published studies, at the molecular level, concerning the biological potential of C. album berries and their nutritional value is still scarce, e.g., less than 10 papers, using Science Direct and Scopus digital databases by searching for specific keywords within the title (“Corema album”) and (“antioxidant”) in abstract. A thorough characterization of these berries is essential for understanding their activity, as well as to allow their safe consumption either as fresh fruits or processed in the form of juices, jams, or jellies. Martin et al. (2020) reported the first spectroscopic study of fresh C. album berries, assigning distinct vibrational fingerprints to the skin and the seeds that revealed the differences in their content in phenolic derivatives, unsaturated fatty acids, and waxy polymers [13].
Since the evaluation of the biological properties of the different parts of C. album berries, as well as their spectroscopic characterization, is still scarce, the present study aims at filling this gap, particularly for extracts from fresh pulp, seed residue, and seed oil. Actually, only two studies are found in the literature for C. album pulp and seed [4[4][14],14], with a small number of antioxidant activity tests, without any vibrational spectroscopic characterization, and with no separation between the seed residue and the seed oil. Solvent extraction with methanol was the method of choice since this combination is routinely used for phytochemicals extraction with good yields [15]. Currently, antioxidant activity was measured for isolated extracts from the pulp (also tested for antimicrobial activity), seed residue, and seed oil, and the results were related to the main chemical constituents determined by both Fourier transform infrared (FTIR) and Raman vibrational spectroscopies. Apart from probing its separated constituents, the establishment of a relationship between composition and health beneficial effects is innovative for this edible fruit.
2. Total Phenolic, Flavonoid, and Monomeric Anthocyanin Content
The fresh berries pulp (FBP) extract presents the lower phenolic, flavonoid, and anthocyanin contents when compared to the berries seed residue (BSR) and berries seed oil (BSO) extracts (Table 1). The results show that the seeds are much richer in phenolic and flavonoid compounds and that the reddish BSR extract has the highest total monomeric anthocyanin content (TMAC) value.
Table 1. Total phenolic content (TPC, mg GAE/g extract), total flavonoid content (TFC, mg QCE/g extract), and total monomeric anthocyanin content (TMAC, mg C3GE/g extract) of pulp and seed extracts of C. album berries.
| Extract | TPC | TFC | TMAC |
|---|---|---|---|
| FBP | 9.9 ± 0.1 c | 1.7 ± 0.4 c | 0.06 ± 0.02 b |
| BSR | 41.0 ± 0.5 a | 19.6 ± 0.7 b | 4.6 ± 0.8 a |
| BSO | 17.6 ± 2.1 b | 79.6 ± 2.3 a | 1.6 ± 0.8 b |
FBP, fresh berries pulp; BSR, berries seed residue; BSO, berries seed oil; GAE, gallic acid equivalents; QCE, quercetin equivalents; C3GE, cyanidin-3-glucoside equivalents. Values represent the mean ± standard deviation of three independent experiments. For the same column, different superscript letters indicate significant differences (Tukey’s post hoc test, p < 0.05).
3. Antioxidant Activity
The BSR extract presents a higher radical scavenging ability both against the DPPH radical and the ABTS radical cation (Table 2), followed by the FBP extract and by the BSO, which presents the lowest antioxidant activity. Noteworthy is the EC50 value for the BSR in the DPPH assay, which is in the same range as the EC50 calculated for BHT. Moreover, only the BSR extract presents the capability to inhibit lipid peroxidation, though to a lower extent than the standard antioxidant BHT.
Table 2. Free radical scavenging activity (DPPH and ABTS) and inhibition of lipid peroxidation of pulp and seed extracts of C. album berries presented as EC50 values (mg/mL).
| Extract/Standard | DPPH | ABTS | Lipid Peroxidation |
|---|
Metal chelating activity (EC50, mg/mL) and ferric (FRAP) and cupric (CUPRAC) reducing powers (mg TE/g extract) of pulp and seed extracts of C. album berries.
| Extract/Standard | Metal Chelating Activity | FRAP | CUPRAC | |||
|---|---|---|---|---|---|---|
| FBP | 3.1 ± 0.2 a | >5 | >5 | |||
| FBP | >5 | 12.0 ± 0.7 b | 24.7 ± 2.0 c | |||
| BSR | 0.15 ± 0.04 b | 1.09 ± 0.03 | 2.0 ± 0.2 | |||
| BSR | 4.2 ± 0.2 | 54.7 ± 4.9 a | 146.6 ± 5.9 a | BSO | >5 | >5 |
| BSO | >5 | 6.8 ± 1.1 | >5 | |||
| BHT | 0.10 ± 0.03 b | 0.17 ± 0.03 | 0.009 ± 0.005 |
FBP, fresh berries pulp; BSR, berries seed residue; BSO, berries seed oil. BHT—reference antioxidant. Values represent the mean ± standard deviation of three independent experiments. For the same column, different superscript letters indicate significant differences (Tukey’s post hoc test, p < 0.05).
In the β-carotene–linoleic acid bleaching assay, the BSR extract presents a higher inhibition of the β-carotene oxidation than the other extracts (Figure 1). Nevertheless, it is still considerably less active than BHT (EC50 = 0.005 ± 0.002 mg/mL). The BSO extract showed a higher level of β-carotene oxidation inhibition when compared to the FBP extract.
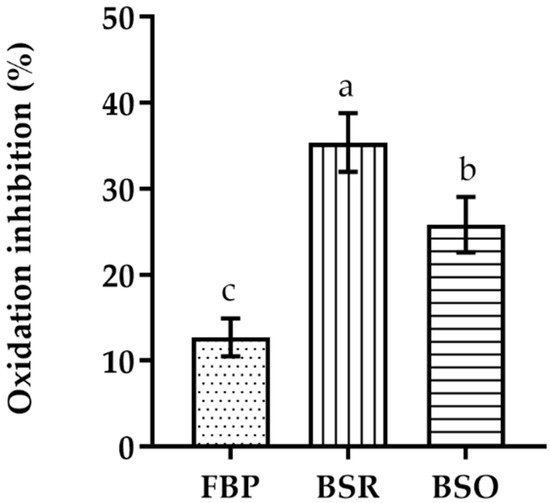

Figure 1. Linoleic acid/β-carotene bleaching inhibitory activity of extracts of different parts of C. album berries. FBP, fresh berries pulp (dotted); BSR, berries seed residue (vertical bar); BSO, berries seed oil (horizontal bar). Values represent the mean ± standard deviation of three independent experiments obtained after 2 h of reaction and for the highest concentration of each extract. Bars with different lowercase letters (a–c) indicate significant differences (Tukey’s post hoc test, p < 0.05).
Regarding the metal ion chelator ability, the results presented in Table 3 reflect the trend already observed for the BSR extract. It shows to be more potent than the other analyzed extracts regarding the ferric and cupric reducing powers, as well as the ability to chelate iron, though to a lower extent than the EDTA chelator. All the extracts are more effective in reducing copper than iron, with FRAP values ranging from 6.8 to 54.7 mg TE/g extract and CUPRAC values in the 24.7–146.6 mg TE/g extract range.
Table 3.
| b | |||
| 127.3 ± 2.4 | |||
| b | |||
| EDTA | 0.015 ± 0 | - | - |
FBP, fresh berries pulp; BSR, berries seed residue; BSO, berries seed oil; TE, Trolox equivalents. EDTA—reference chelating agent. Values represent the mean ± standard deviation of three independent experiments. For the same column, different superscript letters indicate significant differences (Tukey’s post hoc test, p < 0.05).
4. Discussion
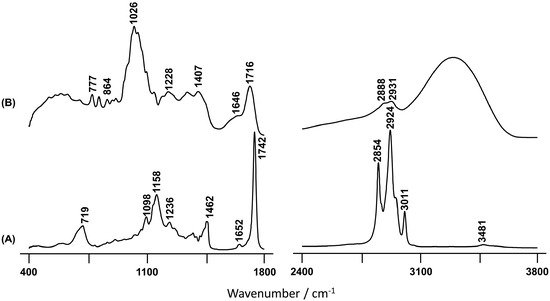
A plethora of methods are described and accepted for the determination of the antioxidant potential of plants, comprising the measurement of the phytochemical composition and different molecular reactions such as radical scavenging, metal chelation, and reducing power. For this study, several of these methodologies were chosen, ranging from the evaluation of free radical scavenging and lipid peroxidation inhibition to metal chelation/reduction potentials and enzymatic inhibitory activity [21][16]. The synthetic antioxidant BHT, commonly employed as a food preservative, was used as a model antioxidant for comparison purposes [22][17]. The berries show great complexity and diversity of phytochemicals, namely phenolic acids (in both their free, ester, and glycosidic forms), flavonoids, and tannins, among others [23][18]. Acetone extracts of C. album berries revealed the presence of this wide variety of compounds: 77.5% phenolic acids, 21.8% flavonoids, and 0.66% anthocyanins [5]. As for other berries of the genera Vaccinium, Sorbus, Empetrum, or Sambucus, the hydroxycinnamate chlorogenic acid is the main phenolic acid found in the C. album berries [5,24][5][19]. However, as the berries have a high proportion of seeds (54.9% of dry weight) with different compositions relative to the other parts of the fruit, the analysis of these separate extracts is particularly relevant. In fact, from the published studies on this type of berry, only two [4,14][4][14] focused on pulp and seeds, while all the others studied the fruit as a whole. In the present study, it was found that the seeds are richer in phenolic and flavonoid compounds, which contrasts with previous data obtained for methanolic extracts of the pulp and seed of freeze-dried white C. album berries [4]. However, the use of different matrices (fresh pulp in the present study versus freeze-dried pulp extracts) might explain these differences. Nevertheless, extraction of the seed components was still more efficient, though different extraction methods were applied, namely magnetic stirring (room temperature, 1 h) versus ultrasonic bath (40 °C, 1 h), both with methanol as the solvent [4]. Nonetheless, acetone/water extracts of dehydrated pulp and seeds confirmed the higher TPC of the seeds [14]. The major flavonoids compounds identified in Portuguese crowberry fruits are quercetin followed by rutin [5] that are compounds with great antioxidant activity, as evidenced by the Trolox equivalent antioxidant capacity (TEAC) of 4.72 and 2.4 mM, respectively [25][20]. In fact, flavonoids are phenolic plant metabolites that play an antioxidant effect, but recently there is evidence that the most abundant flavonoids present in the vegetable matrix have a dual behavior [26,27,28][21][22][23]. Indeed, these antioxidant compounds can act as a prooxidant, inducing oxidative stress under certain conditions such as the concentration of the antioxidant in the matrix, the presence of metal ions and its redox potential [29,30,31][24][25][26]. Specifically, low molecular weight phenolic molecules such as quercetin and gallic acid, which are easily oxidized, have a known pro-oxidant activity [32][27]. The results suggest that some flavonoid compounds present in the BSO extract, as well as their concentration, can induce a prooxidant behavior of the extract. Additionally, the predominant subclasses of flavonoids present in BSO extract can be flavone and flavanone since they have no -OH substitutions that are required for antioxidant activity [33][28]. The extracts were obtained from ripe C. album white berries, already described as presenting only small amounts of anthocyanins, which agrees with the lower TMAC content (Table 1) found in the FBP extract and the lower TPC when compared to other colored berries [5]. Nevertheless, the reddish BSR extract has the highest TMAC value, indicating that this part of the berry concentrates more anthocyanins, which may contribute to its higher TPC in comparison with the other extracts. Despite the low content in anthocyanins of this wild C. album berry, their large amount of total phenolic compounds and high antioxidant capacity are at a similar level to strawberry tree fruit and raspberries [34][29]. Among the three extracts tested, and in agreement with the higher content in total phenolics and anthocyanins, which would confer an enhanced antioxidant activity, the BSR extract presents a higher radical scavenging ability (Table 2) both against the DPPH and the ABTS free radicals. The presence of esters in the berry seeds, clearly detected by FTIR-ATR (Figure 3), may indicate that the hydroxycinnamate chlorogenic acid, which is an efficient antioxidant agent [35[30][31][32],36,37], is mainly concentrated in the seeds. The DPPH radical scavenging activity of the BSR extract (EC50 = 0.15 mg/mL) was found to surpass that obtained for Citrus sinensis seeds (in both hydroethanolic and ethanolic extracts, EC50 = 0.18 and 0.34 mg/mL, respectively) [38][33]. Although BSO displays the highest flavonoid content, it has the lowest antioxidant activity. However, the antioxidant activity of phenolic compounds such as flavonoids significantly depends on the structure and concentration of the molecules in the extract, since intermolecular interactions are prone to affect the redox profile of these antioxidants. Moreover, only the BSR extract presented the capacity to inhibit lipid peroxidation (Table 2), though to a much lower extent than the standard antioxidant BHT, which could be explained by the higher content in polyphenolic compounds in this extract. These results are comparable to those previously reported for black raspberry seed residues extracts (after oil extraction) with EC50 values ranging from 1.132 to 1.255 mg/mL [39][34]. Regarding the β-carotene–linoleic acid bleaching assay (Figure 1), as well as the metal chelating ability and reducing powers (Table 3), the BSR extract presented the highest inhibition of oxidation of β-carotene coupled to a stronger ability to chelate and reduce metals. This is in accordance with the results currently obtained in the other antioxidant tests. Actually, the phenolic compounds in this extract are suggested to be mainly molecules such as hydroxyflavones (e.g., quercetin and rutin) which have the ability to alter their redox potential, thus becoming very effective chelating agents to potentially oxidative metal ions (such as Fe3+ and Cu2+) [25,40][20][35]. Therefore, this type of phenolics will exert their antioxidant activity in a twofold manner. This effect is even more noteworthy in the β-carotene–linoleic acid bleaching assay since this extract was tested at a concentration of 4 mg/mL while the other extracts were used at 5 mg/mL. It is interesting to note that the BSO extract was much more efficient in reducing copper than the FBP extract, which contrasts with their behavior regarding the ferric reducing power. Actually, the BSO extract only presents relevant activities in the CUPRAC and β-carotene–linoleic acid bleaching assays, being almost as potent as the BSR extract. Natural products have been the source of many new drugs (namely against cancer and neurodegenerative disorders), over 119 natural molecules have been shown to be efficient acetylcholinesterase inhibitors, one of the currently available treatment options for Alzheimer’s disease [41][36]. This prompted the evaluation of the inhibitory activity of the C. album extracts against the enzyme AChE, though no significant effect was observed for any of the samples tested. Vibrational spectroscopy techniques have been recognized as powerful tools for identifying functional groups in diverse types of samples, including heterogeneous biological matrices, from a qualitative and semi-quantitative point of view. Thus, it has been largely employed in plant science in the last decades, specifically for the study of edible oils such as olive oil, or unsaturated fatty acids [42,43,44,45,46,47,48][37][38][39][40][41][42][43]. Additionally, these methods have also been applied routinely to the analysis of phenolic compounds [49,50,51][44][45][46]. While it is true that HPLC combined with mass spectrometry is a suitable and very commonly used technique for identifying the main constituents of these types of plant extracts, vibrational spectroscopy (e.g., FTIR and Raman) is able to provide highly accurate chemical data, with unmatched sensitivity and specificity, in a fast and completely non-destructive way, while requiring minimal amounts of extract and no sample preparation. Hence, FTIR is currently a method of choice for evaluating the chemical composition (major constituents) of several types of biological extracts, used routinely in the food industry. The FTIR-ATR spectrum obtained for the BSO extract from the C. album (Figure 2A) presents one prominent band observed at 3011 cm−1, which was assigned to olefinic cis-unsaturation in fatty acids that are known to be present in this kind of edible fruit samples [46,52][41][47]. Thus, this intense band suggests a high content of unsaturated fatty acids in BSO, which is also supported by the observation of a signal at 1652 cm−1 from the C=C stretching mode of cis fatty acids [46][41]. On the other hand, the feature at 1742 cm−1, characteristic of esterified fatty acids [53][48], reveals a considerable amount of esters attending to the quite large shift relative to ν(C=O)acid [54][49]. The Raman spectrum of the BSO is very similar to that of the pure seed previously reported [13], thus showing that the main seed components are those present in the oil. In the light of these results, it is possible to conclude that the efficiency of the extraction procedure currently performed was quantitative, constituting an interesting methodology for future studies of edible fruits.
Figure 2. FTIR-ATR spectra of the BSO (A) and FBP (B) C. album berry extracts.
Regarding the infrared spectrum of the FBP extract (Figure 2B), the results reflect a high content of hydroxylic components, namely phenolic acids. This is clearly evidenced by the broad and intense band centered at 3303 cm−1 and ascribed to the stretching mode from the ring hydroxyls. Additionally, the signal at 777 cm−1 is characteristic of out-of-plane (C-C-H) deformation modes of the aromatic ring [49][44]. This type of compound seems to be absent in the oil extract, as evidenced by the total absence of vibrational bands in the high wavenumber spectral region (Figure 2A). It should be highlighted that the spectrum of the seeds revealed the presence of a significant amount of hydroxylic compounds, as well as water [13], which were most likely removed during the oil extraction process. The broad infrared signal at 1026 cm−1, which is absent in BSO, is due to the ν(C-O) and ν(C-C) modes from polysaccharides and pectins, that are widely present in these types of samples [55,56,57,58][50][51][52][53]. As expected, it is more likely to find sugars in the fresh pulp in comparison with the oleaginous seed part, which is clearly evidenced when BSO and FBP spectra are compared (Figure 2).
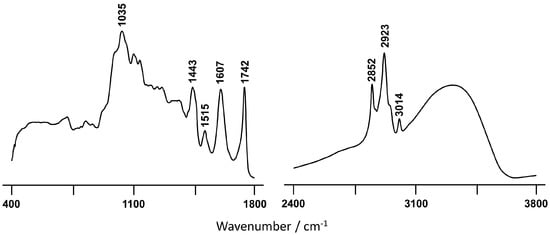
The BSR fraction still contains a significant amount of esterified fatty acids, as evidenced by the infrared spectral features at 3014 and 1742 cm−1 (Figure 3). However, probably the most remarkable characteristic, in contrast to BSO (Figure 2A) is that the infrared profile of BSR shows several features indicative of significant amounts of phenolic compounds, namely three very distinctive bands at 1443, 1515 cm−1, and 1607 cm−1. The first two are ascribed to ring ν(C-C) conjugated with (C=C), while the last one is assigned to ν(C-C)aromatic [59,60,61][54][55][56]. Furthermore, the presence of more carotenoids in BSR relative to the other extracts, detected by Raman (Figure S1), may contribute to the high antioxidant activity currently determined for this sample. Although Raman spectroscopy does not allow an accurate carotenoid quantification in the samples, it does enable us to determine, in a semi-quantitative manner, in which sample this polyene is present on the highest content. This was carried out by comparing the peak ratio I1528/I1448 [13], which reaches a value higher than 1 for BSR, being below unity in the case of BSO. These observations are in good accordance with the higher antioxidant potential determined for the BSR extract relative to the one obtained for BSO.

Figure 3. FTIR-ATR spectrum of the BSR C. album berry extract.
In the present study, the spectroscopic characterization of the extracts by FTIR-ATR and Raman allowed the detection of fatty acids in the BSO and BSR, and of phenolic compounds in FBP and BSR. The FBP extract was also found to contain sugars, triterpenoids, and polysaccharides. In addition, the presence of glycosidic linkages may also indicate that most of the phenolic acids are conjugated to sugar moieties. In fact, these chemical characteristics evidence the complexity of the sugar polymers present in the sample [62,63][57][58].
Finally, it may be interesting to compare the presently obtained results with those from studies previously performed by other authors on C. album. In particular, León-González et al. [7] analyzed the phenolic content of the berries using different extraction methodologies. A large number of phenolic acids was identified by these authors (by HPLC and MS), in some cases reaching ca. 2260 mg per kg of extract [5]. Despite the fact that the solvents were different from the ones used in the current study, the main extracted compounds were found to be the same.
Since C. album has been used by some ancient civilizations to eliminate intestinal worms [1], the present study aimed to evaluate the antimicrobial activity of the FBP sample, since certain antibiotics are also used to treat intestinal parasites (e.g., metronidazole) [64][59]. Comparison of the current results with the few studies in the literature describing the evaluation of antimicrobial activity for plant extracts of the same taxonomic class allows us to conclude that the FBP extract of C. album displays promising antibacterial activity: a MIC = 17 mg/mL for extracts of Lythrum salicaria against Pseudomonas aeruginosa [65][60] relative to a MIC = 12.5 mg/mL (Table 4), and the lowest activity of Tamarix gallica extracts observed against Escherichia coli using disk diffusion method [66][61], relative to a MIC = 6.25 mg/mL (Table 4), which contrasts with the lack of antibacterial activity obtained for acetone/water extracts of C. album pulp testing lower concentrations and using the disk diffusion method [14]. The inhibitory effect mechanism of FBP against the several strains currently tested is not known, however, based on some known compounds that may be present namely hydroxycinnamic acids, vanillic acid, and quercetin [5], could be related to their antioxidant mechanisms. Studies on the antimicrobial activity of these phenolic compounds and isolated flavonoids, showed significant antimicrobial activity against E. coli, P. aeruginosa, K. pneumoniae, and S. aureus, among others [67][62]. The recognized inhibitory effect of phenolic compounds towards bacterial growth can be explained by their ability to increase the cell membrane permeability, this effect varying for different bacterial strains due to differences in their structure and lipophilic character [68][63]. It may also be due to their ability to adsorb to cell membranes, interact with enzymes and other biological substrates, sequester metal ions that, in general, affect the normal cellular function [67][62]. In future work, it will be relevant to understand which components within the FBP extract are responsible for the measured antimicrobial effect and how they can lead to cellular damage in different types of microorganisms.
Table 4. Minimum inhibitory activity (MIC) of the FBP extract against bacterial strains.
| Bacterial Strains | MIC of the FBP Extract (mg/mL) |
|---|---|
| Escherichia coli ATCC 8739 | 6.25 |
| Staphylococcus aureus ATCC 29213 | 12.5 |
| Pseudomonas aeruginosa | 12.5 |
| Klebsiella oxytoca | 25 |
| Enterococcus faecalis | 3.125 |
| Escherichia coli ESβL | 50 |
| Methicillin-resistant Staphylococcus aureus | 12.5 |
| Klebsiella pneumoniae KPC | 6.25 |
References
- De Oliveira, P.B.; Dale, A. Corema album (L.) D. Don, the white crowberry-a new crop. J. Berry Res. 2012, 2, 123–133.
- Martínez-Varea, C.M.; Ferrer-Gallego, P.P.; Dolores, M.; Badal, E.; Ferrando-Pardo, I.; Laguna, E.; Real, C.; Roman, D.; Villaverde, V. Corema album archaeobotanical remains in western Mediterranean basin. Assessing fruit consumption during Upper Palaeolithic in Cova de les Cendres (Alicante, Spain). Quat. Sci. Rev. 2019, 207, 1–12.
- Mazzoni, L.; Scalzo, J.; Di Vittori, L.; Mezzetti, B.; Bottino, M. Berries. In Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd ed.; Yahia, E.M., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; Volume 2, pp. 883–908.
- Andrade, S.C.; Guine, R.P.F.; Goncalves, F.J.A. Evaluation of phenolic compounds, antioxidant activity and bioaccessibility in white crowberry (Corema album). J. Food Meas. Charact. 2017, 11, 1936–1946.
- León-González, A.J.; Truchado, P.; Tomás-Barberán, F.A.; López-Lázaro, M.; Barradas, M.C.D.; Martín-Cordero, C. Phenolic acids, flavonols and anthocyanins in Corema album (L.) D. Don berries. J. Food Compost. Anal. 2013, 29, 58–63.
- Pimpão, R.C.; Dew, T.; Oliveira, P.B.; Williamson, G.; Ferreira, R.B.; Santos, C.N. Analysis of phenolic compounds in portuguese wild and commercial berries after multienzyme hydrolysis. J. Agric. Food Chem. 2013, 61, 4053–4062.
- León-González, A.J.; Mateos, R.; Ramos, S.; Martín, M.Á.; Sarriá, B.; Martín-Cordero, C.; López-Lázaro, M.; Bravo, L.; Goya, L. Chemo-protective activity and characterization of phenolic extracts from Corema album. Food Res. Int. 2012, 49, 728–738.
- Minatel, I.O.; Borges, C.V.; Ferreira, M.I.; Gomez, H.A.G.; Chen, C.-Y.O.; Lima, G.P.P. Phenolic Compounds: Functional Properties, Impact of Processing and Bioavailability. In Phenolic Compounds—Biological Activity; Soto-Hernández, M., Palma-Tenango, M., García-Mateos, R., Eds.; IntechOpen: London, UK, 2017.
- Andrade, S.C.; Gonçalves, F.; Guiné, R.P.F. Contribution for the physical-chemical characterization of Portuguese Crowberry (Corema album). Int. J. Food Sci. Nutr. 2017, 2, 9–14.
- Johnson, T. CRC Ethnobotany Desk Reference; CRC Press: Boca Raton, FL, USA, 1998.
- López-Dóriga, I. The Archaeobotany and Ethnobotany of Portuguese or White Crowberry (Corema album (L.) D. Don). Ethnobiol. Lett. 2018, 9, 19–32.
- Moreira da Silva, A.; Barroca, M.J.; Guiné, R.P.F. Knowledge and Consumption Habits Related with White Crowberries (Corema album L.). Appl. Sci. 2021, 11, 5463.
- Martin, D.; Marques, J.; Amado, A.M.; Barroca, M.J.; Moreira da Silva, A.; Batista de Carvalho, L.A.E.; Marques, M.P.M. Shedding light into the health-beneficial properties of Corema album—A vibrational spectroscopy study. J. Raman Spectrosc. 2020, 51, 313–322.
- Brito, C.; Bertotti, T.; Primitivo, M.J.; Neves, M.; Pires, C.L.; Cruz, P.F.; Martins, P.A.T.; Rodrigues, A.C.; Moreno, M.J.; Brito, R.M.M.; et al. Corema album spp.: Edible wild crowberries with a high content in minerals and organic acids. Food Chem. 2021, 345, 128732.
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20.
- Zhong, Y.; Shahidi, F. Methods for the assessment of antioxidant activity in foods. In Handbook of Antioxidants for Food Preservation; Shahidi, F., Ed.; Woodhead Publishing: England, UK, 2015; pp. 287–333.
- Yehye, W.A.; Rahman, N.A.; Ariffin, A.; Abd Hamid, S.B.; Alhadi, A.A.; Kadir, F.A.; Yaeghoobi, M. Understanding the chemistry behind the antioxidant activities of butylated hydroxytoluene (BHT): A review. Eur. J. Med. Chem. 2015, 101, 295–312.
- Paredes-López, O.; Cervantes-Ceja, M.L.; Vigna-Pérez, M.; Hernández-Pérez, T. Berries: Improving Human Health and Healthy Aging, and Promoting Quality Life—A Review. Plant Foods Hum. Nutr. 2010, 65, 299–308.
- Määttä-Riihinen, K.R.; Kamal-Eldin, A.; Mattila, P.H.; González-Paramás, A.M.; Törrönen, A.R. Distribution and contents of phenolic compounds in eighteen Scandinavian berry species. J. Agric. Food Chem. 2004, 52, 4477–4486.
- Vázquez-Flores, L.F.; Casas-Grajales, S.; Hernández-Aquino, E.; Vargas-Pozada, E.E.; Muriel, P. Chapter 47—Antioxidant, Antiinflammatory, and Antifibrotic Properties of Quercetin in the Liver. In Liver Pathophysiology; Muriel, P., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 653–674.
- Yordi, E.G.; Pérez, E.M.; Matos, M.J.; Villares, E.U. Antioxidant and Pro-Oxidant Effects of Polyphenolic Compounds and Structure-Activity Relationship Evidence. In Nutrition, Well-Being and Health; Bouayed, J., Bohn, T., Eds.; IntechOpen: London, UK, 2012; pp. 23–48.
- Salehi, B.; Azzini, E.; Zucca, P.; Maria Varoni, E.; Anil Kumar, N.V.; Dini, L.; Panzarini, E.; Rajkovic, J.; Valere Tsouh Fokou, P.; Peluso, I.; et al. Plant-Derived Bioactives and Oxidative Stress-Related Disorders: A Key Trend towards Healthy Aging and Longevity Promotion. Appl. Sci. 2020, 10, 947.
- Jomová, K.; Hudecova, L.; Lauro, P.; Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Valko, M. A Switch between Antioxidant and Prooxidant Properties of the Phenolic Compounds Myricetin, Morin, 3′,4′-Dihydroxyflavone, Taxifolin and 4-Hydroxy-Coumarin in the Presence of Copper(II) Ions: A Spectroscopic, Absorption Titration and DNA Damage Study. Molecules 2019, 24, 4335.
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25.
- Sotler, R.; Poljsak, B.; Dahmane, R.; Jukic, T.; Pavan Jukic, D.; Rotim, C.; Trebse, P.; Starc, A. Prooxidant Activities of Antioxidants and Their Impact on Health. Acta Clin. Croat. 2019, 58, 726–736.
- Chobot, V.; Hadacek, F. Exploration of pro-oxidant and antioxidant activities of the flavonoid myricetin. Redox Rep. 2011, 16, 242–247.
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352.
- Ahmad, M.S.; Fazal, F.; Rahman, A.; Hadi, S.M.; Parish, J.H. Activities of flavonoids for the cleavage of DNA in the presence of Cu(II): Correlation with generation of active oxygen species. Carcinogenesis 1992, 13, 605–608.
- Pimpão, R.C. Exploring the Bioavailability of Polyphenols from Berries and Their Potential Activities in Humans. Ph.D. Thesis, Universidade Nova de Lisboa, Lisbon, Portugal, 2014.
- Razzaghi-Asl, N.; Garrido, J.; Khazraei, H.; Borges, F.; Firuzi, O. Antioxidant properties of hydroxycinnamic acids: A review of structure-activity relationships. Curr. Med. Chem. 2013, 20, 4436–4450.
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138.
- Zhao, Z.; Moghadasian, M.H. Bioavailability of hydroxycinnamates: A brief review of in vivo and in vitro studies. Phytochem. Rev. 2010, 9, 133–145.
- Oikeh, E.I.; Oriakhi, K.; Omoregie, E.S. Phenolic Content and in vitro Antioxidant Activities of Sweet Orange (Citrus sinensis L.) Fruit Wastes. Arch. Basic Appl. Med. 2014, 2, 119–126.
- Choi, M.H.; Shim, S.M.; Kim, G.H. Protective effect of black raspberry seed containing anthocyanins against oxidative damage to DNA, protein, and lipid. J. Food Sci. Technol. 2016, 53, 1214–1221.
- Kessler, M.; Ubeaud, G.; Jung, L. Anti- and pro-oxidant activity of rutin and quercetin derivatives. J. Pharm. Pharmacol. 2003, 55, 131–142.
- Braidy, N.; Poljak, A.; Jayasena, T.; Sachdev, P. Natural Plant-Derived Acetylcholinesterase Inhibitors: Relevance for Alzheimer’s Disease. In Natural Products Targeting Clinically Relevant Enzymes, 1st ed.; Andrade, P.B., Valentão, P., Pereira, D.M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; pp. 297–318.
- van de Voort, F.R.; Ismail, A.A.; Sedman, J.; Emo, G. Monitoring the oxidation of edible oils by Fourier transform infrared spectroscopy. J. Am. Oil Chem. Soc. 1994, 71, 243–253.
- Nzai, J.M.; Proctor, A. Determination of phospholipids in vegetable oil by Fourier transform infrared spectroscopy. J. Am. Oil Chem. Soc. 1998, 75, 1281–1289.
- Che Man, Y.B.; Setiowaty, G.; van de Voort, F.R. Determination of iodine value of palm oil by fourier transform infrared spectroscopy. J. Am. Oil Chem. Soc. 1999, 76, 693–699.
- Heise, H.M.; Damm, U.; Lampen, P.; Davies, A.N.; McIntyre, P.S. Spectral variable selection for partial least squares calibration applied to authentication and quantification of extra virgin olive oils using Fourier transform Raman spectroscopy. Appl. Spectrosc. 2005, 59, 1286–1294.
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25.
- Machado, N.F.L.; Batista de Carvalho, L.A.E.B.; Otero, J.C.; Marques, M.P.M. The autooxidation process in linoleic acid screened by Raman spectroscopy. J. Raman Spectrosc. 2012, 43, 1991–2000.
- Senesi, R.; Andreani, C.; Baglioni, P.; de Carvalho, L.A.E.B.; Licoccia, S.; Marques, M.P.M.; Moretti, G.; Noce, A.; Paolesse, R.; Parker, S.F.; et al. Looking for Minor Phenolic Compounds in Extra Virgin Olive Oils Using Neutron and Raman Spectroscopies. Antioxidants 2021, 10, 643.
- Foo, L.Y. Proanthocyanidins: Gross chemical structures by infrared spectra. Phytochemistry 1981, 20, 1397–1402.
- Ramirez, F.J.; Luque, P.; Heredia, A.; Bukovac, M.J. Fourier transform IR study of enzymatically isolated tomato fruit cuticular membrane. Biopolymers 1992, 32, 1425–1429.
- España, L.; Heredia-Guerrero, J.A.; Segado, P.; Benítez, J.J.; Heredia, A.; Domínguez, E. Biomechanical properties of the tomato (Solanum lycopersicum) fruit cuticle during development are modulated by changes in the relative amounts of its components. New Phytol. 2014, 202, 790–802.
- Yoshida, S.; Yoshida, H. Nondestructive analyses of unsaturated fatty acid species in dietary oils by attenuated total reflectance with Fourier transform IR spectroscopy. Biopolymers 2003, 70, 604–613.
- Martin, D.; Lopes, T.; Correia, S.; Canhoto, J.; Marques, M.P.M.; Batista de Carvalho, L.A.E. Nutraceutical properties of tamarillo fruits: A vibrational study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 252, 119501.
- Da Silva, C.E.; Vandenabeele, P.; Edwards, H.G.M.; Cappa De Oliveira, L.F. NIR-FT-Raman spectroscopic analytical characterization of the fruits, seeds, and phytotherapeutic oils from rosehips. Anal. Bioanal. Chem. 2008, 392, 1489–1496.
- Lupoi, J.S.; Singh, S.; Parthasarathi, R.; Simmons, B.A.; Henry, R.J. Recent innovations in analytical methods for the qualitative and quantitative assessment of lignin. Renew. Sustain. Energy Rev. 2015, 49, 871–906.
- Nogales-Bueno, J.; Baca-Bocanegra, B.; Rooney, A.; Hernández-Hierro, J.M.; Byrne, H.J.; Heredia, F.J. Study of phenolic extractability in grape seeds by means of ATR-FTIR and Raman spectroscopy. Food Chem. 2017, 232, 602–609.
- Szymanska-Chargot, M.; Zdunek, A. Use of FT-IR Spectra and PCA to the Bulk Characterization of Cell Wall Residues of Fruits and Vegetables Along a Fraction Process. Food Biophys. 2013, 8, 29–42.
- Wilson, R.H.; Smith, A.C.; Kačuráková, M.; Saunders, P.K.; Wellner, N.; Waldron, K.W. The Mechanical Properties and Molecular Dynamics of Plant Cell Wall Polysaccharides Studied by Fourier-Transform Infrared Spectroscopy. Plant Physiol. 2000, 124, 397–406.
- Abbas, O.; Compère, G.; Larondelle, Y.; Pompeu, D.; Rogez, H.; Baeten, V. Phenolic compound explorer: A mid-infrared spectroscopy database. Vib. Spectrosc. 2017, 92, 111–118.
- Amado, A.M.; Azevedo, C.; Ribeiro-Claro, P.J.A. Conformational and vibrational reassessment of solid paracetamol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 183, 431–438.
- Heredia-Guerrero, J.A.; Benítez, J.J.; Domínguez, E.; Bayer, I.S.; Cingolani, R.; Athanassiou, A.; Heredia, A. Infrared and Raman spectroscopic features of plant cuticles: A review. Front. Plant Sci. 2014, 5, 305.
- Mutter, S.T.; Blanch, E.W. Carbohydrate Secondary and Tertiary Structure Using Raman Spectroscopy. In Polysaccharides: Bioactivity and Biotechnology; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1181–1218.
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and infrared spectroscopy of carbohydrates: A review. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 185, 317–335.
- Samuelson, J. Why metronidazole is active against both bacteria and parasites. Antimicrob. Agents Chemother. 1999, 43, 1533–1541.
- Guclu, E.; Genc, H.; Zengin, M.; Karabay, O. Antibacterial Activity of Lythrum salicaria against Multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Annu. Res. Rev. Biol. 2014, 4, 1099–1105.
- Ksouri, R.; Falleh, H.; Megdiche, W.; Trabelsi, N.; Mhamdi, B.; Chaieb, K.; Bakrouf, A.; Magné, C.; Abdelly, C. Antioxidant and antimicrobial activities of the edible medicinal halophyte Tamarix gallica L. and related polyphenolic constituents. Food Chem. Toxicol. 2009, 47, 2083–2091.
- Vaquero, M.J.R.; Alberto, M.R.; de Nadra, M.C.M. Antibacterial effect of phenolic compounds from different wines. Food Control 2007, 18, 93–101.
- Campos, F.M.; Couto, J.A.; Figueiredo, A.R.; Tóth, I.V.; Rangel, A.O.; Hogg, T.A. Cell membrane damage induced by phenolic acids on wine lactic acid bacteria. Int. J. Food Microbiol. 2009, 135, 144–151.
More
