Thyroid diseases, including neoplasms, autoimmune diseases and thyroid dysfunctions, are becoming a serious social problem with rapidly increasing prevalence. The latter is increasingly linked to oxidative stress. There are many methods for determining the biomarkers of oxidative stress, making it possible to evaluate the oxidative profile in patients with thyroid diseases compared to the healthy population. This opens up a new perspective for investigating the role of elevated parameters of oxidative stress and damage in people with thyroid diseases, especially of neoplastic nature. An imbalance between oxidants and antioxidants is observed at different stages and in different types of thyroid diseases. The organ, which is part of the endocrine system, uses free radicals (reactive oxygen species, ROS) to produce hormones. Thyroid cells release enzymes that catalyse ROS generation; therefore, a key role is played by the internal defence system and non-enzymatic antioxidants that counteract excess ROS not utilised to produce thyroid hormones, acting as a buffer to neutralise free radicals and ensure whole-body homeostasis. An excess of free radicals causes structural cell damage, undermining genomic stability. Looking at the negative effects of ROS accumulation, oxidative stress appears to be implicated in both the initiation and progression of carcinogenesis.
1. Introduction
Reactive oxygen species (ROS) are molecules capable of independent existence, which contain an oxygen atom and unpaired electrons
[1]. ROS arise mainly as by-products in a series of bioenergetic processes of ATP synthesis in mitochondrial respiratory chains
[2][3][2,3]. Inflammatory processes are an additional source of ROS
[1][4][1,4]. The most common reactive oxygen species include radicals derived from the electron reduction of molecular oxygen–superoxide anion (O
2•−), hydrogen peroxide (H
2O
2) and the more reactive hydroxyl radical (HO
•), released in reactions involving metal ions
[5].
Obrona antyoksydacyjna organizmu przed negatywnymi skutkami ROS działa na wielu różnych platformach. Polega na zapobieganiu powstawaniu rodników, ich wymiataniu i naprawie uszkodzeń wywołanych przez ROS. Wiodącą rolę w systemie obronnym organizmu odgrywają enzymy antyoksydacyjne, rozkładające cząsteczki ROS i tym samym chroniące komórki przed nadmierną ekspozycją na ROS [ 6 , 7 , 8 ]. System naprawy uszkodzeń wywołanych przez ROS częściowo opiera się na procesach autofagii i apoptozy, eliminując uszkodzone komórki [ 9 , 10 , 11]. Pomimo szeregu wewnętrznych mechanizmów regulacji enzymatycznej, system obrony antyoksydacyjnej powinien być również wspierany przez mechanizmy nieenzymatyczne. Te ostatnie obejmują działanie cząsteczek o silnych właściwościach antyoksydacyjnych, w tym przede wszystkim glutationu, koenzymu Q10, a także substancji egzogennych – związków polifenolowych, kwasu askorbinowego, retinolu, β-karotenu i tokoferolu. Egzogennych substancji o potwierdzonych właściwościach przeciwutleniających wzmocnienia obrony antyoksydacyjnej ciała zwiększa całkowitą zdolność przeciwutleniającą [ 7 , 12 , 13 , 14 ].
Oxidative stress is an effect of redox imbalance between reactive oxygen species and antioxidant defence
[6][7][9,15]. It may be caused both by the excessive production of ROS and by an inefficient antioxidant system, resulting in molecular damage
[8][16]. Additionally, ROS generation in different subcellular compartments likely involves a positive feedback mechanism, creating a vicious circle of pathological conditions related to oxidative stress
[9][10][11][17,18,19]. Redox homeostasis requires an equilibrium of ROS production and scavenging
[12][20]. Even though the concept of oxidative stress was introduced in the 1980s, its definition and scope of research have been continually elaborated and expanded
[13][6].
Thyroid diseases are a common health problem worldwide, especially among women. The occurrence of subclinical thyroid disorders, which often remain undiagnosed, is also significant
[14][15][16][17][21,22,23,24]. Thyroid diseases are increasingly linked to oxidative stress
[18][19][20][21][25,26,27,28]. It has been shown that thyroid dysfunction can co-occur with metabolic disorders, including obesity
[22][23][24][29,30,31]. Obesity is a metabolic disease involving mitochondrial dysfunction and chronic oxidative stress, as in several metabolic disorders
[25][26][27][28][29][30][31][32,33,34,35,36,37,38]. Since the incidence of thyroid diseases is increased in individuals with increased body weight, the related substrate of metabolic disorders and thyroid dysfunction seems relevant
[23][24][32][30,31,39]. However, current reports do not distinguish between the causes and consequences of metabolic abnormalities, so there is a need to develop research on the pathogenesis of thyroid disorders.
2. Physiological Redox Signalling and the Role of ROS in Thyroid Function
Signalling functions in immune responses are initiated when molecular oxygen is oxidised to the reactive superoxide anion radical by the NADPH oxidase (NOX) complex, itself an additional source of ROS
[4]. Subsequently, the superoxide is converted by superoxide dismutase (SOD) to H
2O
2. Hydrogen peroxide is associated with a signalling function regulating cellular processes, due to its capacity to reversibly modify cysteine residues
[12][20]. The process alters redox signalling
[9][17]. Accumulation of excessive concentrations of H
2O
2 activates thiolate anion (Cys-S-) oxidation pathways. This is an irreversible process, resulting in permanent protein damage
[33][40]. Antioxidant systems serve a protective function, preventing intracellular accumulation of ROS by reversing the modification of cysteine residues
[12][20].
The role (physiological or pathological) played by ROS depends largely on their concentration and the conditions accompanying biochemical transformations. The initial concentration dictates downstream responses
[34][7]. Excessive amounts of ROS at the subcellular level activates pathways leading to damage in particularly susceptible cell structures or apoptosis
[33][40]. In turn, at low physiological levels, ROS play a signalling role, essential for normal cellular processes
[35][36][8,41]. Reactive oxygen species also serve as intracellular mediators produced in phagocytic cells, controlling the inflammatory response and antimicrobial defence
[4].
ROS play an important role in normal thyroid function. Thyroid cells release oxidases, which catalyse ROS production
[37][38][39][42,43,44]. Inositols are also involved in thyroid hormone synthesis and normal thyroid function, activating a cascade of processes including regulating TSH-dependent signalling (as a TSH transmitter) and generating H
2O
2 production used for iodination and coupling of iodotyrosine and iodothyronine
[40][41][42][43][45,46,47,48]. Inositol deficiency or impairment of inositol cascades may result in insufficient synthesis of thyroid hormones, leading to hypothyroidism, which may be further compounded by an increased need for inositols in response to high TSH levels
[40][43][45,48]. Myoinositol supplementation in hypothyroid patients effectively lowers TSH levels. Its effect has been demonstrated in combination with metformin and selenium compared to treatment without inositol
[44][45][49,50].
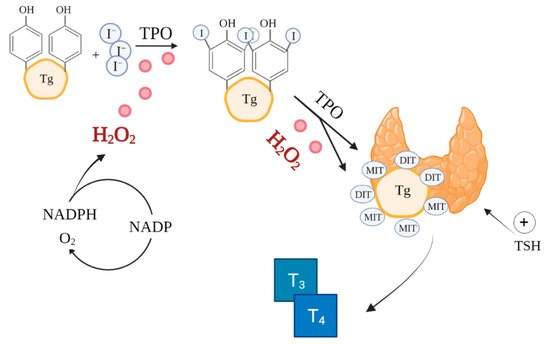
The synthesis of thyroxine (T4) and triiodothyronine (T3) catalysed by thyroid peroxidase (TPO) in thyroid follicles is a very complex process involving ROS, notably, H
2O
2 (
Figure 1)
[46][51]. ROS are already essential in the initial stages of thyroid hormone production, during iodide oxidation
[47][52]. Additionally, thyroid hormones perform a metabolic regulatory function by affecting mitochondrial activity
[48][53]. Because of the reliance on ROS in its function, the thyroid is particularly exposed to oxidative damage
[49][54]. Therefore, the antioxidant defence system of the thyroid must effectively regulate ROS production and scavenging
[19][50][26,55].
Figure 1. Role of ROS in thyroid hormones synthesis. Based on
[42][51][47,56]. Created with BioRender.com.(accessed on 26/08/2021) I—iodine, TPO—thyroid peroxidase, Tg—thyroglobulin, MIT—monoiodotyrosine, DIT—diiodotyrosine, T
3—triiodothyronine, T
4—thyroxine.
3. Biomarkers of Oxidative Stress in Thyroid Diseases
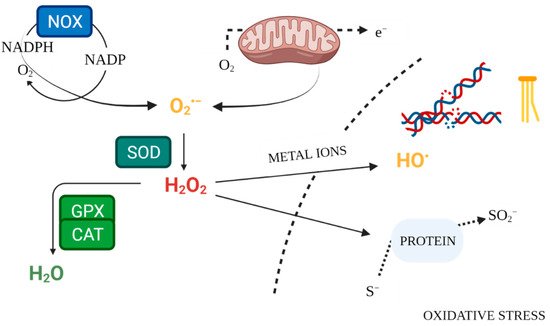
Enzymatic mechanisms of antioxidant defence constitute the internal system for maintaining ROS homeostasis (
Figure 2). Superoxide dismutases (SOD1, SOD2, SOD3) are antioxidant enzymes, neutralising O
2•− [9][52][17,57]. The key enzyme responsible for neutralising hydrogen peroxide is catalase (CAT), which converts it to water and oxygen
[53][58]. Likewise, glutathione peroxidase (GPX) scavenges and detoxifies H
2O
2 [12][20]. Glutathione serves as an intracellular buffer against oxidation. In response to excessive ROS release, it forms an oxidised dimer structure by bridging two glutathione molecules. Glutathione reductase (GR) then restores the reduced form of glutathione, lowering its reactivity
[54][59]. Measurement of antioxidant enzyme activity in serum makes it possible to evaluate the condition of the antioxidant defence system. Lower levels of this activity, compared to the control, may be a sign of inadequate defence against free radicals
[55][60].
Figure 2. Free Radical Physiology. Created with
BioRender.com. (accessed on 26 July 2021).
Biomarkers of oxidative stress also include prooxidant enzymes—NADPH oxidases (NOX), which are an endogenous source of ROS, especially in thyroid tissue
[41][46]. Their increased activity is associated with elevated concentrations of reactive oxygen species in pathological conditions. Direct measurement of ROS concentrations may be a helpful marker in the evaluation of medical conditions, yet its utility may be limited given the short half-life of these molecules
[7][10][15,18].
Malondialdehyde (MDA) is a product of lipid peroxidation by ROS. The marker can be used to evaluate oxidative damage and measure whole-body or tissue-specific oxidative stress
[56][57][61,62]. Advanced glycation end products (AGE) are believed to be associated with the onset and progression of metabolic disorders, notably diabetes and obesity, due to their formation both through lipid peroxidation and glycoxidation reactions; that is, in response to an increased intake of simple carbohydrates
[7][58][15,63]. Elevated levels are observed in ROS-damaged tissues, as the final product of peroxidation, making them markers of oxidative stress in the body
[59][64]. Among DNA bases, guanine is the most easily oxidised, due to its relatively low redox potential. Its oxidised form (8-oxo-2′-deoxyguanosine) may therefore serve as a measurement of DNA damage in cells exposed to oxidative stress and in carcinogenesis. 8-oxo-2′-deoxyguanosine has mutagenic potential
[6][60][9,65].
Total antioxidant capacity (TAC) is a parameter indicative of the body’s overall ability to neutralise oxidants. It takes into account all the antioxidants contained in bodily fluids, including exogenous and endogenous compounds
[7][15]. In turn, total oxidant status (TOS) is based on the oxidation of ferrous ion to ferric ion in the presence of various oxidants. It reflects the oxidation state of bodily fluids, represented by the level of radicals
[61][66]. Oxidative stress index (OSI) is a measure of oxidative stress, calculated as the ratio of total oxidant status to total antioxidant status and therefore represents the overall oxidation state of the body
[62][67].
All the biomarkers employed in the determination of the role of oxidative stress in thyroid diseases in this review are listed in
Table 1.