An important direction in the design of tetrapyrrole macrocyclic receptors for a certain substrate type is modification of the macrocycle periphery with bulky substituents or molecular fragments of different natures. Bulky highly-branched lateral substituents are capable of forming additional complexing cavities that can be used for identification and selective binding of substrates of a certain type.
- porphyrin
- receptor
- molecular recognition
- host–guest interactions
1. Introduction
Molecular recognition is a process in which host molecules (receptors) select and bind guest molecules (substrates) into structurally highly organized systems through multipoint intermolecular interactions [1,2,3,4,5,6,7][1][2][3][4][5][6][7]. Porphyrins are extremely useful for this purpose. Their unique properties are the result of the unusual geometric and electronic structure of the porphyrin macrocycle, containing a developed aromatic conjugated system. Selective chemical modification of porphyrins by fragments of other classes of compounds makes it possible to synthesize molecular systems that are different in their nature and positions of the reaction centers relative to each other [8,9,10,11,12,13,14][8][9][10][11][12][13][14]. This entreviewy discusses the progress made so far in the design and sensing and binding abilities of porphyrins modified with calix[4]arenes, calix[4]pyrroles, oxacalix-[2]-arenes-[2], and resorcinarenes and with bulky carbazolylphenyl substituents of different generations towards substrates of different natures. The molecular structures, sensing mechanism, and successful applications of these macrocyclic compounds with well-defined geometries as heterotopic receptors for molecular recognition and reversible binding of anions, cations, and small organic molecules are discussed in detail. Data on the ion-assisted binding ability of tetrapyrrole macroheterocyclic compounds decorated with additional complexing cavities and channels are of particular interest. It is shown that the binding ability of the porphyrin core depends on the conformation mobility of the substituents and the possibility of formation of intramolecular cavities and channels of various shapes within them.
2. Molecular Receptors Based on Porphyrin Conjugates with Macrocyclic Compounds of Different Natures
Interest in the chemistry of porphyrins was first sparked by the participation of these compounds in photosynthesis. However, it has now turned in another direction as porphyrins can be used as selective molecular receptors for certain types of substrates [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35]. The fact that it is possible to chemically modify the tetrapyrrole molecule has enabled researchers to synthesize porphyrin conjugates with other macrocyclic compounds(porphyrin conjugates with calix[n]arenes [36[36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54],37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54], cyclodextrines [55[55][56][57][58][59][60][61][62][63][64][65][66],56,57,58,59,60,61,62,63,64,65,66], calix[n]pyrroles, fullerenes, ferrocenes, and resorcinarenes [66,67,68,69,70,71,72,73,74,75,76,77,78,79,80][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80]) possessing their own properties, allowing them to form complexes with ions and organic molecules of different natures. Table 1 and Table 2 present some data on “molecular tweezer” macrocyclic receptors and the substrates they bind, based on results of the analysis of the most interesting works in this field.
| “Molecular Tweezers” | Substrates | Ref. | “Molecular Tweezers” | Substrates | Ref. | ||
|---|---|---|---|---|---|---|---|
| MPorph. | Linked | MPorph. | Linked | ||||
| H2Porph.1 ZnPorph.2 |
 |
 |
[36] [37] |
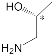
H2Porph.1 | 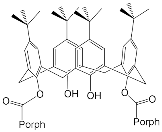 |
 |
[38] |
| H2Porph.1 | 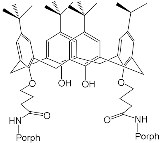 |
 |
[38] | H2Porph.1 H2Porph.3 H2Porph.4 |
 |
 |
[38] |
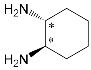
| ZnPorph.6 | 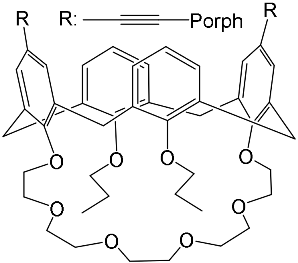 |
 |
[39] | ZnPorph.7 | 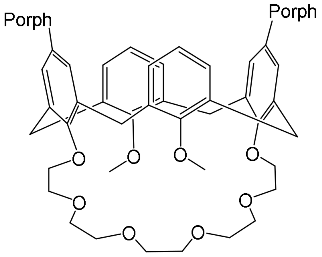 |
 |
[40] |
| ZnPorph.7 | 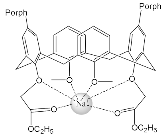 |
 |
[41] | H2Porph.8 | 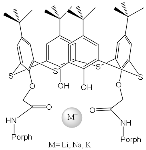 |
Regulation of the interporphyrin distance by the introduction of metal cations | [42] |
| ZnPorph.3 | 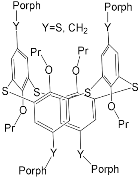 |
 |
[43] | ZnPorph.3 | 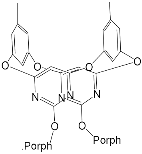 |
 |
[44] |
| H2Porph.9 | 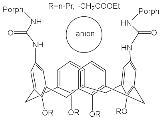 |
Anions Cl−, Br−, I−, NO3− |
[45] | H2Porph.10 ZnPorph.10 |
 |
Fluorescent detection of Cu(II), Al(III), Cr(III), Zn(II), Cd(II) | [46] |
| ZnPorph.11 |  |
 |
[67] | ZnPorph.9 | 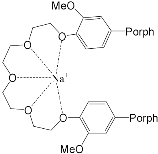 |
 |
[68] |
| ZnPorph.3 | 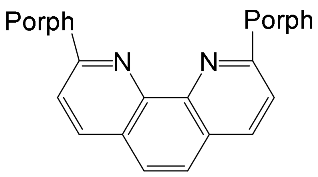 |
H2Porph.12 | [71] | ZnPorph.3 | 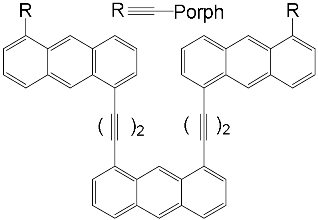 |
H2Porph.12 | [70] |
| ZnPorph.6 | 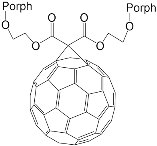 |
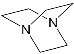
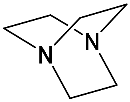
DABCO | [71] | ZnPorph.9 | 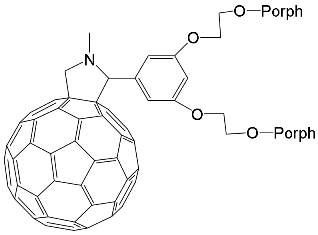 |
DABCO | [72] |
| ZnPorph.13 | 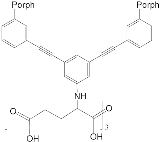 |
 |
[73] | ZnPorph.14 | 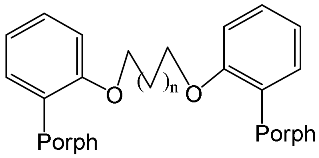 |
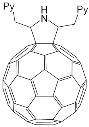 |
[74] |
| Bis-porphyrin “molecular tweezers” for recognition of chiral guest molecules. | |||||||
| ZnPorph.9 | 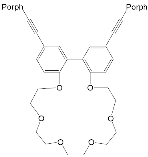 |
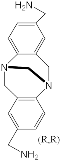 |
[75] | ZnPorph.9 | 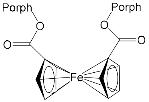 |
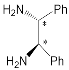 |
[76] |
| ZnPorph.15 MgPorph.15 |
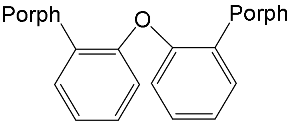 |
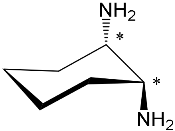 |
[15] | ZnPorph.15 MgPorph.15 |
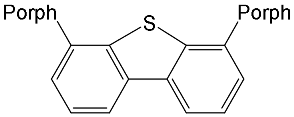 |
 |
[15] |
| ZnPorph.14 | 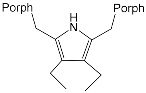 |
 |
[15] | ZnPorph.9 | 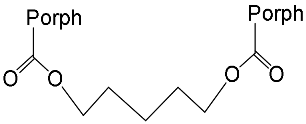 |
 |
[15] |
| MPorph. | R5, R10,R15 | R20 | R2,3,7,8,12,13,17,18 | MPorph. | R5, R10,R15 | R20 | R2,3,7,8,12,13,17,18 | 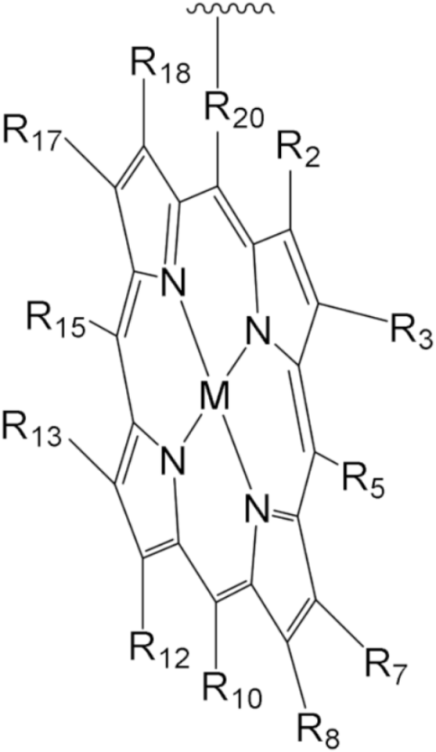 |
| 1 | 4-Me-Ph | Ph | H | |||||
| 2 | 4-Me-Ph | – | 9 | Ph | H | |||
| 3 | 3,5-But-Ph | Ph | 10 | H | – | 2,3,7,13,17,18-H, 8,12-n-C5H14 | ||
| 4 | (2,3,4,5,6-F-Ph) | 11 | Et | |||||
| 5 | 2,4,6-Me-Ph | 13 | 4-COOH-Ph | Ph | H | |||
| 6 | 5,15-H;10-(3,5But-Ph) | 2,8,12,18-Me, 3,7,13,17-Bu | 12 | 5,10-Py, 15-Ph | ||||
| 7 | 2,15-H, 10-Ph | – | 2,8,12,18-Me,3,7,13,17-Et | 14 | Ph | – | ||
| 8 | H | 2,3,17,18-H, 7,8,12,13-Et | 15 | H | 2,8,12,18-Me, 3,7,13,17-Et | |||
The 1H-NMR spectra of porphyrinate 7 in the presence of pyridine (Py) and imidazole (Im) have two rows of signals of aromatic protons belonging to the free and bound porphyrinate molecules of the substrate ( Figure 1 ). The ring current shielding effect of the porphyrin macrocycle results in an up-field shift of the aromatic protons of the bound substrate molecules compared with the respective protons of the unbound substrate molecules.
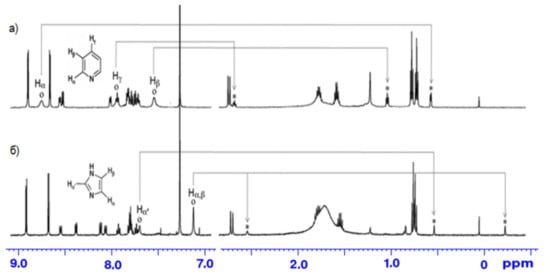
Thus, Zn-porphyrin 7 capped with calix[4]arene offers in its cavity a guest-binding environment for selective binding of small organic molecules, which makes it possible to distinguish even small structural differences between pyridine and 4-methylpyridine. Guests that are shaped in a way that is complementary to the confined cavity are capable of fitting into and binding to the cavity by van der Waals attractive interactions.
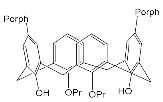
The synthesis and binding ability of a Zn-porphyrin ( 12) with a calix[5]arene cap were presented in [49]. In order to synthesize such a bridged porphyrin, 5,15-Bis-(carboxynaphtyl)-10,20-diphenylporphyrin ( 13) with syn arrangement of two naphthalene rings was prepared. Reaction of the porphyrin 13 with calix[5]arene with two amino functions ( 14) and subsequent treatment of the reaction product with zinc acetate gave the calix[5]arene-capped Zn-porphyrin 12 ( Scheme 4 ).
3. Molecular Receptors Based on Porphyrins Modified at the Macrocycle Periphery with Bulky Substituents
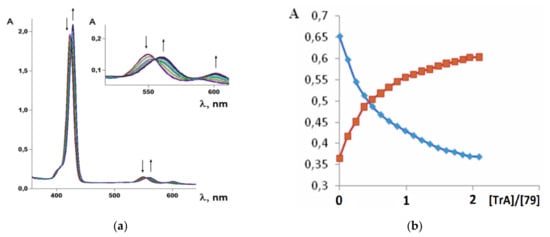
It was established by the spectrophotometric titration and 1H-NMR methods that the formation of a complex between zinc porphyrinate ( 79) and triazole (TrA) ( Scheme 26 ) leads to the formation of one family of spectral curves corresponding to its own set of isobestic points ( Figure 182 a).
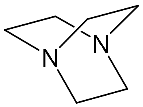
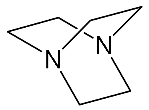
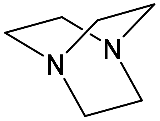
It should be said that complex formation is accompanied by considerable changes in the cryptand conformation, according to [111][81]. Figure 23 shows an example of complex formation between cryptand-[2,2,2][2][2][2] and a potassium cation and conformational changes accompanying this process in the complex.
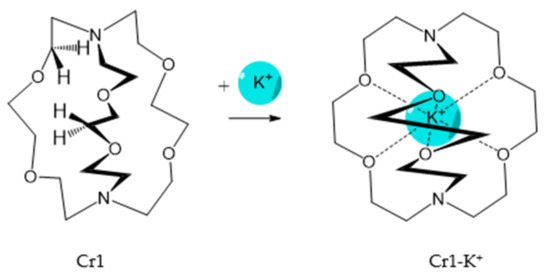
It should be also said that the 79-Cr1 complex is destroyed as well when trifluoroacetic acid is added to the solution ( Scheme 30 ). If the porphyrinate–ligand complex destruction under the action of the metal cation is caused by changes in the cryptate geometry in comparison with that of the cryptand, the complex destruction under the action of the acid is, evidently, caused by the breakage of the zinc–nitrogen bond between the porphyrinate metal cation and cryptand nitrogen upon protonation of the cryptand nitrogen atom.
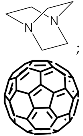
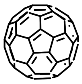
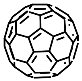
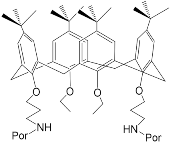
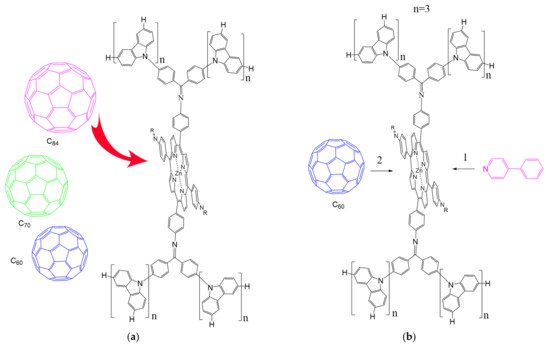
According to [113][82], the formation of a complex between the porphyrinate zinc and the phenyl-pyridine nitrogen atom ( Figure 29 4 ) causing conformational changes in the macrocycle dendrimer environment affects the ability of the outer carbazole layer of the 87G 2 receptor to form complexes with C 60.
References
- Beletskaya, I.; Tyurin, V.S.; Tsivadze, A.Y.; Gulard, R.; Stern, C. Supramolecular Chemistry of Metalloporphyrins. Chem. Rev. 2009, 109, 1659–1713.
- Guchhait, T.; Sasmal, S.; Khan, F.S.T.; Rath, S.P. Oxo- and hydroxo-bridged diiron(III) porphyrin dimers: Inorganic and bio-inorganic perspectives and effects of intermacrocyclic interactions. Coord. Chem. Rev. 2017, 337, 112–144.
- Mamardashvili, G.M.; Kaigorodova, E.Y.; Dmitrieva, O.; Mamardashvili, N.Z.; Koifman, O.I. Molecular recognition of imidazole derivatives by Co(III)-porphyrins in phosphate buffer (pH = 7.4) and cetylpyridinium chloride containing solutions. Molecules 2021, 26, 868.
- Mamardashvili, N.Z.; Koifman, M.O. Synthesis of Monohydroxy-Substituted Diarylporphyrins and Their Binding Ability towards Aminobenzoic Acids. Macroheterocycles 2011, 4, 30–33.
- Gil-Ramirez, G.; Karlen, S.D.; Shundo, A.; Porfyrakis, K.; Ito, Y.; Briggs, G.A.D.; Morton, J.J.; Anderson, H.L. A Cyclic Porphyrin Trimer as a Receptor for Fullerenes. Org. Lett. 2010, 12, 3544–3547.
- Hu, J.; Liu, S. Engineering Responsive Polymer Building Blocks with Host–Guest Molecular Recognition for Functional Applications. Acc. Chem. Res. 2014, 47, 2084–2095.
- Chandramouli, N.; Ferrand, Y.; Lautrette, G.; Kauffmann, B.; Mackereth, C.D.; Laguerre, M.; Dubreuil, D.; Huc, I. Iterative design of a helically folded aromatic oligoamide sequence for the selective encapsulation of fructose. Nat. Chem. 2015, 7, 334–341.
- Margetić, D. Host-guest Studies of Bis-porphyrins. Curr. Org. Chem. 2012, 16, 829–851.
- Davis, C.M.; Ohkubo, K.; Lammer, A.D.; Kim, D.S.; Kawashima, Y.; Sessler, J.L.; Fukuzumi, S. Photoinduced electron transfer in a supramolecular triad produced by porphyrin anion-induced electron transfer from tetrathiafulvalene calix[4]pyrrole to [email protected]60. Chem. Commun. 2015, 51, 9789–9792.
- Liu, Q.; Dong, J.; Sun, Q.; Zhao, S.; Chen, Y.; Jiang, J. A novel calix[4]arene-modified porphyrin-baseddual-mode sensor for the specific detection ofdopamine with excellent performance. New J. Chem. 2019, 43, 10376–10381.
- Nguyen, N.T.; Hofkens, J.; Scheblykin, I.G.; Kruk, M.; Dehaen, W. Click Reaction Synthesis and Photophysical Studies of Dendritic Metalloporphyrins. Eur. J. Org. Chem. 2014, 2014, 1766–1777.
- Merkas, S.; Bouatra, S.; Rein, R.; Piantanida, I.; Zinic, M.; Solladié, N. Pre-organized dinucleosides with pendant porphyrins for the formation of sandwich type complexes with DABCO with high association constants. J. Porphyr. Phthalocyanines 2015, 19, 535–546.
- Higashino, T.; Fujimori, Y.; Sugiura, K.; Tsuji, Y.; Ito, S.; Imahori, H. Synthesis of push–pull porphyrin with two electron-donating and two electron-withdrawing groups and its application to dye-sensitized solar cell. J. Porphyr. Phthalocyanines 2015, 19, 140–149.
- Sharma, G.D.; Zervaki, G.E.; Ladomenou, L.; Koukaras, E.N.; Panagiotis, P.; Angaridis, P.P.; Coutsolelos, A.G. Donor-π-acceptor, triazine-linked porphyrin dyads as sensitizers for dye-sensitized solar cells. J. Porphyr. Phthalocyanines 2015, 19, 175–191.
- Dhamija, A.; Mondal, P.; Saha, B.; Rath, S.P. Induction, control, and rationalization of supramolecular chirogenesis using metalloporphyrin tweezers: A structure-function correlation. Dalton Trans. 2020, 49, 10679–10700.
- Milanesio, M.E.; Alvarez, M.G.; Durantini, E.N. Methoxyphenyl Porphyrin Derivatives as Phototherapeutic Agents. Curr. Bioact. Compd. 2010, 6, 97–105.
- De Visser, S.P.; Valentine, J.S.; Nam, W. A Biomimetic Ferric Hydroperoxo Porphyrin Intermediate. Angew. Chem. Int. Ed. Engl. 2010, 49, 2099–2101.
- Gonsalves, A.M.A.R.; Serra, A.C.; Pineiro, M. The small stones of Coimbra in the huge tetrapyrrolic chemistry building. J. Porphyr. Phthalocyanines 2009, 13, 429–445.
- Maeda, C.; Kamada, T.; Aratani, N.; Osuka, A. Chiral self-discriminative self-assembling of meso-meso linked diporphyrins. Coord. Chem. Rev. 2007, 251, 2743–2752.
- Hori, T.; Nakamura, Y.; Aratani, N.; Osuka, A. Exploration of electronically interactive cyclic porphyrin arrays. J. Organomet. Chem. 2007, 692, 148–155.
- Mamardasvili, G.M.; Mamardashvili, N.Z.; Koifman, O.I. Self-assembling systems based on porphirins. Russ. Chem. Rev. 2008, 77, 765–782.
- Mamardashvili, G.M.; Mamardashvili, N.Z.; Koifman, O.I. Synthesis and receptor properties of calix[4]pyrroles. Russ. Chem. Rev. 2015, 84, 275–287.
- Balaban, T.S. Tailoring Porphyrins and Chlorins for Self-Assembly in Biomimetic Artificial Antenna Systems. Acc. Chem. Res. 2005, 38, 612–623.
- Carofiglio, T.; Lubian, E.; Varotto, A. Synthesis, heterogenization and sensing properties of melamine-bridged Bis-porphyrin dimers. J. Porphyr. Phthalocyanines 2010, 14, 701–707.
- Samaroo, D.; Vinodu, M.; Chen, X.; Drain, C.M. meso-Tetra(pentafluorophenyl)porphyrin as an Efficient Platform for Combinatorial Synthesis and the Selection of New Photodynamic Therapeutics using a Cancer Cell Line. J. Comb. Chem. 2007, 9, 998–1011.
- Tsuda, A. Design of Porphyrin Nanoclusters toward Discovery of Novel Properties and Functions Bull. Chem. Soc. Jpn. 2009, 82, 11–28.
- Goldberg, I. Crystal engineering of nanoporous architectures and chiralporphyrinassemblies. CrystEngComm 2008, 10, 637–645.
- Boyd, P.D.W.; Reed, C.A. Fullerene−Porphyrin Constructs. Acc. Chem. Res. 2005, 38, 235–242.
- Jurow, M.; Schuckman, A.E.; Batteas, J.D.; Drain, C.M. Porphyrins as molecular electronic components of functional devices. Coord. Chem. Rev. 2010, 254, 2297–2310.
- Yua, G.; Zhub, B.; Shaoc, L.; Zhouc, J.; Sahad, M.L.; Shid, B.; Zhang, Z.; Hong, T.; Lib, S.; Chena, X.; et al. Host−guest complexation-mediated codelivery of anticancer drug and photosensitizer for cancer photo-chemotherapy. Proc. Natl. Acad. Sci. USA 2019, 116, 6618–6623.
- Vinodh, M.; Alipour, F.H.; Abdirahman, A.M.; Talal, F. Al-Azemi Molecular Assemblies of Porphyrins and Macrocyclic Receptors: Recent Developments in Their Synthesis and Applications. Molecules 2012, 17, 11763–11799.
- Blom, M.; Norrehed, S.; Andersson, C.-H.; Huang, H.; Light, M.E.; Bergquist, J.; Grennberg, H.; Gogoll, A. Synthesis and Properties of Bis-Porphyrin Molecular Tweezers: Effects of Spacer Flexibility on Binding and Supramolecular Chirogenesis. Molecules 2016, 21, 16.
- Liang, X.; Honglin Zhang, H.; Luo, H.; Qin, M.; Li, M.; Zhu, W. Bio-Inspired Ni(II) Porphyrin Dimers with a Bridging Diphenyl Moiety: Facile Synthesis and Molecular Inherent Chirality. Macroheterocycles 2019, 12, 35–39.
- Huang, X.; Borhan, B.; Rickman, B.H.; Nakanishi, K.; Berova, N. Binding of Cationic Bis-porphyrins Linked with p- or m- Xylylenediamine and Their Zinc(II) Complexes to Duplex DNA. Molecules 2008, 13, 3117–3128.
- Ballester, P.; Costa, A.; Castilla, A.M.; Dey, P.M.; Frontera, A.; Gomila, R.M.; Hunter, C.A. DABCO-Directed Self-Assembly of Bisporphyrins (DABCO=1,4-Diazabicyclo[2.2.2]octane). Chem. Eur. J. 2005, 11, 2196.
- Dudic, M.; Lhoták, P.; Proškova, P.; Stibor, I.; Lang, K.; Sýkora, J. Calixarene-based metalloporphyrins: Molecular tweezers for complexation of DABCO. Tetrahedron 2003, 59, 2409–2415.
- Káš, M.; Lang, K.; Stibor, I.; Lhoták, P. Novel fullerene receptors based on calixarene-porphyrin conjugates. Tetrahedron Lett. 2007, 48, 477–481.
- Hosseini, A.; Taylor, S.; Accorsi, G.; Armaroli, N.; Reed, C.A.; Boyd, P.D. Calix[4]arene-Linked Bisporphyrin Hosts for Fullerenes: Binding Strength, Solvation Effects, and Porphyrin−Fullerene Charge Transfer Bands. J. Am. Chem. Soc. 2006, 128, 15903–15913.
- Jokic, D.; Zouhair Asfari, Z.; Weiss, J. The First Versatile Synthetic Approach to Cofacial Bis-Porphyrins with Calixarene Spacers. Org. Lett. 2002, 4, 2129–2132.
- Mamardashvili, G.M.; Shinkar’, I.A.; Mamardashvili, N.Z.; Koifman, O.I. Calix[4]arene-Porphyrin Molecular Receptors for Selective Binding of Ethylenediamines. Russ. J. Coord. Chem. 2007, 33, 774–778.
- Mamardashvili, G.M.; Mamardashvili, N.Z.; Koifman, O.I. Cation-Dependent Binding of Zinc Diethoxycarbonylcalix[4]arene-Bis(porphyrinate) Triethylenediamine. Russ. J. Coord. Chem. 2011, 37, 195–201.
- Dudic, M.; Lhoták, P.; Stibor, I.; Dvoráková, H. Lang Kamil Synthesis and spectroscopic properties of porphyrin-(thia)calix[4]arene conjugates. Tetrahedron 2002, 58, 5475–5482.
- Kundrat, O.; Kas, M.; Tkadlecova, M.; Lang, K.; Cvacka, J.; Stibor, I.; Lhotak, P. Thiacalix[4]arene–porphyrin conjugates with high selectivity towards fullerene C70. Tetrahedron Lett. 2007, 48, 6620–6623.
- Rossom, W.V.; Kundrát, O.; Ngo, T.H.; Lhoták, P.; Dehaen, W.; Maes, W. An oxacalix[2]arene[2]pyrimidine-Bis(Zn-porphyrin) tweezer as a selective receptor towards fullerene C70. Tetrahedron Lett. 2010, 51, 2423–2426.
- Dudić, M.; Lhoták, P.; Stibor, I.; Lang, K.; Proškova, P. Calix[4]arene-porphyrin Conjugates as Versatile Molecular Receptors for Anions. Org. Lett. 2003, 5, 149–152.
- Yakushev, A.A.; Averin, A.D.; Sakovich, M.V.; Vatsouro, I.M.; Kovalev, V.V.; Syrbu, S.A.; Koifman, O.I.; Beletskaya, I.P. Synthesisoftheporphyrin-calix[4]areneconjugates via Pd-catalyzedaminationandtheirevaluationasfluorescentchemosensors. J. Porphyr. 2019, 23, 1551–1562.
- Iwamoto, H.; Nishi, S.; Haino, T. Highly shape-selective guest encapsulation in the precisely defined cavity of a calix[4]arene-capped metalloporphyrin. Chem. Commun. 2011, 47, 12670–12672.
- Nagasaki, T.; Fujishima, H.; Shinkai, S. Calix[4]arene-Capped Tetraphenylporphyrin. Synthetic Approach to a Chiral Capped Porphyrin with Regular C4 Symmetry. Chem. Lett. 1994, 23, 989–992.
- Iwamoto, H.; Yukimasa, Y.; Yoshimasa Fukazawa, Y. Synthesis and binding behavior of a Zn(II)-porphyrin having calix[5]arene cap. Tetrahedron Lett. 2002, 43, 8191–8194.
- Mibradt, R.; Weiss, J. Efficient Combination of Calix[4]arenes and meso-Diphenylporphyrins. Tetrahedron Lett. 1995, 36, 2999–3002.
- Pognon, G.; Boudon, C.; Schenk, K.J.; Bonin, M.; Bach, B.; Weiss, J. Electrochemically Triggered Open and Closed Pacman Bis-metalloporphyrins. J. Am. Chem. Soc. 2006, 128, 3488–3489.
- Holler, M.; Schmitt, M.; Nierengarten, J.-F. Synthesis and conformational analysis of porphyrin derivatives substituted with calix[4]arene subunits. J. Porphyr. Phthalocyanines 2011, 15, 1183–1188.
- Mamardashvili, N.Z.; Mamardashvili, G.M.; Weiss, J. Complexation of Zinc Porphyrintes with 1,4-Diazabicyclo[2,2,2]octane. Russ. J. Inorg. Chem. 2005, 50, 218–224.
- Mamardashvili, N.Z.; Koifman, O.I. Cation and anion assisted binding of triethylenediamine by zinc Bis-porphyrinates. Russ. Chem. Bull. 2013, 62, 123–132.
- Puglisi, A.; Purrello, R.; Rizzarelli, E.; Sortino, S.; Vecchio, G. Spectroscopic and self-association behavior of a porphyrin-β-cyclodextrinconjugate. New J. Chem. 2007, 31, 1499–1506.
- Hosokawa, K.; Miura, Y.; Kiba, T.; Kakuchi, T.; Sato, S. Fluorescence Resonance Energy Transfer in Host–Guest Inclusion Complexes of Cyclodextrin–Porphyrin Composite in Aqueous Solution. Chem. Lett. 2008, 37, 60–61.
- Fathalla, M.; Li, S.; Diebold, U.; Alb, A.; Jayawickramarajah, J. Water-soluble nanorods self-assembledviapristine C60 and porphyrin moieties. Chem. Commun. 2009, 28, 4209–4211.
- Kralova, J.; Kejik, Z.; Briza, T.; Pouckova, P.; Kral, A.; Martasek, P.; Kral, V. Porphyrin−Cyclodextrin Conjugates as a Nanosystem for Versatile Drug Delivery and Multimodal Cancer Therapy. J. Med. Chem. 2010, 53, 128–138.
- Guo, Y.; Zhang, P.; Chao, J.; Shuang, S.; Dong, C. Study on the supramolecular system of 5-(p-hydroxyphenyl)-10,15,20-tris-(4-chlorophenyl)porphyrin with cyclodextrins and its analytical characteristics. Spectrochim. Acta A 2008, 71A, 946–950.
- Fathalla, M.; Neuberger, A.; Li, S.-C.; Schmehl, R.; Diebold, U.; Jayawickramarajah, J. Straightforward Self-Assembly of Porphyrin Nanowires in Water: Harnessing Adamantane/β-Cyclodextrin Interactions. J. Am. Chem. Soc. 2010, 132, 9966–9967.
- Guo, Y.-J.; Chao, J.-B.; Pan, J.-H. Study on the interaction of 5-pyridine-10,15,20-tris-(p-chlorophenyl)porphyrin with cyclodextrins and DNA by spectroscopy. Spectrochim. Acta A 2007, 68A, 231–236.
- Kiba, T.; Suzuki, H.; Hosokawa, K.; Kobayashi, H.; Baba, S.; Kakuchi, T.; Sato, S. SupramolecularJ-Aggregate Assembly of a Covalently Linked Zinc Porphyrin−β-cyclodextrin Conjugate in a Water/Ethanol Binary Mixture. J. Phys. Chem. B 2009, 113, 11560–11563.
- Ermilov, E.A.; Menting, R.; Lau, J.T.F.; Leng, X.; Roeder, B.; Ng, D.K.P. Switching the photoinduced processes in host–guest complexes of β-cyclodextrin-substituted silicon(iv) phthalocyanines and a tetrasulfonated porphyrin. Phys. Chem. Chem. Phys. 2011, 13, 17633–17641.
- Xu, H.; Ermilov, E.A.; Roeder, B.; Ng, D.K.P. Formation and energy transfer property of a subphthalocyanine–porphyrin complex held by host–guest interactions. Phys. Chem. Chem. Phys. 2010, 12, 7366–7370.
- Khavasi, H.R.; Sasan, K.; Safari, N. Inclusion complex of ironmeso-tetrakis(p-sulfonatophenyl)porphyrin and 2-hydroxypropyl-β-cyclodextrin as a functional model of cytochrome P-450: Study on a supramolecular formation and its application in aqueous oxidation of styrene. J. Porphyr. Phthalocyanines 2007, 11, 874–882.
- Liu, Y.; Ke, C.-F.; Zhang, H.-Y.; Cui, J.; Ding, F. Complexation-Induced Transition of Nanorod to Network Aggregates: Alternate Porphyrin and Cyclodextrin Arrays. J. Am. Chem. Soc. 2008, 130, 600–605.
- Mamardashvili, G.M.; Mamardashvili, N.Z.; Koifman, O.I. Anion-Dependent Binding of Zinc Calix[4]pyrrole-Bisporphyrinate Triethylenediamine. Russ. J. Coord. Chem. 2011, 37, 872–877.
- Donato Monti, D.; Monica, L.L.; Anita Scipioni, A.; Mancini, G. Effect of the inclusion of sodium cations on the binding properties of a switchable diporphyrin receptor. New J. Chem. 2001, 25, 780–782.
- Iengo, E.; Zangrando, E.; Alessio, E.; Chambron, J.-C.; Heitz, V.; Flamigni, L.; Sauvage, J.-P. A Functionalized Noncovalent Macrocyclic Multiporphyrin Assembly from a Dizinc(II) Bis-Porphyrin Receptor and a Free-Base Dipyridylporphyrin. Chem. Eur. J. 2003, 9, 5879.
- Flamigni, L.; Talarico, A.M.; Ventura, B.; Rein, R.; Solladié, N. A Versatile Bis-Porphyrin Tweezer Host for the Assembly of Noncovalent Photoactive Architectures: A Photophysical Characterization of the Tweezers and Their Association with Porphyrins and Other Guests. Chem. Eur. J. 2006, 12, 701.
- Guldi, D.M.; Luo, C.; Swartz, A.; Scheloske, M.; Hirsch, A. Selforganisation in photoactive fullerene porphyrin based donor–acceptor ensembles. Chem. Commun. 2001, 1066–1067.
- El-Khouly, M.E.; Hasegawa, J.; Momotake, A.; Sasaki, M.; Araki, Y.; Ito, O.; Arai, T. Intramolecular photoinduced processes of newly synthesized dual zinc porphyrin-fullerene triad with flexible linkers. J. Porph. Phthaloc. 2006, 10, 1380–1391.
- Tashiro, K.; Aida, T. Metalloporphyrin hosts for supramolecular chemistry of fullerens. Chem. Soc. Rev. 2007, 36, 189–197.
- D’Souza, F.; Gadde, S.; Zandler, M.E.; Itou, M.; Araki, Y.; Ito, O. Supramolecular complex composed of a covalently linked zinc porphyrin dimer and fulleropyrrolidine bearing two axially coordinating pyridine entities. Chem. Commun. 2004, 2276–2277.
- Kubo, Y.; Ohno, T.; Yamanaka, J.; Tokita, S.; Iida, T.; Ishimaru, Y. Chirality-Transfer Control Using a Heterotopic Zinc(II) Porphyrin Dimer. J. Am. Chem. Soc. 2001, 123, 12700.
- Brahma, S.; Ikbal, A.S.; Dhamija, A.; Rath, S.P. Highly Enhanced Bisignate Circular Dichroism of Ferrocene-Bridged Zn(II) Bisporphyrin Tweezer with Extended Chiral Substrates due to Well-Matched Host–Guest System. Inorg. Chem. 2014, 53, 2381–2395.
- Nakazawa, J.; Mizuki, M.; Shimazaki, Y.; Tani, F.; Naruta, Y. Encapsulation of Small Molecules by a Cavitand Porphyrin Self-Assembled via Quadruple Hydrogen Bonds. Org. Lett. 2006, 8, 4275–4278.
- Churakhina, Y.I.; Ivanova, Y.B.; Maltseva, O.V.; Mamardashvili, N.Z. Basic properties of porphyrins with polyethylenoxide spacers of various length. Russ. J. Gen. Chem. 2009, 79, 2435–2439.
- Ho, K.-H.L.; Hijazi, I.; Rivier, L.; Gautier, C.; Jousselme, B.; de Miguel, G.; Romero-Nieto, C.; Guldi, D.M.; Heinrich, B.; Donnio, B.; et al. Host–Guest Complexation of [60]Fullerenes and Porphyrins Enabled by “Click Chemistry”. Chem. Eur. J. 2013, 19, 11374–11381.
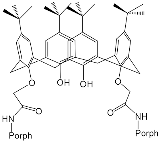
- Al-Azemi, T.F.; Vinodh, M. Synthesis of porphyrin conjugates based on conformationally rigid and flexible resorcin[4]arene frameworks. Tetrahedron 2011, 67, 2585–2590.
- Bukhzam, A.; Bade, N. Crown Ethers: Their Complexes and Analytical Applications. J. Applic. Chem. 2014, 3, 237–244.
- Albrecht, K.; Kasai, Y.; Kuramoto, Y.; Yamamoto, K. Dynamic control of dendrimer–fullereneassociation by axial coordination to the core. Chem. Com. 2013, 46, 6861–6863.
