Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Nicolas de Prost and Version 2 by Amina Yu.
Necrotizing soft tissue infections (NSTIs) are rare life-threatening bacterial infections characterized by an extensive necrosis of skin and subcutaneous tissues. Initial urgent management of NSTIs relies on broad-spectrum antibiotic therapy, rapid surgical debridement of all infected tissues and, when present, treatment of associated organ failures in the intensive care unit.
- necrotizing soft tissue infections
- antibiotic
- pharmacokinetics
- pharmacodynamics
- tissue diffusion
- anti-toxinic
- piperacillin-tazobactam
- clindamycin
- beta-lactam
1. Introduction
Necrotizing soft tissue infections (NSTIs) are rare life-threatening bacterial infections characterized by an extensive necrosis of skin and subcutaneous tissues. NSTIs can affect any part of the body but the extremities—particularly the lower limbs—are most frequently involved [1][2][3][4][1,2,3,4]. Most patients developing NSTIs have previous comorbidities, including diabetes mellitus, obesity, cardiovascular disease, intravenous drug use, and immunosuppression [1][2][3][1,2,3]. Infection can spread after traumatic injuries, minor breaches of the skin or mucosa, and even non-penetrating soft tissue injuries [1]. Mortality ranges from 10 to 30% according to initial patient severity, and morbidity among survivors includes potential amputations and profound impact on long-term health-related quality of life [5][6][7][5,6,7]. Initial urgent management of NSTIs relies on broad-spectrum antibiotic therapy, rapid surgical debridement of all infected tissues and, when present, treatment of associated organ failures in the intensive care unit. The time to surgery is one of the main modifiable prognostic factors with, in a recent meta-analysis, a significantly lower mortality rate when surgery was performed within six hours of hospital admission [8]. High-volume centers, caring for at least three patients per year, may also contribute to improving prognosis [9].
As a consequence of their rarity, data on optimal antibiotic treatment in NSTI are scarce [10] and current guidelines [11][12][13][14][11,12,13,14] are mainly derived from observational studies and experimental data. Antibiotic therapy for NSTI patients faces several challenges and should ideally achieve the following goals: (1) carry broad-spectrum activity against gram-positive and gram-negative pathogens because of frequent polymicrobial infections, considering extended coverage for multidrug resistance in selected cases; (2) decrease toxin production, mainly in proven or suspected group A streptococcus (GAS) infections; and (3) achieve the best possible tissular diffusion in the face of impaired regional perfusion, tissue necrosis, and pharmacokinetic and pharmacodynamic alterations among these frequently critically-ill patients.
2. Microbial Documentation of NSTIs: A Challenge for Microbiologists
Because of their uncertain contribution to the microbiological diagnosis, superficial samples should be avoided [15][26]. Contrariwise, deep samples collected at the interface between healthy and necrotized tissues by the surgeon during initial debridement and blood cultures are paramount, allowing for the identification of causative pathogens in approximatively 90% of cases [16][17][18][19][17,19,27,28]. As for any other deep suppuration, surgical samples are affixed on slides and Gram stained for extemporaneous microscopic direct examination. The detection of yeasts or the visualization of staphylococci, possibly leading to the use of rapid molecular tools aiming to detect methicillin resistant Staphylococcus aureus (MRSA), can have an immediate impact on antimicrobial treatment. As recommended by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) [20][29], samples are seeded on solid and liquid media and incubated for up to 5 days, in order to grow all the present bacteria, including anaerobes and difficult-to-grow bacteria. Because of the deep nature of the studied samples and the severity of NSTI, all growing bacteria of medical interest should be identified, and their antibiotic susceptibility tested. Nevertheless, as presented in Table 1, pathogenecity of different micro-organisms is still a matter of debate [21][22][25,30].
Table 1. Most frequently cultured microorganisms in NSTI. Classification adapted from Bruun et al. [21][25].
| Type of Pathogens | Primary | Secondary | Polymicrobial | Commensals |
|---|---|---|---|---|
| Pathogenicity | May cause NSTI in patients without known risk factors | May cause infection in patients with risk factors | Rarely pathogen in the absence of a primary or secondary pathogen | Do not cause NSTI although sometimes identified with other pathogens |
| Species | Group A Streptococcus Staphylococcus aureus Vibrio vulnificus Clostridium perfringens |
Other Streptococcus (group B, C, G, anginosus) Pneumococcus Haemophilus influenzae Neisseria meningitidis Enterobacteriaceae Nonfermenting gram-negative bacilli Other anaerobes (Bacteroides, Prevotella, Fusobacterium) |
Enterococcus | Bacillus Corynebacterium Micrococcus Coagulase negative Staphylococci |
The emergence of next-generation sequencing (NGS) methods is transforming medical diagnosis, including in infectious diseases. These methods include targeted metagenomics in which a PCR step (aiming to restrict the panel of microorganisms searched similarly to the 16S metagenomics for bacteria) precedes the sequencing, and shotgun metagenomics, a method consisting in sequencing the whole extracted nucleic acids (DNA and/or RNA). In the latter case, after subtraction of human sequences, analysis of the remaining reads leads to the identification of all the microorganisms present, including bacteria, viruses, yeasts, or parasites. Like in many other situations [23][24][25][31,32,33], shotgun metagenomics has demonstrated its better ability to detect a broad range of pathogens, particularly strict anaerobes compared to other methods [26][34]. Interest for this method is enhanced by the recent description of a complex pathobiome in NSTIs, urging to broadly identify all present microorganism both in necrotic and macroscopically healthy tissue [27][20]. Furthermore, ongoing developments of shotgun metagenomics may in the future add genomic information by detecting virulence or resistance determinants that could improve clinical management [28][35].
3. Suggested Empiric Treatment for Suspected NSTI Based on Basic Microbiology
The treatment of NSTIs relies on antibiotics and early surgical debridement, which is one of the most important modifiable prognostic factors [8]. With more than 50% of patients presenting with septic shock, urgent and bactericidal intravenous antibiotics are recommended [11][12][11,12]. NSTIs are often polymicrobial, and although some admission characteristics have been correlated with monomicrobial forms [29][30][31][18,121,122], even the site of infection is insufficient to guide empiric antibiotic treatment [4][17][32][33][4,19,123,124]. This should cover both gram positive, gram-negative, and anaerobic bacteria, usually with a broad-spectrum β-lactam (e.g., piperacillin-tazobactam). As discussed above, high-loading doses, continuous infusion, and therapeutic drug monitoring could improve the outcome. According to local ecology and individual risk factors, coverage of MRSA by glycopeptides or daptomycin, or of resistant gram-negatives by carbapenems should be considered on a case-by-case basis. Aminoglycosides should be reserved to broadening spectrum in case of septic shock. Finally, clindamycin adjunction seems adequate in the case of proven or suspected GAS infection (limb infection, features of streptococcal toxic shock, absence of comorbidities, blunt trauma, absence of chronic skin lesions, homelessness, injectable drug use, and non-steroidal anti-inflammatory drug use). In the absence of data, de-escalation of spectrum according to documentation seems reasonable, and suggested treatment duration is of 48–72 h after last surgery in case of clinical improvement [11][12][11,12]. A suggestion for management of antibiotic treatment in NSTI as well as future perspectives is presented in Figure 12.
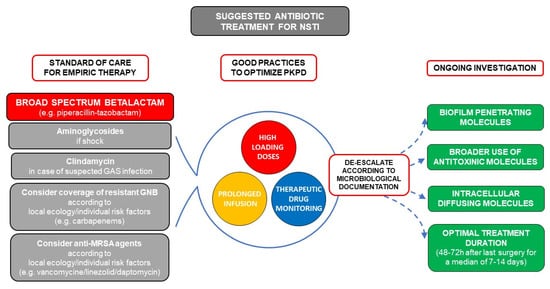

Figure 12. Suggested antibiotic treatment for necrotizing soft tissue infection (NSTI) and future perspectives. The mainstem of empiric treatment is a broad-spectrum beta-lactam (e.g., piperacillin-tazobactam) with additional aminoglycosides in case of septic shock. Clindamycin should be added in case of documented or suspected group A streptococcus (GAS) infection (limb infection, features of streptococcal toxic shock, absence of comorbidities, blunt trauma, absence of chronic skin lesions, homelessness, injectable drug use, non-steroidal anti-inflammatory drug use). Coverage of resistant gram-negative bacilli by carbapenems should be used according to local ecology and individual risk factors (hospital acquired infection, beta-lactam, or quinolone exposure in the previous 3 months, history of extended spectrum beta-lactamase (ESBL) carrying, germ colonization/infection or travel to high ESBL endemicity aeras in the previous 3 months). Similarly, use of anti-methicillin resistant Staphylococcus aureus (MRSA) drugs such as vancomycin, linezolid, or daptomycin should be considered in case of local endemicity, residence in a long-stay care facility, chronic dialysis, permanent transcutaneous medical devices or prior MRSA infection/colonization. Pharmacokinetics (PK) and Pharmacodynamics (PD) should be optimized by use of high-loading doses and prolonged infusions for molecules with time-dependent bactericidal activity such as beta-lactams, and therapeutic drug monitoring should be used when available.
4. Conclusions
Together with urgent surgical removal of necrotic tissues, antibiotics are the cornerstone of NSTI management, its mainstem being urgent bactericidal intravenous administration of a broad-spectrum beta-lactam such as piperacillin-tazobactam. Though no literature specifically focusing on NSTI exists, clinicians face the association of the profound PK/PD alterations associated with sepsis (i.e., distribution volume increase, tissue necrosis, locally altered perfusion) hindering drug diffusion. Administration modalities should definitely be optimized with high initial loading doses, continuous antibiotic perfusions and therapeutic drug monitoring when available. Future research focusing on antibiotic strategies in NSTI patients should assess the use of drugs with higher reported tissue diffusion, broader use of anti-toxinic, biofilm, or intracellular penetrating molecules as well as the effect of different/personalized treatment durations.
