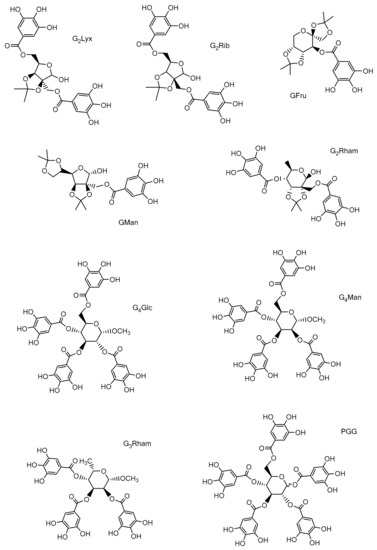
Nature has been a source of inspiration for the development of new pharmaceutically active agents. Polyphenols, including gallotannins, are widely studied as they protect cells against oxidative damage and pathogen attack. A series of new unnatural gallotannins (GTs), derived from D-lyxose, D-ribose, D-rhamnose, D-mannose, and D-fructose have been designed and synthesized i order to study the protective and antimicrobial effects of synthetic polyphenols that are structurally related to plant-derived products. Apart from spectral analysis, their antioxidant activity was evaluated. Structurally different GTs were screened in vitro for their antimicrobial properties against a spectrum of staphylococci, enterococci, and mycobacteria. Furthermore, the antibiofilm activity of GTs against S. aureus, and their ability to inhibit sortase A were inspected. Experimental data suggest that synthetic GTs could be considered as promising candidates for pharmacological, biomedical, and food industry applications.
- unnatural gallotannins
- antioxidant potential
- S. aureus
- antibiofilm activity
- antimicrobial effect
1. Introduction
2. Results and Discussion on New Unnatural Gallotannins
2.1. Chemistry
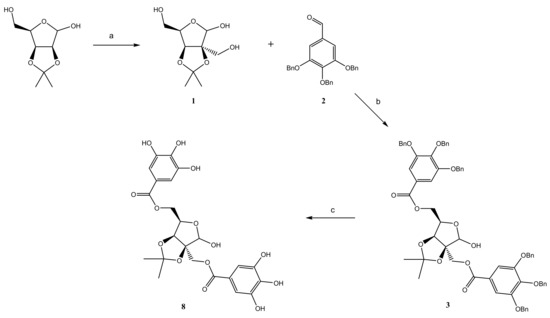
2.2. Antioxidant Activity
| Comp. | Concentration (mM) |
DPPH Scavenging Activity (%) | Reducing Power (Absorbance) |
|---|---|---|---|
| GFru | |||
| 0.1 | |||
| 27.67± 1.25 *** | |||
| 0.485 ± 0.044 * | |||
2.3. Antibacterial and Antimycobacterial Activity
| Comp. | MIC ([µg/mL]/[mM]) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SA | MRSA-1 | MRSA-2 | MRSA-3 | EF | VRE-1 | VRE-2 | VRE-3 | MT | MK | MS | |||||
| G2Lyx | |||||||||||||||
| G | 0.1 | 449.97 ± 1.14 | Glc | 256/ 3180.675 ± 0.041 |
|||||||||||
| 256/ | 318 | 64/ 79.7 |
64/ 79.7 |
32/ 39.8 |
64/ 79.7 |
256/ 318 |
256/ 318 |
>128/ >159 |
256 318 |
256/ 318 |
0.25 | 63.98 ± 1.05 | 0.886 ± 0.015 | ||
| G4Man | 64/ 79.7 |
256/ 318 |
64/ 79.7 |
64/ 79.7 |
16/ 19.9 |
32/ 39.8 |
256/ 318 |
256/ 318 |
>128/ >159 |
128/ 159 |
256/ 318 |
0.5 | 75.35 ± 1.61 | 1.263 ± 0.053 | |
| G3Rham | 128/ 201 |
256/ 403 |
128/ 201 |
128/ 201 |
32/ 50.4 |
128/ 201 |
256/ 403 |
256/ 403 |
>128/ >201 |
128/ 201 |
256/ 403 |
1 | 87.36 ± 1.35 | 1.699 ± 0.082 | |
| G2Lyx | >256/ >504 |
>256/ >504 |
>256/ >504 |
>256/ >504 |
>256/ >504 |
>256/ >504 |
>256/ >504 |
>256/ >504 |
>128/ >252 |
>256/ 504 |
>256/ 504 |
G2Rib | 0.1 | ||
| G2Rham | 52.67 ± 1.27 * | 256/ 456 |
256/ 4560.698 ± 0.028 |
||||||||||||
| 256/ | 456 | 256/ | 456 |
>256/ >456 |
>256/ >456 |
>256/ >456 |
>256/ >456 |
>256/ >456 |
>256/ >456 |
>256/ >456 |
0.25 | 67.55 ± 1.08 * | 0.979 ± 0.015 | ||
| G2Rib | 128/ 244 |
256/ 488.2 |
256/ 488 |
128/ 244 |
>256/ >488 |
>256/ >488 |
>256/ >488 |
>256/ >488 |
>128/ >244 |
>256/ >488 |
>256/ >488 |
0.5 | 78.39 ± 1.11 | ||
| GMan | 128/ 289 | 1.185 ± 0.031 | |||||||||||||
| 256/ | 578 | 256/ 578 |
>578 | >256/ | >578 | >128/ >289 |
>256/ >578 |
>256/ | 1 | 87.95 ± 1.39 | 1.761 ± 0.102 | ||||
| 128/ | >578 | ||||||||||||||
| GFru | 128/ 310 |
128/ 310 |
256/ 620 |
128/ 310 |
>256/ >620 |
>256/ >620 |
>256/ >620 |
>256/ >620 |
>128/ >310 |
>256/ >620 |
>256/ >620 |
G2Rham | 0.1 | 49.68 ± 2.13 | 0.622 ± 0.036 |
| PGG | 32/ 34.0 |
128/ 136 |
32/ 34.0 |
16/ 17.0 |
16/ 17.0 |
64/ 68.0 |
128/ 136 |
256/ 272 |
>128/ >136 |
256/ 272 |
>256/ >272 |
0.25 | 67.04 ± 1.72 | 0.904 ± 0.045 | |
| GA | 256/ 1487 |
64/ 371 |
64/ 371 |
32/ 185 |
>256/ >1487 |
>256/ >1487 |
>256/ >1487 |
>256/ >1487 |
>128/ >743 |
256/ 1487 |
>256/ >1487 |
0.5 | 79.63 ± 1.21 | 1.325 ± 0.018 | |
| AMP | 2/ 5.72 |
16/ 45.8 |
>16/ >45.8 |
>16/ >45.8 |
1/ 2.86 |
4/ 11.5 |
4/ 11.5 |
4/ 11.5 |
– | – | –1 | 85.18 ± 1.03 | 1.711 ± 0.072 | ||
| GMan | 0.1 | 33.57 ± 1.66 *** | 0.534 ± 0.081 * | ||||||||||||
| 0.25 | 48.19 ± 2.09 ** | 0.871 ± 0.016 * | |||||||||||||
| 0.5 | 0.25 | 39.29 ± 1.08 *** | 0.621 ± 0.073 ** | ||||||||||||
| INH | – | – | – | – | – | – | – | – | 8 58.0 |
4 | 0.5 | 62.18 ± 1.13 *** | 0.823 ± 0.051 ** | ||
| 1 | 73.36± 2.19 *** | 1.105 ± 0.069 ** | |||||||||||||
| G4Glc | 0.1 | 69.72 ± 1.59 *** | 0.826 ± 0.017 * | ||||||||||||
| 0.25 | 75.35 ± 1.98 ** | 1.297 ± 0.044 ** | |||||||||||||
| 0.5 | 84.49 ± 2.27 * | 1.999 ± 0.081 ** | |||||||||||||
| 1 | 95.96 ± 1.77 ** | 2.267 ± 0.086 ** | |||||||||||||
| 29.1 | 16 | 117 | G4Man | 0.1 | 68.19 ± 1.54 *** | 0.779 ± 0.051 | |||||||||
| 0.25 | 77.02 ± 2.11 ** | 1.307 ± 0.079 *** | |||||||||||||
| 289 | >256/ | >578 | >256/ | >578 |
>256/68.42 ± 1.74 * | ||||||||||
2.4. Antibiofilm Activity
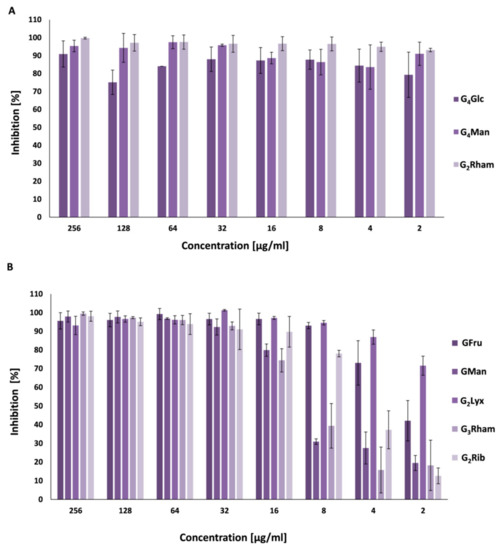
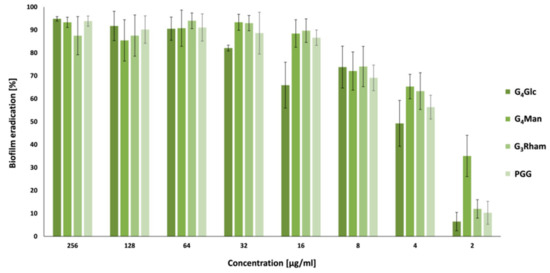
2.5. Quorum Sensing Inhibition
| Comp. (conc. 10 mg/mL) |
Diameter of Inhibition Zone on Agar [mm] |
||
|---|---|---|---|
| G4Glc | 5.00 ± 0.01 | ||
| G4Man | 8.50 ± 2.90 | ||
| G3Rham | 7.00 ± 1.40 | ||
| G2Rham | – | ||
| G2Rib | 8.30 ± 0.62 | ||
| G2Lyx | 5.00 ± 1.01 | ||
| GMan | 3.00 ± 0.02 | ||
| GFru | 6.50 ± 1.31 | ||
| 1.054 ± 0.037 * | |||
| 1 | |||
| 71.28 ± 1.16 ** | |||
| 1.128 ± 0.057 ** | |||
| 0.5 | |||
| 83.76 ± 1.99 * | |||
| 1.878 ± 0.068 ** | |||
| 1 | |||
| 95.01 ± 1.63 ** | |||
| 2.238 ± 0.039 ** | |||
| G3Rham | 0.1 | 71.38 ± 1.74 *** | 0.801 ± 0.073 * |
| 0.25 | 78.14 ± 1.49 ** | 1.464 ± 0.057 ** | |
| 0.5 | 86.63 ± 2.08 * | 1.935 ± 0.108 *** | |
| 1 | 94.13 ± 1.27 ** | 2.197 ± 0.096 ** | |
| PGG | 0.1 | 75.32 ± 2.01 *** | 0.918 ± 0.025 * |
| 0.25 | 80.27 ± 1.14 *** | 1.506 ± 0.037 ** | |
| 0.5 | 92.35 ± 1.58 ** | 2.104 ± 0.093 *** | |
| 1 | 97.88 ± 1.22 ** | 2.307 ± 0.034 ** | |
| GA | 0.1 | 47.56 ± 1.45 | 0.688 ± 0.015 |
| 0.25 | 63.03 ± 2.06 | 1.002 ± 0.071 | |
| 0.5 | 76.89 ± 1.73 | 1.289 ± 0.059 | |
| 1 | 88.14 ± 1.16 | 1.736 ± 0.084 |
| PGG |
| 6.00 ± 0.01 |
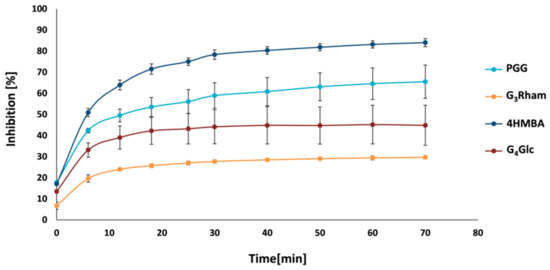
2.6. Inhibition of Sortase A
3. Future directions
References
- Khanbabaee, K.; van Ree, T. Tannins: classification and definition. Nat. Prod. Rep. 2001, 18, 641–649.
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586 – 621.
- Barbehenn, R.V.; Constabel, C.P. Tannins in plant–herbivore interactions. Phytochemistry. 2011, 72, 1551–1565.
- Chung, K.T.; Wong, T.Y.; Wei, C.I.; Huang, Y.W.; Lin, Y. Tannins and Human Health: A Review.Critical Reviews in Food Science and Nutrition1998, 38, 421–464.
- Kiss, A.K.; Piwowarski, J.P. Ellagitannins, gallotannins and their metabolites - The contribution to the Anti-Inflammatory effect of food products and medicinal plants.Curr. Med. Chem. 2018, 25, 4946–4967.
- Lee, S.J.; Lee, H.K.; Jung, M.K.; Mar, W. In vitro antiviral activity of 1,2,3,4,6-Penta-O-galloyl-b-D-glucose against hepatitis B virus. Biol. Pharm. Bull. 2006, 29, 2131–2134.
- Zhang, J.; Li, L.; Kim, S.H.; Hagerman, A.E.; Lü, J. Anti-Cancer, Anti-Diabetic and Other Pharmacologic and Biological Activities of Penta Galloyl-Glucose. Pharmaceut. Res. 2009, 26, 2066–2080.
- Yin, S.; Dong, Y.; Li, J.; Lü, J.; Hu, H. Penta-1,2,3,4,6-O-galloyl-β-D-glucose induces senescence-like terminal S-phase arrest in Human hepatoma and breast cancer cells. Mol. Carcinog. 2011, 50, 592–600.
- Jourdes, M.; Pouysegu, L.; Deffieux, D.; Teissedre, P.-L.; Quideau, S. Hydrolyzable tannins: gallotannins and ellagitannins. In: Ramawat K.G., Merillon J.M. (Eds.) Natural products: phytochemistry, botany and metabolism of alkaloids, phenolics and terpenes. Springer, Berlin, 2013, 1975–2010.
- Wu, Y.P.; Liu, X.Y.; Bai, J.R.; Xie, H.C.; Ye, S.L.; Zhong, K.; Huang, Y.N.; Gao, H. Inhibitory effect of a natural phenolic compound, 3-p-trans-coumaroyl-2-hydroxyquinic acid against the attachment phase of biofilm formation of Staphylococcus aureus through targeting sortase A. RSC Advances, 2019, 9, 32453–32461.
- Blando, F.; Russo, R.; Negro, C.; De Bellis, L.; Frassinetti, S. Antimicrobial and Antibiofilm Activity against Staphylococcus aureus of Opuntiaficus-indica (L.) Mill. Cladode Polyphenolic Extracts. Antioxidants, 2019, 8, 117.
- Gotz F. Staphylococcus and biofilms. Molec. Microbiol. 2002, 43, 1367–1378.
- Zheng, Y.; He, L.; Asiamah, T.K.; Otto, M. Colonization of medical devices by staphylococci. Environm. Microbiol. 2018, 20, 3141–3153.
- Takó, M.; Kerekes, E.B.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant Phenolics and Phenolic-Enriched Extracts as Antimicrobial Agents against Food Contaminating Microorganisms. Antioxidants, 2020, 9, 165.
- Amakura, Y.; Yoshimura, M.; Sugimoto, N.; Yamazaki, T.; Yoshida, T. Marker constituents of the natural antioxidant eucalyptus leaf extract for the evaluation of food additives. Biosci. Biotechnol. Biochem. 2009, 73, 1060–1065.
- Abou-Zaid, M.M.; Nozzolillo, C. 1-O-galloyl-α-l-rhamnose from Acer rubrum. Phytochemistry 1999, 52, 1629–1631.
- Xie, Y.; Zhao, Y. Synthesis of 7-O-galloyl-D-sedoheptulose. Carbohydr. Res. 2007, 342, 1510–1513.
- Ren, Y.; Himmeldirk, K.; Chen, X. Synthesis and structure-activity relationship study of antidiabetic penta-O-galloyl-D-glucopyranose and its analogues. J. Med. Chem. 2006, 49, 2829–2837.
- Cao, Y.; Himmeldirk, K.; Qian, Y.; Ren, Y.; Malki, A; Chen, X. Biological and biomedical functions of Penta-O-galloyl-D-glucose and its derivatives. J. Nat. Med. 2014, 68, 465–472.
- González-Sarrías, A.; Yuan, T.; Seeram, N.P. Cytotoxicity and structure activity relationship studies of maplexins A–I, gallotannins from red maple (Acer rubrum). Food Chem. Toxicol. 2012, 50, 1369–1376.
- Torres-León, C.; Ventura-Sobrevilla, J.; Serna-Cock, L.; Ascacio-Valdés, J.A.; Contreras-Esquivel, J.; Aguilar, C.N. Pentagalloylglucose (PGG): A valuable phenolic compound with functional properties. J. Funct. Foods 2017, 37, 176–189.
- Jiamboonsri, P; Pithayanukul, P.; Bavovada, R.; Chomnawang, M.T. The Inhibitory Potential of Thai Mango Seed Kernel Extract against Methicillin-Resistant Staphylococcus Aureus. Molecules 2011, 16, 6255–6270.
- Lin, M.H.; Chang, F.R.; Hua, M.Y.; Wu, Y.C.; Liu, S.T. Inhibitory Effects of 1,2,3,4,6-Penta-O-Galloyl-β-d-Glucopyranose on Biofilm Formation by Staphylococcus aureus. Antimicrob. Agents and Chemotherapy, 2011, 55, 1021–1027.
- Zhao, Y.; Wang, B.; Zhang, S.; Yang, S.; Wang, H.; Ren, A.; Yi, E. Isolation of antifungal compound from Paeoniasuffruticosa and its antifungal mechanism. Chinese J. Integrat. Med. 2014, 21, 211–216.
- Shafizadeh F. Branched-chain sugars of natural occurrence. Adv. Carbohydr. Chem. 1956, 48, 263–283.
- Grisebach, H.; Schmid, R. Chemistry and Biochemistry of Branched-Chain Sugars Angew. Chem. Internat. Edit. 1972, 1, 159–248.
- Beck, E.; Hopf, H. Branched-chain Sugars and Sugar Alcohols. Carbohydrates 1990, 235–289.
- Masaki, H.; Atsumi, T.; Sakurai, H. Hamamelitannin as a new potent active oxygen scavenger. Phytochemistry1994, 37, 337–343.
- Masaki, H.; Atsumi, T.; Sakurai, H. Peroxyl radical scavenging activities of hamamelitannin in chemical and biological systems. Free. Rad. Res. 1995, 22, 419–430.
- Lizárraga, D.; Touriño, S.; Reyes-Zurita, F.J.; de Kok, T.M.; van Delft, J.H.; Maas, L.M.; Briedé, J.J.; Centelles, J.J.; Torres, J.L.; Cascante, M. Witch hazel (Hamamelis virginiana) fractions and the importance of gallate moieties-electron transfer capacities in their antitumoral properties. J. Agric. Food. Chem.2008, 56, 11675–11682.
- Erdelmeier, C.; Cinatl, J.; Rabenau, H.; Doerr, H.; Biber, A.; Koch, E. Antiviral and Antiphlogistic Activities of Hamamelisvirginiana Bark. Planta Medica, 1996, 62, 241–245.
- Sánchez-Tena, S.; Fernández-Cachón, M.F.; Carreras, A.A.; Mateos-Martín, M.L.; Costoya, N.; Moyer, M.P.; Nuñez, M.J.; Torres, J.L. Cascante M. Hamamelitannin from Witch Hazel (Hamamelis virginiana) Displays Specific Cytotoxic Activity against Colon Cancer Cells. J. Nat. Prod. 2012, 75, 26–33.
- Vermote, A.; Brackman, G.; Risseeuw, M.D.P.; Cappoen, D.; Cos, P.; Coenye, T.; Van Calenbergh, S. Novel Potentiators for Vancomycin in the Treatment of Biofilm-Related MRSA Infections via a Mix and Match Approach. ACS Med. Chem. Lett. 2016, 8, 38–42.
- Brackman, G.; Breyne, K.; De Rycke, R.; Vermote, A.; Van Nieuwerburgh, F.; Meyer, E.; Van Calenbergh, S.; Coenye, T. The quorum sensing inhibitor hamamelitannin increases antibiotic susceptibility of Staphylococcus aureus bioflms by affecting peptidoglycan biosynthesis and eDNA release. Sci. Rep. 2016, 6, 20321.
- Hricovíniová, J.; Ševčovičová, A.; Hricovíniová, Z. Evaluation of the genotoxic, DNA-protective and antioxidant profile of synthetic alkyl gallates and gallotannins using in vitro assays. Toxicol in Vitro, 2020,65, 104789.
- Siquet, C.; Paiva-Martins, F.; Lima, J.L.F.C.; Reis, S.; Borges, F. Antioxidant profile of dihydroxy- and trihydroxyphenolic acids - A structure-activity relationship study. Free Rad. Res. 2006, 40, 433–442.
- Jing, P.; Zhao, S.J.; Jian, W.J.; Qian, B.J.; Dong, Y.; Pang, J. Quantitative studies on structure-DPPH• scavenging activity relationships of food phenolic acids. Molecules 2012, 17, 12910–12924.
- Chan, E.W.L.; Gray, A.I.; Igoli, J.O.; Lee, S.M.; Goh, J.K. Galloylatedflavonolrhamnosides from the leaves of Calliandra tergemina with antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA). Phytochemistry, 2014, 107, 148–154.
- Bag, A.; Bhattacharyya, S.K.; Chattopadhyay, R.R. Isolation and identification of a gallotannin 1,2,6-tri-O-galloyl-β-d-glucopyranose from hydroalcoholic extract of Terminalia chebulafruits effective against multidrug-resistant uropathogens. J. Appl. Microbiol. 2013, 115, 390–397.
- Aguilar-Galvez, A.; Noratto, G.; Chambi, F.; Debaste, F.; Campos, D. Potential of tara (Caesalpinia spinosa) gallotannins and hydrolysates as natural antibacterial compounds. Food Chem. 2014, 156, 301–304.
- Maisetta, G.; Batoni, G.; Caboni, P.; Esin, S.; Rinaldi, A.C.; Zucca, P. Tannin profile, antioxidant properties, and antimicrobial activity of extracts from two Mediterranean species of parasitic plant Cytinus. BMC Complement. Alternat. Med. 2019, 19.
- Bag, A.; Chattopadhyay R.R. Synergistic antibiofilm efficacy of a gallotannin 1,2,6-tri-O-galloyl-β-D-glucopyranose from Terminalia chebula fruit in combination with gentamicinand trimethoprim against multidrug resistant uropathogenic Escherichia coli biofilms. PLOS ONE, 2017, 12, e0178712.
- Ta, C.; Arnason, J. Mini Review of Phytochemicals and Plant Taxa with Activity as Microbial Biofilm and Quorum Sensing Inhibitors. Molecules, 2015, 21, 29.
- Narla, A.V.; Borenstein, D.B.; Wingreen, N.S. A biophysical limit for quorum sensing in biofilms. PNAS, 2021, 118, e2022818118.
- Wang, G.; Gao, Y.; Wang, H.; Niu, X.; Wang, J. Baicalin weakens Staphylococcus aureus pathogenicity by targeting sortase B. Front. Cell. Infect. Microbiol. 2018, 8, 418.
- Oh, I.; Yang, W.-Y.; Chung, S.-C.; Kim, T.-Y.; Oh, K.-B.; Shin, J. In vitro sortasea inhibitory and antimicrobial activity of flavonoids isolated from the roots of Sophora flavescens. Arch. Pharm. Res. 2011, 34, 217–222.
- Mu, D.; Xiang, H.; Dong, H.; Wang, D.; Wang, T. Isovitexin, a potential candidate inhibitor of sortase A of Staphylococcus aureus USA 300. J. Microbiol. Biotechnol. 2018, 28, 1426–1432.
- Wang, J.; Shi, Y.; Jing, S.; Dong, H.; Wang, D.; Wang, T. Astilbin inhibits the activity of Sortase A from Streptococcus mutans. Molecules, 2019, 24, 465.
- Dong, J.; Zhang, L.; Xu, N.; Zhou, S.; Song, Y.; Yang, Q.; Liu, Y.; Yang, Y.; Ai X. Rutin reduces the pathogenicity of Streptococcus agalactiae to tilapia by inhibiting the activity of sortase A. Aquaculture, 2021,530,735743.
