LAMP is highly specific and produces a large yield of amplicon in a short period of time, with reagents that are less expensive and more readily available. In addition, the CRISPR technology is a highly specific approach for detecting nucleic acids rapidly and accurately. Therefore, this review focuses on the CRISPR-based detection of SARS-CoV-2 RNA using Cas12 and Cas13 nucleases integrated with reverse-transcription LAMP (RT-LAMP).
- COVID-19
- SARS-CoV-2
- RT-LAMP
- CRISPR
- Cas12
- Cas13
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) , the causative agent for coronavirus disease 2019 (COVID-19), emerged in December 2019 in Wuhan, China, and caused a pandemic [1]. Coronaviruses are large, positive-stranded ribonucleic acid (RNA) viruses with genome sizes ranging from ~27 to ~32 kilobase pairs (kbp), which is the largest genome size for RNA viruses [2]. Individuals above the age of 70, as well as those with underlying comorbidities, are at a higher risk of developing severe COVID-19, which includes pneumonia and acute respiratory distress syndrome (ARDS). Severe COVID-19 patients require mechanical ventilators to improve their breathing. Many others experience mild-to-moderate symptoms, including fever, lethargy, dry cough, and dyspnea or have no symptoms at all [3]. Over half of the human-to-human transmissions of SARS-CoV-2 occur from asymptomatic carriers. Therefore, the aggressive contact tracking and isolation of asymptomatic carriers have proven to be quite efficient in limiting the virus spread [4]. Thus, rapid and accurate diagnostic tests are essential for detecting SAR-CoV-2 to fight against the pandemic.
The COVID-19 pandemic can be monitored and managed more effectively if the virus is detected early. Reverse transcription–quantitative polymerase chain reaction (RT-qPCR) has been considered the gold standard method and is the most widely used detection approach for SARS -CoV-2 [5]. Even though RT-qPCR has high sensitivity and reliability in detection, it is not suited for large-scale point-of-care (POC) diagnostics because it requires highly skilled workers, expensive equipment, and a long reaction time (turnaround time, TAT: 2–4 h) [5][6][7][5,6,7]. In addition, COVID-19 diagnosis by RT-qPCR is more challenging in certain low-resource areas [8]. Clinical samples must be processed in a biosafety level 2 (BSL-2) laboratory with unidirectional airflow or a biosafety level 3 (BSL-3) facility for the isolation of SARS-CoV-2 RNA virus before performing the RT-qPCR test. Therefore, this technique is challenging to execute during emergency scenarios where hundreds of samples must be evaluated as soon as possible to assess treatment choices and control an outbreak.
As a result, approaches that meet the ASSURED (Affordable, Sensitive, Specific, User-friendly, Rapid and Robust, Equipment-free, and Deliverable to end-users) criteria for POC diagnostics are urgently required to combat the COVID-19 pandemic. CRISPR-Cas (clustered regularly interspaced short palindromic repeats and CRISPR-associated proteins) systems are molecular immunity mechanisms that protect bacteria and archaea against invading nucleic acids, such as phages and conjugative plasmids [9][10][9,10], and these systems have been used as powerful tools for genome and transcriptome editing, gene therapy, and nucleic acid detection [11]. Effector proteins of CRISPR-Cas systems are targeted to DNA or RNA sequences under the guidance of a CRISPR-RNA (crRNA). Among the single effector Cas endonucleases, Cas13 and Cas12 perform indiscriminate RNA and single-stranded deoxyribonucleic acid (ssDNA) cleavage, respectively, when they are activated with the crRNA target sequences [12][13][12,13]. This feature has been harnessed for reporting the presence of a defined RNA or DNA sequence in a sample, giving rise to the concept of CRISPR-based diagnostics [13][14][15][16][13,14,15,16]. CRISPR technology is a highly specific approach for detecting nucleic acids rapidly and accurately. CRISPR-based diagnostic methods offer ultrasensitive, less expensive, and portable diagnostic tests for evaluating suspected COVID-19 cases to aid in the diagnosis of SARS-CoV-2 infection. CRISPR-based diagnostic methods take advantage of diverse isothermal amplification approaches such as loop-mediated isothermal amplification (LAMP) and recombinase polymerase amplification (RPA), which yield the highly specific and sensitive amplification of a few copies of the targeted nucleic acid in a short period of time at a constant temperature, obviating the need for thermocycling steps. Thus, they are preferred for POC diagnostics where low cost and ease of use are required [4].
CRISPR-based techniques for the detection of SARS-CoV-2 RNA have been developed by combining RT-RPA with CRISPR-mediated detection. However, this combined method has some limitations, including being performed in two separate reaction steps, requiring a long incubation period (120 min), the generation of weak signals for low template concentrations, and having a strong background signal due to the multiple enzymes in the RPA system [7]. Furthermore, there are difficulties in the supply chain for the RPA reagents that are available on the market and challenges in developing a rapid and one-pot RPA test for the sensitive detection of SARS -CoV-2 [17]. However, LAMP is highly specific and produces a large yield of amplicon in a short period of time, with reagents that are less expensive and more readily available. Therefore, this review focuses on the CRISPR-based detection of SARS-CoV-2 RNA using Cas12 and Cas13 nucleases integrated with reverse-transcription LAMP (RT-LAMP).
2. RT-LAMP CRISPR-Cas Workflow
The first stage in RT-LAMP CRISPR-based SARS-CoV-2 nucleic acid detection is sample collection from patients. Upper respiratory samples (nasopharyngeal swabs (NPSs), nasal swabs, oropharyngeal swabs (OPSs), nasopharyngeal aspirates, and nasal aspirates) are the most common forms of SARS-CoV-2 samples, followed by saliva, bronchoalveolar lavage, and sputum. Although NPS remains the gold standard for diagnostic testing for SARS-CoV-2, saliva has been proposed as an alternate sample source for SARS-CoV-2 detection. Saliva collection does not need specific consumables (swabs) and personal protective equipment (gloves and facemasks), causes less patient discomfort (non-invasive), and lowers the exposure risk for health professionals (direct interaction) [17][18][19][17,18,19]. As shown in Table 1 , the number of clinical samples tested in the studies related to RT-LAMP CRISPR-based SARS-CoV-2 detection ranged from 8 to 378 samples. Various viral RNA extraction protocols have been authorized by the Centers for Disease Control and Prevention-Emergency Use Authorization (CDC-EUA). These include the DIRECTZOL KIT (Zymo Research, CA, USA), Qiagen DSP Viral RNA Mini kit (Qiagen, Hilden, Germany), and PureLink™ Viral RNA Mini Kit (Thermo Fisher Scientific, Waltham, MA, USA). The RNA isolation processes usually consist of three stages: lysis, separation, and the elution of RNA. However, as the number of COVID-19 cases increases, RNA extraction kits, and consumables become scarce. Therefore, several groups have achieved simple viral RNA extraction by combining chemical- and heat-based methods. For example, after the addition of lysis solution, the incubation of swab samples at 42 °C for 20 min and 64 °C for 5 min enabled RT-LAMP and Cas12a-based SARS-CoV-2 detection [20]. Similarly, the treatment of saliva samples with TCEP/EDTA, followed by heating at 95 °C for 10 min achieved viral RNA extraction without commercial kits [21]. Both studies showed notably high clinical sensitivity and specificity ( Table 1 ), demonstrating that rapid and cheap viral RNA extraction methods are suitable for RT-LAMP CRISPR-based SARS-CoV-2 detection methods.
Summary of Cas12- and Cas13-based detection of SARS-CoV-2 nucleic acid using RT-LAMP as an amplification method.
| Name of the Method | Cas Enzyme |
Target Region |
Type of Clinical Samples |
Number of Steps | Readout Method | Instrument Requirement |
Assay Time * | Limit of Detection | Number of Clinical Samples | Sensitivity and Specificity (%) |
ASSURED Criteria | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| opvCRISPR | Cas12a | S | Nasopharyngeal swab | One | Fluorescence | Blue light | 45 min | 5 copies | 50 | 100 and 100 | Yes | [7] |
| iSCAN | Cas12a and Cas12b | N, E | Nasopharyngeal swab | One or two | Fluorescence or LFA | Fluorescence reader | 60 min | 10 copies/reaction | 24 | 86 and 100 | Yes (LFA) | [4] |
| DETECTR | Cas12a | N, E | Nasopharyngeal swab | Two | Fluorescence or LFA | Fluorescence reader | 45 min | 10 copies/µL | 82 | 95 and 100 | Yes (LFA) | [22][24] |
| - | Cas12a | ORF1ab | Respiratory swab | One | Fluorescence | Smartphone and 3D printing instrument | 45 min | 20 copies/reaction | 10 | 100 and 100 | Yes | [8] |
| CRISPR-ENHANCE | Cas12a with 3′DNA7-modified crRNA | N | - | Two | Fluorescence or LFA | Fluorescence reader | 40 min | 3–300 copies | - | - | Yes (LFA) | [23][25] |
| DETECTR | Cas12a | N | Nasopharyngeal swab, bronchoalveolar lavage, sputum | Two | Fluorescence or LFA | Fluorescence reader | 30 min | 50 copies | 378 | 93 and 95.5 | Yes (LFA) | [6] |
| ITP-CRISPR | Cas12a | N, E | Nasopharyngeal swab | One | Fluorescence | Inverted epifluorescence microscope | 30 min | 10 copies/µL | 8 | 75 and 100 | No a | [24][22] |
| VaNGuard | Cas12a | S | Nasopharyngeal swab | Two | Fluorescence or LFA | Fluorescence reader | 30 min | 93 copies/reaction | - | - | Yes (LFA) | [25][28] |
| - | Cas12a | N, E | Respiratory swab | One | Fluorescence | Handheld UV lamp | 40 min | N-30 E-45 copies/µL |
100 | 94 and 100 | Yes | [26][27] |
| STOPCovid | Cas12b | N | Nasopharyngeal swab, saliva | One | Fluorescence or LFA | Fluorescence reader | 40–70 min | 100 copies/reaction | 17 | 91.7 and 100 | Yes (LFA) | [17] |
| STOPCovid.v2 | Cas12b | N | Nasopharyngeal swab, anterior nasal swab | One | Fluorescence | Fluorescence reader | 45 min | 0.033 copies/µL | 402 | 93.1 and 98.5 | No a | [27][29] |
| - | Cas12a | N | Nasal swab | Two | Fluorescence | Blue-light transilluminator | 40 min | 16 copies/µL | 12 | 100 and 100 | No a | [20] |
| RCSMS | Cas12a | E | Saliva | Two | Fluorescence and LFA | Fluorescence reader | 40 min | 5 copies/reaction | 276 | 93.8 and 99 | Yes (LFA) | [21] |
| CLAP | Cas12a | N | - | Two | Colorimetry | - | 40 min | 4 copies/µL | - | - | Yes | [28][30] |
| - | Cas12a | N, E | Nasopharyngeal swab | Two | Colorimetry | - | 45 min | 225 copies/µL | 54 | 92.6 and 100 | Yes | [29][31] |
| WS-CRISPR | Cas12a | N | - | One | Fluorescence | LED blue light or UV light | 90 min | 50 copies/μL | 32 | - | No b | [30][32] |
| dWS-CRISPR | Nasal swabs and saliva | 5 copies/μL | No b | |||||||||
| SherlockTM CRISPR SARS-CoV-2 | Cas13a | ORF1ab & N | Nasopharyngeal swab | Two | Fluorescence | Fluorescence reader | 1 h | ORF1ab-6.75 N-1.35 copies/µL |
60 | 100 and 100 | No a | [31][33] |
| DISCoVER | Cas13a | N | Nasal swab, saliva | Two | Fluorescence | Microfluidic cartridge, compact fluorescence reader | 35 min | 40 copies/μL | 63 | 93.9 and 100 | No a | [32][34] |
SARS-CoV-2: Severe acute respiratory syndrome coronavirus-2; RT-LAMP: Reverse transcription loop-mediated isothermal amplification; LFA: Lateral flow assay; UV: Ultraviolet; crRNA: CRISPR RNA; CRISPR: Clustered regularly interspaced short palindromic repeats.
: required non-portable complex instruments for on-site testing;
: required QuantStudio 3D digital chip. * Assay time excluding RNA extraction.
After viral RNA isolation, an amplification step was adopted by performing RT-LAMP to improve the sensitivity of the assay. RT followed by CRISPR-Cas-mediated detection was insufficient to detect viral RNA in samples with low SARS-CoV-2 viral loads [17], highlighting the importance of performing additional amplification before CRISPR-Cas detection. RT-LAMP was used to convert viral RNA into complementary DNA (cDNA), which was then amplified using a constant (60–65 °C) temperature. RT-LAMP does not require the use of a thermocycler, and the reaction time is shorter than that for the RT-qPCR approach. Furthermore, LAMP reagents are more widely available from various commercial sources, and the LAMP buffers are well-defined and can be systematically optimized with the Cas enzyme [17]. However, for the detection of SARS-CoV-2 RNA, RT-LAMP is performed through two sequential reactions: (1) the amplification of the viral RNA via RT-LAMP is performed; (2) a Cas endonuclease is used to detect the resultant amplicons [22][23][24,25]. In the two-pot reaction, the opening of tubes is required after RT-LAMP, increasing the risk of amplicon contamination. The amount of target nucleic acid in the tube following the RT-LAMP reaction was high. When the tubes are opened, the amplicons may generate aerosol contamination, which can lead to high false-positive rates. Thus, RT-LAMP-based detection is not permitted outside well-controlled laboratory settings [33][26]. As a result, various research has been performed to develop a one-pot approach to POC testing that simplifies operations and eliminates the contamination risk in the detection of SARS-CoV-2.
Cas12 proteins are effector nucleases of the Class 2 type V CRISPR-Cas systems. Cas12 enzyme performs two types of cleavage activity such as cis-cleavage (specific) activity against dsDNA targets and trans-cleavage (collateral or non-specific) activity on ssDNA non-targeted sequences. The collateral activity is the foundation for very specific and sensitive nucleic acid detection methods. In COVID-19 diagnosis, Cas12 endonucleases can randomly cleave the non-target DNA (reporter probe) once activated by a single- or double-stranded DNA target sequence complementary to their crRNA [4][34][4,36]. Two orthologs of the Cas12 family, Cas12a, and Cas12b, have been widely used to detect SARS-CoV-2 nucleic acid. Cas12b is significantly smaller and more thermostable than Cas12a [35][36][37,38]. In the RT-LAMP and Cas12-based SARS-CoV-2 RNA detection assays, the RT-LAMP amplicons are introduced to the Cas12/crRNA complex. After the specific binding of crRNA to the target DNA amplicon, Cas12 nuclease performs collateral cleavage on the non-target reporters, as shown in Figure 1 [4][22][4,24]. The fluorescence reporter probe is made up of a short ssDNA with a fluorophore on one end and a quencher on the other. Fluorescence is suppressed until Cas12 performs collateral cleavage and degrades the reporter probe. The fluorophore is then liberated, resulting in the production of a fluorescent signal.
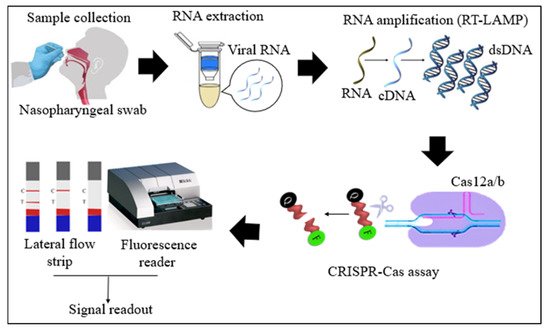
Figure 1. Schematic diagram of RT-LAMP CRISPR-Cas12a/b-based detection of SARS-CoV-2. Samples (nasopharyngeal swabs) were collected from symptomatic and asymptomatic individuals, and viral RNAs were extracted. With the RT-LAMP step, viral RNAs are first converted into cDNAs, which are subsequently amplified. The amplicons were targeted in CRISPR-Cas-based detection, and the results of the tests were visualized via colorimetry or a fluorescence assay.
Cas13 proteins are effector nucleases of the Class 2 type VI CRISPR-Cas systems. Unlike other Cas nucleases of Class 2 CRISPR-Cas systems, Cas13 targets single-stranded RNA (ssRNA) instead of DNA [4]. Cas13 also demonstrates collateral RNA cleavage activity when activated by a target RNA complementary to its crRNA. The stem-loop structure of the crRNA is critical for ssRNA cleavage. Therefore, Cas13a crRNA constructed with a single stem-loop specific to Cas13a and a protospacer domain-specific to the target [12]. The crRNAs must have minimal sequence overlap with the primer sequences; thus, a comparative in-silico analysis of RT-LAMP primers and guide RNAs with the targeted viral regions should be performed to avoid detection of non-specific amplification products by Cas13 to ensure specificity of assay [32][34]. As Cas13a proteins are triggered only by RNA targets, an additional T7 transcription step is required to convert the DNA amplicons to RNAs after the RT-LAMP reaction (Figure 2) [31][33]. In addition, as triggered Cas13a cleaves ssRNA rather than ssDNA, the reporter probe for the Cas13a system should be introduced as an ssRNA rather than ssDNA. Compared to Cas12, Cas13 enzymes have been used less frequently in RT-LAMP CRISPR-based SARS-CoV-2 detection studies because Cas13 requires an extra T7 transcription step and operates at lower temperatures than those at which RT-LAMP occurs.
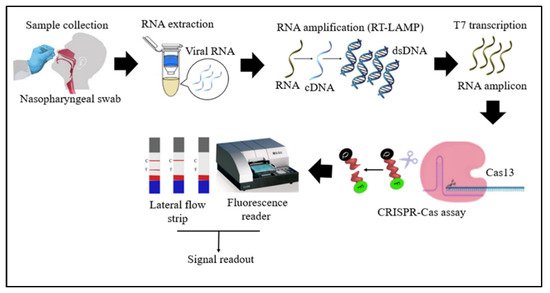
Figure 2. Schematic diagram of RT-LAMP CRISPR-Cas13-based detection of SARS-CoV-2. Samples (nasopharyngeal swabs) were collected from symptomatic and asymptomatic individuals, and viral RNAs were extracted. With the RT-LAMP step, viral RNAs are first converted into cDNAs, which are subsequently amplified. An additional step was required for Cas13-based detection: T7 transcription to convert DNA amplicons to RNA amplicons, which were then targeted in Cas13-based detection; the results of the test were visualized via colorimetry or a fluorescence assay.
Various readout mechanisms, especially fluorescence [8] and colorimetric [22][24] methods, have been developed for CRISPR-based assays to detect SARS-CoV-2. However, fluorescence signal measurement requires special instruments, such as a fluorescence reader, inverted epifluorescence microscope, handheld UV lamp, or 3D printing device, which are typically large, costly, and unsuitable for POC applications [4][8][24][4,8,22]. On the other hand, colorimetric assays are ideal for POC applications because these approaches are simple to use, inexpensive, and readily available. The lateral flow assay (LFA) seems to be the most popular colorimetric readout technique [22][24]. The LFA also enables POC testing solutions that can be used in areas where SARS-CoV-2 infection is most likely to spread—airports, public hospitals, and regional medical centers—especially in countries with limited resources. The LFA is appropriate for large-scale testing for the early diagnosis of SARS-CoV-2 carriers, enabling them to be efficiently isolated and quarantined, hence minimizing the transmission of the virus.
3. Conclusions
The use of RT-LAMP and CRISPR technologies in the detection of SAR-CoV-2 is a novel technique that has made significant progress and has the potential to be further developed. In most CRISPR-Cas-mediated SARS-CoV-2 detection methods, Cas12 or Cas13 proteins have been used as the CRISPR effectors. Patients might be able to obtain their COVID-19 testing results in less than an hour at a reasonable cost if this technique becomes available in hospital settings, which would become a vital tool during this disease outbreak. With these methods, on-site screening could be applied more widely to prevent asymptomatic carriers from spreading the infection to others unintentionally. Following the necessary improvements in the RT-LAMP CRISPR-based detection with a combination of LFA, it is expected to be offered in healthcare environments or as a diagnostic kit for use at home possibly in the future. To date, the FDA has only approved two CRISPR-based COVID-19 diagnostic tools for emergency use: SHERLOCK and DETECTR. Both are still two-step methods excluding RNA extraction, and SHERLOCK evaluates the data using a fluorescence reader, which is less effective for a POC test. Our recommendations for RT-LAMP CRISPR-mediated SARS-CoV-2 detection are (i) applying a rapid RNA extraction method or simplifying the RNA-extraction step followed by RT-LAMP amplification and CRISPR-mediated detection in one pot; (ii) performing CRISPR-Cas-based detection using thermostable Cas12b with double crRNA and optimized buffer conditions; and (iii) finally, visualizing the assay results using LFA. These optimizations will make CRISPR-based COVID-19 diagnostic tests more sensitive and easier to use and will further facilitate the applicability of these tests at the POC.
