The producers of essential oils from the Republic of Moldova care about the quality of their products and at the same time, try to capitalize on the waste from processing. The purpose of the present study was to analyze the chemical composition of lavender (Lavanda angustifolia L.) essential oil and some by-products derived from its production (residual water, residual herbs), as well as to assess their “in vitro” antimicrobial activity. The gas chromatography-mass spectrometry analysis of essential oils produced by seven industrial manufacturers led to the identification of 41 constituents that meant 96.80–99.79% of the total. The main constituents are monoterpenes (84.08–92.55%), followed by sesquiterpenes (3.30–13.45%), and some aliphatic compounds (1.42–3.90%). The high-performance liquid chromatography analysis allowed the quantification of known triterpenes, ursolic, and oleanolic acids, in freshly dried lavender plants and in the residual by-products after hydrodistillation of the essential oil. The lavender essential oil showed good antibacterial activity against Bacillus subtilis, Pseudomonas fluorescens, Xanthomonas campestris, Erwinia carotovora at 300 μg/mL concentration, and Erwinia amylovora, Candida utilis at 150 μg/mL concentration, respectively.
- Lavandula angustifolia L.
- essential oil
- by-products
- terpenic compounds
- chromatographic analyses
- antimicrobial activity
- statistical data analysis
1. Introduction
2. Discussion
2.1. Chemical Composition of Lavender Essential Oils
| No. |
|---|
| 96.80 |
| 97.62 |
| 99.11 |
| 98.51 |
| Class | Subclass | Content, (%) |
|---|---|---|
| Terpenic compounds | 94.89–97.77 | |
| Monoterpenes | 84.08–92.55 | |
| Monoterpene hydrocarbons | 8.72–15.32 | |
| Oxygenated monoterpenes | 69.00–83.83 | |
| Esters | ||
| 0.91–2.09 | ||
| Total | ||
| 96.80–99.79 |
| RT* |
|---|
| (min) |
|---|
| Component |
|---|
| Producer, Content (%) | |||||||
|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | |
| 1 | 4.416 | α-Pinene | 0.36 | 0.57 | 0.36 | 0.18 | 0.09 |
2.2. Chemical Composition of Lavender Plant Material
| Lavender Plant Material | Extract Yield (%) | Concentration (mg/g Extract) |
Concentration (mg/100 g Lavender Plant Material, DW) |
||
|---|---|---|---|---|---|
| OA | UA | OA | UA | ||
| LPM 1 | 9.94 | 16.19 | 37.46 | 160.95 | 372.36 |
| LPM 2 | 8.83 | 19.09 | 60.82 | 168.57 | 537.00 |
| LPM 3 | 9.91 | 13.43 | 33.28 | 133.11 | |
| Sesquiterpenes | 3.30–13.45 | ||||
| 329.83 | Sesquiterpene hydrocarbons | 3.09–12.83 | |||
| Oxygenated sesquiterpenes | 0.19–1.26 | ||||
| Aliphatic compounds | 1.42–3.90 | ||||
| Hydrocarbons | 0.17 | ||||
| Alcohols | 0.13–1.01 | ||||
| Ketones | 0.25–0.80 | ||||
| (mg/100 g Dry Solid Residue) | |||||
|---|---|---|---|---|---|
| OA | UA | OA | UA | ||
| SR 1 | 3.88 | 29.21 | 80.82 | 113.47 | 313.95 |
| SR 2 | 3.68 | 39.37 | 135.56 | 144.98 | 499.15 |
| SR 3 | 4.15 | 27.48 | 87.90 | 114.07 | 364.89 |
| 0.26 |
| 0.57 |
| 2 | 4.710 | Camphene | 0.34 | 0.47 | 0.30 | 0.09 | 0.09 | 0.26 | 0.63 |
| 3 | 5.179 | Sabinene | 0.14 | - | - | - | - | - | - |
| 4 | 5.240 | 1-Octen-3-ol | 0.83 | - | - | - | 0.15 | 0.27 | |
| 5 | 5.263 | β-Pinene | 0.51 | - | 0.34 | 0.39 | 0.38 | 0.22 | 0.57 |
| 6 | 5.398 | Octan-3-one | 0.28 | 0.51 | 0.31 | 0.25 | 0.39 | 0.12 | 0.21 |
| 7 | 5.489 | β-Myrcene | 1.06 | 1.50 | 0.96 | 0.80 | 0.89 | 0.62 | 0.89 |
| 8 | 5.577 | Octan-3-ol | 0.13 | 0.18 | 0.20 | 0.17 | 0.20 | - | - |
| 9 | 5.962 | n-Hexyl acetate | 0.59 | 1.27 | 0.31 | 0.42 | 0.55 | 0.52 | 1.17 |
| 10 | 6.284 | p-Cymene | 0.22 | 0.46 | 0.22 | 0.14 | 0.10 | 0.24 | 0.39 |
| 11 | 6.400 | Limonene | 0.52 | 1.79 | 0.79 | 0.45 | 0.55 | 1.17 | 1.93 |
| 12 | 6.455 | 1,8-Cineol (eucalyptol) | 5.00 | 3.81 | 2.22 | 3.73 | 4.44 | 3.83 | 9.29 |
| 13 | 6.574 | (E)-Ocimene | 8.06 | 5.87 | 6.85 | 7.86 | 4.37 | 5.25 | 7.15 |
| 14 | 6.807 | (Z)-Ocimene | 3.74 | 3.45 | 2.59 | 2.55 | 1.86 | 1.76 | 2.16 |
| 15 | 7.087 | γ-Terpinene | 0.10 | 0.29 | 0.29 | 0.06 | 0.07 | 0.07 | 0.15 |
| 16 | 7.443 | Linalool oxide | - | 0.17 | 0.07 | 0.11 | 0.12 | - | - |
| 17 | 7.824 | δ-Terpinene | 0.27 | - | - | - | - | - | - |
| 18 | 7.825 | α-Terpinolene | - | 0.58 | 0.25 | 0.21 | 0.32 | 0.16 | 0.23 |
| 19 | 8.238 | Linalool | 23.54 | 27.98 | 29.06 | 25.57 | 40.68 | 33.29 | 26.19 |
| 20 | 8.392 | Oct-1-en-3-yl acetate | 0.56 | 0.82 | 0.60 | 0.71 | 0.63 | 0.39 | 0.58 |
| 21 | 9.308 | Camphor | 0.47 | 0.47 | 0.37 | 0.36 | 0.30 | 0.32 | 0.61 |
| 22 | 9.847 | Borneol | 1.92 | 2.15 | 1.68 | 1.28 | 1.65 | 1.41 | 2.40 |
| 23 | 10.00 | (3E,5Z)-Undeca-1,3,5-triene | 0.17 | - | - | - | - | - | - |
| 24 | 10.15 | Terpin-1-en-4-ol | 1.30 | 4.65 | 5.98 | 0.94 | 1.67 | 1.41 | 1.03 |
| 25 | 10.41 | Cryptone | 0.29 | 0.29 | - | - | - | 0.30 | 0.33 |
| 26 | 10.50 | α-Terpineol | 2.42 | 3.31 | 2.02 | 1.42 | 7.95 | 1.49 | 1.61 |
| 27 | 11.49 | Nerol | 0.38 | 0.46 | 0.23 | 0.14 | 1.14 | - | - |
| 28 | 11.84 | p-Cumic aldehyde | 0.13 | 0.15 | - | - | - | 0.18 | - |
| 29 | 12.32 | Linalyl acetate | 26.55 | 20.26 | 28.65 | 32.25 | 16.68 | 33.30 | 28.10 |
| 30 | 13.01 | Bornyl acetate | 0.32 | 0.25 | 0.24 | 0.27 | 0.19 | 0.17 | 0.24 |
| 31 | 13.11 | Lavandulyl acetate | 4.88 | 2.84 | 2.36 | 4.83 | 4.78 | 2.56 | 3.07 |
| 32 | 14.98 | Neryl acetate | 0.78 | 0.91 | 0.39 | 0.33 | 1.53 | 0.31 | 0.37 |
| 33 | 15.47 | Geranyl acetate | 1.31 | 1.69 | 0.79 | 0.73 | 2.70 | 0.59 | 0.67 |
| 34 | 15.66 | α-Zingiberene | 0.15 | - | - | - | - | - | - |
| 35 | 16.47 | β-Caryophyllene | 6.25 | 5.33 | 4.62 | 5.44 | 1.64 | 4.93 | 4.32 |
| 36 | 16.80 | α-Bergamotene | 0.27 | 0.28 | 0.19 | 0.20 | 0.05 | 0.16 | - |
| 37 | 17.31 | (E)-β-Farnesene | 4.86 | 2.59 | 3.65 | 3.94 | 1.23 | 2.46 | 2.45 |
| 38 | 17.97 | β-Cubebene | 1.12 | 0.82 | 1.03 | 0.69 | 0.17 | - | - |
| 39 | 18.75 | γ-Cadinene | 0.18 | 0.53 | - | - | - | 0.68 | 0.65 |
| 40 | 20.39 | Caryophyllene oxide | 0.45 | 0.69 | 0.19 | 0.29 | 0.21 | 0.35 | 0.28 |
| 41 | 21.69 | Cadinol | 0.17 | 0.57 | - | - | - | 0.18 | - |
| Total content, (%) | 99.80 | 98.79 | 98.17 | ||||||
2.3. Chemical Composition of Lavender by-Products
| Lavender by-Product (Solid Residue, SR) | Extract Yield (%) | Concentration (mg/g Extract) |
Concentration | ||
|---|---|---|---|---|---|
2.4. Antimicrobial Assessments
| MBC and MFC, μg/mL | ||||||
|---|---|---|---|---|---|---|
| Sample | Bacillus subtilis |
Pseudomonas fluorescens |
Xantdomonas campestris |
Erwinia amylovora |
Erwinia carotovora |
Candida utilis |
| LEO | 300 | 300 | 300 | 150 | 300 | 150 |
2.5. Statistical Data Analysis
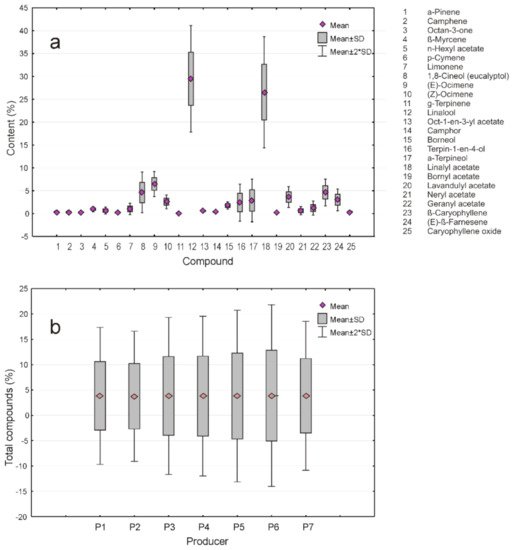
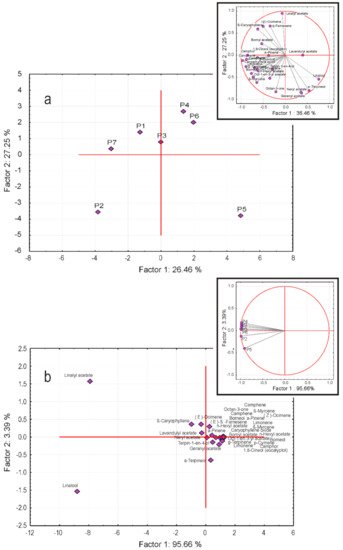
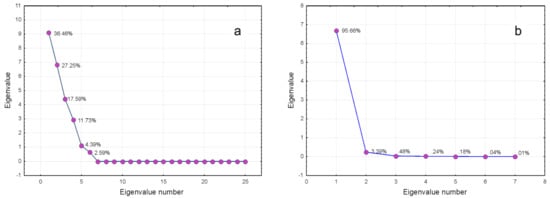
References
- Basch, E.; Foppa, I.; Liebowitz, R.; Nelson, J.; Smith, M.; Sollars, D.; Ulbricht, C. Monograph from National Standard: Lavender (Lavandula angustifolia Miller). J. Herb. Pharmacother. 2004, 4, 63–78.
- Denner, S.S. Lavandula angustifolia Miller. Holist. Nurs. Pract. 2009, 23, 57–64.
- Da Porto, C.; Decorti, D.; Kikic, I. Flavour compounds of Lavandula angustifolia Mill. To use in food manufacturing. Comparison of three different extraction methods. Food Chem. 2009, 112, 1072–1078.
- Erland, L.A.E.; Mahmoud, S.S. Lavender (Lavandula angustifolia) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.E., Ed.; Academic Press: Amsterdam, The Netherlands; pp. 501–507.
- Smigielski, K.; Sikora, M.; Majewska, M.; Raj, A. The application of essentials oils to natural and organic cosmetics. Pol. J. Cosmet. 2008, 11, 89–107.
- Prusinowska, R.; Smigielski, K. Composition, biological activity and therapeutic effects of lavander (Lavandula angustifolia L.): A review. Herba Pol. 2014, 60, 56–66.
- Hajhashemi, V.; Ghannadi, A.; Sharif, B. Anti-inflammatory and analgesic properties of the leaf extracts and essential oil of Lavandula angustifolia Mill. J. Ethnopharm. 2003, 89, 67–81.
- Hamad, K.J.; Al-Shaheen, S.J.A.; Kaskoos, R.A.; Ahanad, J.; Jameel, M.; Mir, S.R. Essential oil composition and antioxidant activity of Lavandula angustifolia from Irak. Int. Res. J. Pharm. 2013, 4, 117–120.
- Djenane, D.; Aıder, M.; Yanguela, J.; Idir, L.; Gomez, D.; Roncales, P. Antioxidant and antibacterial effects of Lavandula and Mentha essential oils in minced beef inoculated with E. coli O157:H7 and S. aureus during storage at abuse refrigeration temperature. Meat Sci. 2012, 92, 667–674.
- Adaszynska-Skwirzynska, M.; Swarcewicz, M.; Dobrowolska, A. The potential of use lavender from vegetable waste as effective antibacterial and sedative agents. Med. Chem. 2014, 4, 734–737.
- Mantovani, A.L.L.; Vieira, G.P.G.; Cunha, W.R.; Groppo, M.; Santos, R.A.; Rodrigues, V.; Magalhaes, L.G.; Crotti, A.E.M. Chemical composition, antischistosomal and cytotoxic effects of the essential oil of Lavandula angustifolia grown in South-eastern Brazil. Rev. Braz. Farmacogn. 2013, 23, 877–884.
- Shou-Dong, S.; Chang-Xu, C.; Ji-Shu, Q.; Ming-Hua, S. Study on antitumor effect of Lavender angustifolia extract. Food Sci. Technol. 2009, 2, 213–215.
- Martucci, J.F.; Gende, L.B.; Neira, L.M.; Ruseckaite, R.A. Oregano and lavender essential oils as antioxidant and antimicrobial additives of biogenic gelatine films. Ind. Crop. Prod. 2015, 71, 205–213.
- Tarek, N.; Hassan, H.M.; AbdelGhani, S.M.M.; Radwan, I.A.; Hammouda, O.; El-Gendy, A.O. Comparative chemical and antimicrobial study of nine essential oils obtained from medicinal plants growing in Egypt. Beni-Seuf Univ. J. Appl. Sci. 2014, 3, 149–156.
- Yamada, K.; Mimaki, Y.; Sashida, Y. Anticonvulsive effects of inhaling lavender oil vapour. Biol. Pharm. Bull. 1994, 17, 359–360.
- Aprotosoaie, A.C.; Gille, E.; Trifan, A.; Luca, V.S.; Miron, A. Essential oil of Lavandula genus: A systematic review of their chemistry. Phytochem. Rev. 2017, 16, 761–799.
- Smigielski, K.; Raj, A.; Krosowiak, K.; Grusca, R. Chemical composition of the essential oil of Lavandula angustifolia cultivated in Poland. J. Essent. Oil-Bear. Plants 2009, 12, 338–347.
- Hassanpouraghdam, M.B.; Hassani, A.; Vojodi, L.; Hajisamadi, A.B.; Rostami, A. Essential oil constituents of Lavandula angustifolia Chaix from Northwest Iran. Chemija 2011, 22, 167–171.
- Belhadj, M.M.; Kabouche, A.; Abaza, I.; Aburjai, T.; Tauzani, R.; Kabouche, Z. Chemotypes investigation of Lavandula essential oils growing at different North African soils. J. Mater. Environ. Sci. 2014, 5, 1896–1901.
- Cong, Y.; Abulizi, P.; Zhi, L.; Wang, X. Chemical composition of the essential oil of Lavandula angustifolia from Xinjiang, China. Chem. Nat. Compd. 2008, 44, 810.
- Renaud, E.N.C.; Denys, C.J.; Simon, J.E. Essential oil quantity and composition from 10 cultivars of organically grown lavender and lavandin. J. Essent. Oil Res. 2001, 13, 269–273.
- Jianu, C.; Pop, G.; Gruia, A.T.; Horhat, F.G. Chemical composition and antimicrobial activity of essential oils of lavender (Lavandula angustifolia) and lavandin (Lavandula x intermedia) grown in western Romania. Int. J. Agric. Biol. 2013, 15, 772–776.
- Zagorcheva, T.; Stanev, S.; Rusanov, K.; Atanassov, I. Comparative GC/MS analysis of lavender (Lavandula angustifolia Mill.) inflorescence and essential oil volatiles. Agric. Sci. Technol. 2013, 5, 459–462.
- Chatzopolou, P.S.; Goliaris, A.H.; Katsiotis, T. Contribution to the analysis of the volatile constituents from some lavender and lavandin cultivars grown in Greece. Sci. Pharm. 2003, 71, 229–234.
- Raina, A.P.; Negi, K.S. Comparative essential oil composition of Lavandula species from India. J. Herb Spice Med. Plants 2012, 18, 268–273.
- Verma, R.S.; Rahman, L.U.; Chanotiya, C.S.; Verma, R.K.; Chauhan, A.; Singh, A.; Yadav, A.K. Essential oil composition of Lavandula angustifolia Mill. cultivated in the mid hills of Uttarakhand. India. J. Serbian Chem. Soc. 2010, 75, 343–348.
- Jaeger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants-rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016–2031.
- Janicsak, G.; Veres, K.; Zoltan Kakasy, A.; Mathe, I. Study of the oleanolic and ursolic acid contents of some species of the Lamiaceae. Biochem. Syst. Ecol. 2006, 34, 392–396.
- Duke, J.A. Handbook of Biologically Active Phytochemicals and Their Activities, 1st ed.; CRC Press: Boca Raton, FL, USA, 1992; p. 208.
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68.
- Wolska, K.; Grudniak, A.; Fiecek, B.; Kraczkiewicz-Dowjat, A.; Kurek, A. Antibacterial activity of oleanolic and ursolic acids and their derivatives. Open Life Sci. 2010, 5, 543–553.
- Hsu, Y.L.; Kuo, P.L.; Lin, C.C. Proliferative inhibition, cell-cycle dysregulation, and induction of apoptosis by ursolic acid in human non-small cell lung cancer A549 cells. Life Sci. 2004, 75, 2303–2316.
- Chiang, L.C.; Chiang, W.; Chang, M.Y.; Ng, L.T.; Lin, C.C. Antileukemic activity of selected natural products in Taiwan. Am. J. Chin. Med. 2003, 31, 37–46.
- Zhang, W.; Hong, D.; Zhou, Y.; Zhang, Y.; Shen, Q.; Li, J.; Hu, L.; Li, J. Ursolic acid and its derivative inhibit protein tyrosine phosphatase 1B, enhancing insulin receptor phosphorylation and stimulating glucose uptake. Biochim. Biophys. Acta 2006, 1760, 1505–1512.
- Tian, Z.; Lin, G.; Zheng, R.X.; Huang, F.; Yang, M.S.; Xiao, P.G. Anti-hepatoma activity and mechanism of ursolic acid and its derivatives isolated from Aralia decaisneana. World J. Gastroenterol. 2006, 12, 874–879.
- Kim, Y.K.; Yoon, S.K.; Ryu, S.Y. Cytotoxic Triterpenes from Stem Bark of Physocarpus intermedius. Planta Med. 2000, 66, 485–486.
- Kashiwada, Y.; Nagao, T.; Hashimoto, A.; Ikeshiro, Y.; Okabe, H.; Cosentino, L.M.; Lee, K.H. Anti-AIDS agents 38. Anti-HIV activity of 3-O-acyl ursolic acid derivatives. J. Nat. Prod. 2000, 63, 1619–1622.
- Yan, S.; Huang, C.; Wu, S.; Yin, M. Oleanolic acid and ursolic acid induce apoptosis in four human liver cancer cell lines. Toxicol. In Vitro 2010, 24, 842–848.
- Kowalski, R. Studies of selected plant raw materials as alternative sources of triterpenes of oleanolic and ursolic acid types. J. Agric. Food Chem. 2007, 55, 656–662.
- Zheljazkov, V.D.; Astatkie, T. Effect of residual distillation water of 15 plants and three plant hormones on Scotch spearmint (Mentha × gracilis Sole). Ind. Crop. Prod. 2011, 33, 704–709.
- Tiliacos, C.; Gaydou, E.M.; Bessiere, J.-M.; Agnel, R. Distilled lavandin (Lavandula intermedia Emeric ex. Loise l) wastes: A rich source of coumarin and herniarin. J. Essent. Oil Res. 2008, 20, 412–413.
- Torras-Claveria, L.; Jauregui, O.; Bastida, J.; Codina, C.; Viladomat, F. Antioxidant Activity and Phenolic Composition of Lavandin (Lavandula x intermedia Emeric ex. Loiseleur). J. Agric. Food Chem. 2007, 55, 8436–8443.
- Khadzhieva, P.; Aleksiev, K.; Topalova, I. Di-and triterpenoids in essential oil waste-from pinus, lavender, and salvia. Int. Conf. Chem. Biotechnol. Biol. Act. Nat. Prod. 1987, 5, 519–524.
- Amorese, V.; Donadu, M.; Usai, D.; Sanna, A.; Milia, F.; Pisanu, F.; Molicotti, P.; Zanetti, S.; Doria, C. In vitro activity of essential oils against Pseudomonas aeruginosa isolated from infected hip implants. J. Infect. Dev. Ctries. 2018, 12, 996–1001.
- Yap, P.S.X.; Krishnan, T.; Yiap, B.C.; Hu, C.P.; Chan, K.-G.; Lim, S.H.E. Membrane disruption and anti-quorum sensing effects of synergistic interaction between Lavandula angustifolia (lavender oil) in combination with antibiotic against plasmid-conferred multi-drug-resistant Escherichia coli. J. Appl. Microbiol. 2014, 116, 1119–1128.
- Yap, P.S.X.; Lim, S.H.E.; Hu, C.P.; Yiap, B.C. Combination of essential oils and antibiotics reduce antibiotic resistance in plasmid-conferred multidrug resistant bacteria. Phytomedicine 2013, 20, 710–713.
- Kunicka-Styczyńska, A.; Śmigielski, K.; Prusinowska, R.; Rajkowska, K.; Kuśmider, B.; Sikora, M. Preservative activity of lavender hydrosols in moisturizing body gels. Lett. Appl. Microbiol. 2015, 60, 27–32.
- ISO 3515:2002 Oil of Lavender (Lavandula Angustifolia Mill.); ISO: Geneva, Switzerland, 2002.
- Ivanov, I.; Petkova, N.; Tumbarski, Y.; Vrancheva, R.; Stoyanova, M. Lavender waste–promising source of triterpenoids and polyphenols with antioxidant and antimicrobial activity. Ind. Technol. 2018, 5, 26–32.
- Piqué, N.; Miñana-Galbis, D.; Merino, S.M.; Tomás, J. Virulence Factors of Erwinia amylovora: A Review. Int. J. Mol. Sci. 2015, 16, 12836–12854.
- Benada, M.; Boumaaza, B.; Boudalia, S.; Khaladi, O.; Guessas, B. Variability of aggressiveness and virulence of Erwinia carotovora subsp. carotovorum causing the soft rot on potato tubers in the western of Algeria. Int. J. Plant Biol. 2018, 9, 52–56.
- Tamir-Ariel, D.; Navon, N.; Burdman, S. Identification of Genes in Xanthomonas campestris pv. vesicatoria induced during its interaction with tomato. J. Bacteriol. 2007, 189, 6359–6371.
- Davies, J.C. Statistics and Data Analysis in Geology; Elsevier: Amsterdam, The Netherlands, 2002.
- Shi, G. Cluster Analysis. In Data Mining and Knowledge Discovery for Geoscientists; Shi, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2014.
