2. Checkpoint Blockade
Checkpoint inhibitors are tumor-directed monoclonal antibodies that have established therapeutic efficacy in the treatment of various solid tumors such as lung, head-and-neck, and renal cancers in addition to melanoma
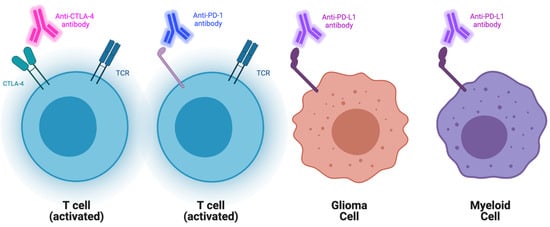
[8][9][10][11][22,23,24,25]. These antibodies specifically target immune checkpoint molecules (ICs), which normally function to attenuate T-cell function (
Figure 1). Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) are two of the most researched ICs. Inhibiting them has been shown to significantly augment the antitumor response
[12][13][26,27]. CheckMate 143 served as the first randomized phase I clinical trial that targeted the PD pathway in recurrent GBM. In this study, nivolumab (a PD-1 inhibitor), in addition to ipilimumab (a CTLA-4 inhibitor), were evaluated for safety and efficacy. All 40 patients in this study received surgical resection, radiation, and temozolomide before being divided into three treatment arms. Objective response rates (ORR) of 11% and 10% were observed in the NIVO3 and NIVO1+IPI3 treatment arms, respectively. The phase I trial highlighted that nivolumab monotherapy was better tolerated than nivolumab plus ipilimumab. Additionally, the tolerability of the combination therapy was seen to be influenced by ipilimumab dosage
[14][28]. The subsequent phase III clinical trial randomized 369 recurrent GBM patients to receive either nivolumab or bevacizumab, an antiangiogenic antibody that targets VEGF-A, which received accelerated FDA approval in recurrent GBM in 2009
[15][16][29,30]. Results from this study did not demonstrate an improved OS in patients with recurrent GBM when compared to bevacizumab monotherapy. When investigating the overall response rate (ORR), which describes the number of patients who have experienced a complete or partial response to therapy, it was noted to be lower with nivolumab than with bevacizumab
[17][31].
Figure 1. Schematic representation of current checkpoint blockades and their targets. Represented aCTLA-4, PD1, and PDL1 blockades and their main cellular targets.
Durvalumab (durva) is a human IgG1 monoclonal antibody that specifically targets PD-L1. Durva has already been approved in the treatment of non-small cell lung cancer in addition to bladder cancer in select patients
[18][19][32,33]. A phase II trial is currently investigating the efficacy and safety of durva in the treatment of newly diagnosed GBM. This trial served as the first study of anti-PD-L1 antibodies in the treatment of newly diagnosed GBM. There are five GBM cohorts; the data was published for cohort A. Cohort A evaluated durva, in addition to the radiotherapy (60 Grays over 30 fractions), followed by durva monotherapy in 40 patients with newly diagnosed GBM. No significant differences in overall survival were noted with this treatment regimen. The treatment was well tolerated when combined with radiotherapy and the adverse events (grade ≥ 3), as defined by the Common Terminology Criteria for Adverse Events (CTCAE), occurred in 35% of patients (14)—with the most common adverse event being the asymptomatic increase in lipase (six patients) and amylase (two patients). Another ongoing phase II study aims to determine the safety and efficacy of tremelimumab (CTLA-4 inhibitor) and durva as monotherapies or in combination as adjuvant therapy for recurrent GBM
[20][34]. The combination of varlilumab, an agonist anti-CD27 monoclonal antibody, and nivolumab has been investigated for refractory solid tumors
[21][35] in a phase I/II trial that monitored for adverse effects, dose-limiting toxicities, and laboratory abnormalities 100 days from the last study drug dose in 36 patients. CD27 is a known costimulatory molecule that is able to stimulate T-cells to proliferate, differentiate, and increase their effector response. The solid tumors investigated were ovarian carcinoma, colorectal cancer, melanoma, and squamous cell carcinoma of the head and neck. There were no unexpected toxicities, and there was encouraging evidence of antitumor activity in subsets of patients prompting further investigation. The subsequent trial will investigate the efficacy of the combination varlilumab and nivolumab at different dosages as measured by the overall survival of 12 months in GBM, along with the other previously investigated solid tumors.
Aside from the Checkmate 143 phase III trial, ORR has been demonstrated in two other checkpoint inhibitor studies
[22][23][36,37]. A single practice case series demonstrated an ORR of 31% in a total of 20 patients with recurrent GBM who were treated with ipilimumab plus bevacizumab
[22][36]. Although two patients could not complete the treatment regimen due to grade 2 adverse events, the treatment combination was well-tolerated overall
[22][36]. A phase I study of atezoliumab, an antibody targeting programmed cell death-ligand 1 (PD-L1), demonstrated an ORR of 6% in 16 patients with recurrent GBM
[23][37]. Despite the limited therapeutic efficacy of the checkpoint blockade in recurrent GBM, there has been enthusiasm in evaluating their efficacy in the neoadjuvant setting. In 2019, a single-arm phase II clinical trial analyzed pre- and post-surgical administration of nivolumab for 30 GBM patients, 27 with recurrent disease and 3 with newly diagnosed tumors. Despite the promising results of higher immune cell infiltration and T-cell receptor clonal diversity among tumor-infiltrating T lymphocytes, no clear clinical benefit was shown following salvage surgery for recurrent cases
[24][38]. Another multi-institutional randomized controlled trial evaluating the PD-1 inhibitor pembrolizumab was conducted by the Ivy Foundation Early Phase Clinical Trials Consortium in 35 patients with recurrent GBM. In this study, patients who received neoadjuvant and adjuvant pembrolizumab had a median OS of 417 days versus a median OS of 228.5 days in patients receiving adjuvant pembrolizumab only
[25][39]. Although limited by small sample size, the early results supported the expansion of the study and the pursuit of future clinical trials.
Ongoing trials continue to investigate CTLA-4, PD-1, and other potential checkpoint inhibitors such as indoleamine 2,3-dioxygenase (IDO). IDO is an endogenous enzyme that plays an important role in the regulation of the immune system functioning to augment suppressor activity of regulatory T-cells and consequently inhibit CD8+ T-cells
[26][27][40,41]. Studies have shown that 50 to 90 percent of GBMs express IDO, which is correlated with poor prognosis
[28][29][42,43]. In vitro, IDO inhibitors have been shown to slow tumor growth through the improvement of anti-tumor T-cell responses
[30][44]. A phase Ib/II trial is currently ongoing to evaluate the IDO inhibitor indoximod in newly diagnosed GBM patients
[31][45]. Their early work in mouse models has highlighted a synergistic effect of indoximod when used in conjunction with temozolomide and radiation. The primary endpoint of the phase II trial will be six months PFS. Epacadostat, another IDO inhibitor, is being investigated in an ongoing phase I/II trial. This trial aims to identify the safety and feasibility when administered with nivolumab in subjects with advanced solid tumors and lymphomas
[32][46]. Phase II will include expansion cohorts in 7 tumor types, including GBM.
3. Limitation
GBM presents unique challenges when approaching treatment from an immunotherapeutic angle. GBM is largely immunosuppressive to the systemic immune system as well as to neighboring cells alike
[33][165]. This largely arises from the complex interactions of cytokines, extracellular matrix proteins, and other diverse cell populations. Although their complex interactions are not fully understood, studies have highlighted numerous examples of the far-reaching consequences of this microenvironment. Microglia and macrophages are examples of important players in the tumor microenvironment and adjacent regions. Along with myeloid-derived suppressor cells (MDSCs), these cells work in conjunction to inhibit cytotoxic T-cells as well as accentuate the effect of Treg cells
[34][35][36][166,167,168]. More than just tumor cells influence immunosuppression; the tumor microenvironment is also a site of chronic inflammation. It is believed that these inflammatory stimuli serve to impact the blood-brain barrier (BBB) as well as activate microglia cells within the CNS
[37][169]. These cells are recruited via various chemoattractants, and they can make up as much as 50% of the tumor mass
[38][170].