Microorganisms can be useful or hazardous, and some microbes show unrecognizable effects. Microbes with beneficial effects are important and are utilized for CO
2 fixations, the degradation of complex organic compounds into basic molecules, fermentation, and many more applications. Furthermore, microorganisms can have a harmful effect, causing infections or diseases such as anthrax, conjunctivitis, ringworm, influenza, and many more
[1]. Treatment of such health issues caused by microbes can be performed by appropriate diagnosis followed by adequate medication or therapy
[2]. Antimicrobial compounds are considered a convenient solution for such health issues and can be of low molecular weight (LMW) or high molecule weight (HMW). Diverse relationships have been observed between the molecular weight and antimicrobial activity of these compounds. However, the majority of researchers report an increase in antimicrobial activity with the decrease in the degree of polymerization (i.e., molecular weight)
[3]. It is expected that the adhesion of HMW antimicrobial polymers to a negatively charged bacterial cell membrane should be highly effective in comparison to the adhesion of LMW antimicrobial polymers. Instead, contradicting results are found, according to which LMW antimicrobial polymers have greater biocidial activity
[4]. Usually for bacterial infections, antibiotics are the most commonly used drug for treatment, which show adverse effects such as reducing immunity, increasing susceptibility to infections, and developing resistance against antimicrobial agents
[5].
Antimicrobial peptides (AMPs) are short-chain (5 to 100) amino acids that possess the ability to counter microbial attacks or any infective agent in all living organisms
[7]. AMPs act as the first line of defense to disrupt bacterial, fungal, yeast, viral, and even cancer cells
[8]. Most AMPs are found to be cationic, and are considered promising agents due to their distinctive mode of action and the inability of microbes to develop resistivity against them
[9][10][9,10]. However, some of the AMPs are also anionic in nature due to the acidic polar residues, and these consist of short Asp-rich sequences
[11]. More than 2500 AMPs are present in nature, which are broadly grouped into four categories: α helical, β sheet, extended, and αβ mixed antimicrobial peptides
[12][13][12,13]. α helical AMPs possess a distance of about 0.15 nm between two adjacent bond angles (for example, LL-37)
[14]. The β Sheet peptides are rigid structures that are more organized in an aqueous solution and do not show conformational change on membrane linkage (for example, Gomesin)
[15]. Extended peptides comprise a high amount of arginine, tryptophan, proline, and/or histidine residues (for example, indolicidin and α1-purothionin)
[16][17][18][16,17,18]. On the basis of activity, AMPs can also be classified as antimicrobial, antiprotozoal, anticancer, insecticidal, and antiparasitic
[19]. AMPs can be harvested from invertebrates, vertebrates, and plants
[20]. The first AMPs derived from certain sources were as follows: Cecropin from Hyalophora cecropia
[21], defensis from rabbit
[22], purothionin from wheat flour
[23], and Gramicidin D from Bacillus brevis
[24]. Among all the sources, some of the most valuable sources of AMPs with future prospects are marine animals
[25], wild plants and weeds
[26], and the black soldier fly
[27].
Similar to AMPs, a group of polymers exists, known as synthetic antimicrobial oligomers, which are cationic and amphiphilic in nature
[28][32]. These antimicrobial agents are fabricated to imitate AMPs
[29][33]. However, these oligomers have a drawback of heterogeneity and innate toxicity
[30][34]. Thus, antimicrobial peptides have the advantage of low toxicity over the synthetic antimicrobial oligomers and are highly effective in microbial disruption
[31][35]. Recent studies have shown the impactful results of using AMPs in the biomedical sector. This article is different from various other papers on AMPs because it not only includes information about the factors affecting the functioning of AMPs and their important role, but along with it, focuses on the implementation techniques of AMPs in the biomedical sector. In this review, smart and intelligent delivery methods are also considered. Thus, this review could be helpful for readers that require compact information about the role of AMPs in biomedical applications. The schematic representation of this review is shown in
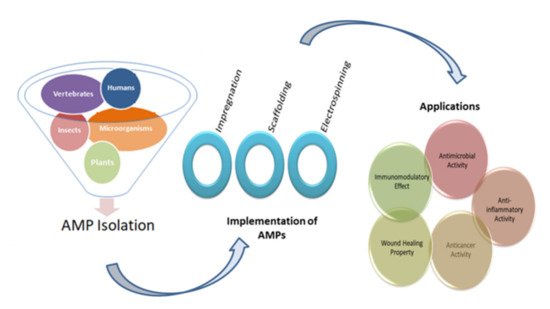
Figure 1.