Flowering, the beginning of the reproductive growth, is a significant stage in the growth and development of plants. Conifers are economically and ecologically important, characterized by straight trunks and a good wood quality and, thus, conifer plantations are widely distributed around the world. In addition, conifer species have a good tolerance to biotic and abiotic stress, and a stronger survival ability. Seeds of some conifer species, such as Pinus koraiensis, are rich in vitamins, amino acids, mineral elements and other nutrients, which are used for food and medicine. Although conifers are the largest (giant sequoia) and oldest living plants (bristlecone pine), their growth cycle is relatively long, and the seed yield is unstable. Flowering and seed yields in conifers are affected by a variety of factors, such as pollen, temperature, light, water availability, nutrients, etc., and a number of management techniques, including topping off, pruning, fertilization, hormone treatment, supplementary pollination, etc. has been developed for improving cone yields.
- conifers
- flowering
- seed production
- pollination
- tree management
- nutrient fertilization
1. Factors Affecting Seed Formation and Development
1.1. Pollen
1.2. Temperature and Light
Low temperature affects germination by regulating the vernalization of seeds [9]. Vernalization is a process that depends on a chilling requirement to produce flowering and a good fruit crop. For example, peaches need a few hundred hours below 7 °C to satisfy the chilling requirement to break dormancy, promote flowering and have successful fruit production (
accessed on 31 March 2021). The overall germination rate and the time needed for germination of seeds varies significantly under different temperature conditions [10][11][12]. A suitable temperature is also a precondition for flower bud formation and an important factor affecting pollen longevity and viability [13]. In addition, the number of male cones is generally more than female cones in conifers, which is not an ideal situation. Many experimental results may be caused by the different mechanisms of male and female flower buds in response to temperature, as temperature indirectly regulates the sex expression of conifer species by changing the hormone balance in flower buds [14][15]. Conifer seeds typically can be stored in the cold, e.g., at around zero degrees + or −2 °C for several months, then planted to break dormancy. Temperature signals can also regulate the activity of various enzymes and affect the metabolism in various biochemical reactions of conifer species; thus, becoming a vital participant in photosynthesis and respiration [16][17][18].
Light is also a crucial environmental signal and the main energy source for the plant’s photosynthesis and respiration [19]. There is a high canopy density with many lateral and dead branches that affect the supply of normal light in conifers. Light intensity, spectral composition and photoperiod are important factors for conifer species growth and development, and they influence numerous physiological and biochemical reactions that cause changes in their morphology and reproductive characteristics [20]. In addition, different tree species have different demands and reaction mechanisms for light, which are affected by external environmental factors as well as their biological characters [21][22]. With the increase in forest age and the crown canopy density, the available light under the canopy will decrease, and flowering and reproduction will inevitably be affected. Therefore, an appropriate planting density, pruning and topping off can ameliorate the insufficient light of conifer species [23].
1.3. Water and Nutrient Fertilization
Water and nutrient fertilization are equally indispensable for plant seed formation and development [24]. Plant roots absorb nutrients from the soil, which is rich in minerals and organic matter to feed seed formation. Nutrient fertilization typically provides nitrogen, phosphate and potassium. Nitrogen deficiency often limits growth due to the need for substantial amounts being needed for biosynthesis of proteins and nucleic acids, while phosphorous is needed for energy metabolism and nucleic acid biosynthesis. Potassium is needed for salt balance, transport of water and nutrients. Fertilization maintains the stability of mineral circulation in the soil, which ensures the support capacity of the soil for plants and promotes the biosynthesis of proteins, amino acids and vitamins [25][26]. Plant growth and development are the result of the interaction of water and fertilizer. Water provides a good moist environment for plant growth, which determines the activity of roots and microbes, and contributes to the construction of a good root system [27]. In the Southeastern US, with extensive plantations of southern pines, fertilization is a common practice. In this region, chronic deficiencies are found for both nitrogen and phosphorus [28]. The internal rate of return from mid-rotation fertilization of nitrogen and phosphorus was calculated to be 16%. Because most conifer species grow in mountainous areas with poor soil and harsh environmental conditions, the nutrients are typically limited in the soil [29]. Water and fertilization not only can effectively improve the soil environment, but can regulate the nutrient supply and growth, photosynthesis and other metabolic processes [30][31].
1.4. Molecular Mechanisms
The molecular mechanisms of flowering and seed production are complex, and the related genes can directly or indirectly interfere with the sex differentiation of flower, time of anthesis and seed development, resulting in the seed formation differences among individuals or species. Flower-related genes, such as
(Flowering locus T) and MADS-box (
,
,
and
box), have been identified to play a crucial role in flowering in conifer trees.
In the study of
and
genes by RT-PCR and RACE technology, and found that the two genes were highly expressed during the development of male and female cones, respectively, suggesting that they were involved in the development of flowers and seed formation. The
gene is an important member of the regulation of sunshine length between the circadian clock and flowering time genes, which can combine light signals with circadian clock signals to regularly activate the expression of the
gene; thus, inducing flowering. In
, the study of the effects of photoperiod on the
gene transcription and seedling growth showed that
activates
transcription to control flowering [34][35]. The plant
gene encodes a class of plant-specific transcription factors, which play an important role in the transition from vegetative to reproductive growth of flowering plants. The
and
genes have a similar function in conifers; the
gene interacts with the
gene by cloning and yeast two-hybrid techniques, and it is speculated that the
gene in
may act as a transcriptional cofactor to regulate the
gene activity and, thus, participate in the regulation of the flower meristem development [36]. MADS-box genes, a class of important transcriptional regulatory factors in eukaryotes (animals, plants and fungi), play an important role in growth and development regulation and signal transduction [37][38]. There are studies that show that three MADS-box genes (
,
and
),
and
(
) were highly participated in the early stages of initiation and differentiation of
male and female cone buds, as well as vegetative buds [39]. In another study, a transcriptome data analysis showed that the enhanced transcriptional activity of MADS-box transcription factors was also closely related to the formation of early cones, in particular the process of sex reversal [40][41]. Thus, it can be also speculated that MADS-box transcription factors are a key gene family in the molecular mechanism of conifer flowering and seed production. The research on the molecular mechanism of flowering and seed production of conifers is still scarce. Therefore, it is still the mainstream direction of future research. Despite a long growth cycle and large genome compared with the broad-leaved trees, conifer species were developed and used with some traditional tree breeding methods.
2. Technical Measures of Breeding and Management
In forest tree breeding, selecting parents with good characters is the basis of tree improvement. Efficient management technology is the guarantee of a high quality and yield. Conventional methods, including artificial pollination, hormone treatment, pruning, water and fertilization coupling, can promote the flowering and reproduction of conifer species. One technical measure that greatly improves the efficient management of a genetic improvement program is genetic fingerprinting, which provides a high level of quality control on genetic selection [42]. In addition to providing an accurate inventory, DNA markers can estimate genetic diversity, define genetic load, estimate the degree of inbreeding, identify quantitative trait loci (QTLs) and using high throughput genomic markers allow for a highly efficient genomic selection where many genomic markers associated with favorable traits can be selected simultaneously [43].
2.1. Supplementary Pollination
Plant inbreeding refers to the combination of male and female gametophytes on the same plant, or the mating between individuals with the same genotype [44]. Because of the high genetic diversity and load, close genetic relationships can lead to poor selection. Inbred lines are rare in conifer breeding programs [45][46]. The genetic characters of inbred offspring are mediocre, including growth, cone and wood traits [47]. Most conifer species are monoecious with a high possibility of inbreeding, which causes a high abortion rate of flowers and low cone yields [48]. Therefore, supplemental pollination avoids inbreeding and increases the efficiency of crosses. This method can be applied to open pollination or to controlled crosses to effectively make up for low natural pollination. The pollen with desired genes is scattered on the stigma of female flowers to create more fertilization events [47]. Understanding the details of flowering is needed to improve the sustainability and effectiveness of pollination. In the study of Douglas fir, (
), the rate of external pollination and the inbreeding rates were 10% to 28% and 12% to 17%, respectively [49]. In addition to high inbreeding, pollen contamination from unwanted genotypes is a serious detriment to defined breeding. In Scots pine (
), a hand pollinator was used on female cones at the end of May. The amount of pollen per individual plant was 0.06~0.08 mL, and the success rate was 66–84%; whereas the success rate was 10–23% when the pollination amount per plant was 0.03–0.05 mL that was applied by a long aluminum pole duster [50]. The highest success rate of pollination was 69% using pressurized backpack sprayers to pollinate the female flowers. Therefore, methods and amounts are also critical factors affecting the success of pollination.
Supplementary pollination can introduce elite pollen into female cones to broaden the genetic base, reduce pollen contamination, increase cone yield and maximize genetic gain [51]. Understanding the flowering habits of each individual and selecting elite parents are crucial for the efficient improvement of conifer species. It is essential to select the best plants with good characters, fast growth, a high yield and allowing a robust pollen collection. Furthermore, weather condition is a key factor affecting the success rate of pollination [52]. As Additionally, the well-timed isolation of cones (method and bags) of selected mother trees has a crucial importance in the process of artificial pollination.
2.2. Topping Off
Topping off (cutting off the apical meristem) inhibits apical dominance, controls shape and height, improves light conditions and increases seed yields of trees [53]. When topping off was carried out on
for seven consecutive years, the cone yields for each individual tree increased by 95.5% and quality by 17.2% [54]. In
, the upper two-wheel branches were topped off, and the results showed an average growth rate for female cones and male cones increased by 372% and 238% [55]. Sun [56] carried out topping off on
for three years, and the best result for topping off was to cut the first wheel branch, and then the numbers of female and male cones increased by 2–4-fold. Similar results were obtained in Chen et al. [57] and Tan et al. [58] studies on
.
Topping off can reduce the tree height of conifers, which is more convenient for cone collection and improves cone quality. The topping off treatment should be carried out in late autumn or early spring because conifer species grow slowly and have low physiological activity during those periods [55][56]. The target trees should be 10~20 m tall and have vigorous growth. For such trees, it is advisable to cut off a fifth to quarter of the tree height. For some conifer species with a height of 20~30 m, a quarter to third of the overall height can be topped off. For particularly tall trees, the topping off height should not exceed one half of the tree height to avoid inhibition of further growth. After a topping off treatment, some new branches of conifer species will regenerate at the top in the second year, which will serve as cone setting branches, thereby increasing cone yields [55].
Adult conifer species with big crowns hardly form ideal tree shapes under natural conditions. Thus, some measures such as topping off and branch pruning should be used to control the tree shape and improve cone yields [59] (
1). Many new branches are induced from new lateral meristems after topping off in the first year, then the trees create a new leader and regain apical dominance. Therefore, the topping off treatment should be implemented on lateral branches in consecutive years. The final ideal tree shape is umbrella-like and has a large canopy, low tree height, many lateral branches and new shoots [57]. When conifer species reach the mature seed production age, the number of flowers and fertile seeds increases significantly.
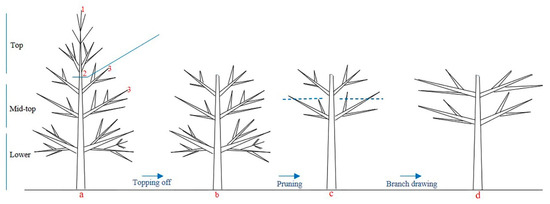
A schematic presentation of the topping off (
), pruning (
) and branch drawing (
) practices to improve flowering, panel (
) shows ideal tree shape, where in panel a 1 represents terminal buds, 2 represents topping off position on trunk and 3 represents lateral branch.
2.3. Thinning and Pruning
Thinning, a primary forestry tree management method, is the main treatment to reduce stand density. Its main function for trees is to provide light and to improve nutrient utilization efficiency [60][61][62], as well as carbon uptake and drought resistance [63]. Thinning allows faster growth rates, reduces root competition and combined with pruning results in longer rotation times and great improvements in wood quality. Thinning treatment of
, retaining 300 trees per hectare, with 75% intensity, increased the cone yield of each individual tree by 8~10-fold. When 750 trees per hectare were reduced by 37.5% thinning intensity, the total yield of cones increased three-fold compared to an un-thinned stand [64]. In
(stone pine), when 350 trees were retained per hectare, the cone yield of each individual tree increased by 1.48 times, while a high thinning intensity reduced the cone yield [65]. Appropriate timing and reasonable intensity are the basic requirements of thinning. Based on the evaluation of different traits such as growth, wood and cone characters, some individuals with poor performance were selected as the thinning targets [66][67]. Based on aforementioned research, the optimal thinning time was before the stage of tree sap flow or after cone shedding. For different site conditions and species characteristics, optimal thinning intensity may be different, and a poor choice of thinning methods may result in the reduction in yield [63]. In summary, conifer forests with a high density should maintain 500~700 trees per hectare after thinning in the first year, and thinning intensity should maintain 300~400 trees per hectare after 2~3 years.
Pruning is another effective measure to improve the light transmittance of trees in plantations. It refers to the removal of parts or redundant lateral branches, which can improve tree shape and increase the effect of ventilation and light transmittance [68]. Pruning may have dramatic effects on wood quality by reducing knots and promoting the growth of long straight stems. The content of trace elements in needles changes after pruning, which can improve the biomass and productivity of trees [69][70][71]. In
and
, the yields and quality of individual trees were significantly improved after pruning [72][73]. In another study, 3–4 main branches were kept in each round of the
crown while the remaining branches were cut off results in an increase in the number of cones of each individual tree by 14.3% [74]. Therefore, reasonable pruning plays a key role in the growth and development of conifers [75][76]. The stage of tree sap flow is the optimal time for pruning because the ability of trees to recover is strong and wound healing is fast.
2.4. Girdling and Cutting Roots
Girdling is a method involving wounding of the phloem of the trunk or main branches to regulate the distribution of nutrients in the trees so as to promote flowering and reproduction. Studies on girdling in conifers are focused on its impacts on cell activity [77], wood characteristics [78][79] and photosynthesis and respiration [80], but only a few investigate its effect on flowering and reproduction dynamics. In a study on Japanese larch (
), girdling carried out at 1.2 m above the ground in April resulted in a significantly higher average cone yield than the control group [81].
Girdling should be carried out on trees growing in good soil conditions, with vigorous growth and no infestation/infection by pests or pathogens. The girdling time should be one month before bud differentiation. The specific method would be to girdle the bark of the trunk or main branches with a knife around the trunk with a width of 1~2 cm below the DBH (100~120 cm from the ground) and cut the bark to the xylem in depth. If the tree is very large, the girdling width can be enlarged. After 3~5 years, the number of flowers and developing seeds will improve. Girdling does damage to the tree itself, which may lead to a weakened resistance to pests and pathogens, and nutrients should be added in time to accelerate conifer growth after girdling.
Conifer species absorb nutrients from the soil by their roots. Root cutting is the process of removing excess virtual roots from the ground and limiting root growth. The study conducted on winter wheat (
) has shown that the water consumption and water use efficiency were low after root cutting [82][83]. It also affects the photosynthesis, translocation and stress resistant of plants [84]. However, root cutting is rarely conducted in conifer species. In a study of
, root cutting was carried out in three seed orchards of different ages in May at about 2 m from the trunk on both sides of each tree with sharp steel plates to a soil depth of 40 cm. There was no significant difference in the number of male cones, but the number of female cones was doubled [85]. In
, a circular groove was opened at a distance of 1.7 m from the trunk to cut off roots in April, and the number of female and male flowers increased two to three-fold and one to two-fold, respectively [56]. Summing up the above, the treatment time of conifer species generally chosen before the stage of tree sap flow and an annular groove is dug around the tree 1.5~2 m away from the trunk of the tree.
References
- Huber, M.; Maschewsky-Schneider, U. Studies on the pollination characteristics and pollination level of Chinese fir seed orchard. Silvae. Genet. 2004, 53, 7–11.
- Owens, J.N.; Takaso, T.; Runions, C.J. Pollination in conifers. Trends Plant Sci. 1998, 3, 479–485.
- Williams, C.G. Long-distance pine pollen still germinates after meso-scale dispersal. Am. J. Bot. 2010, 97, 846–855.
- Chybicki, I.; Oleksa, A. Seed and pollen gene dispersal in Taxus baccata, a dioecious conifer in the face of strong population fragmentation. Ann. Bot. 2018, 122, 409–421.
- Anderson, E.; Owens, J. Microsporogenesis, pollination, pollen germination and male gametophyte development in Taxus brevifolia. Ann. Bot. 2000, 86, 1033–1042.
- Tretyakova, I.N.; Noskova, N.E. Scotch Pine pollen under conditions of environmental stress. Russ. J. Ecol. 2004, 35, 20–26.
- Batos, B.; Miljković, D. The vitality of the Serbian spruce (Picea omorika) pollen during the long-term cryopreservation. Grana 2019, 58, 433–446.
- Gottardini, E.; Cristofori, A.; Cristofolini, F.; Maccherini, S.; Ferretti, M. Ambient levels of nitrogen dioxide (NO2) may reduce pollen viability in Austrian pine (Pinus nigra Arnold) trees—Correlative evidence from a field study. Sci. Total Environ. 2008, 402, 299–305.
- Almqvist, C.; Bergsten, U.; Bondesson, L.; Eriksson, U. Predicting germination capacity of Pinus sylvestris and Picea abies seeds using temperature data from weather stations. Can. J. For. Res. 1998, 28, 1530–1535.
- Chanyenga, T.F.; Geldenhuys, C.J.; Sileshi, G.W. Germination response and viability of an endangered tropical conifer Widdringtonia whytei seeds to temperature and light. S. Afr. J. Bot. 2012, 81, 25–28.
- Henderson, I.R.; Shindo, C.; Dean, C. The need for winter in the switch to Flowering. Annu. Rev. Genet. 2003, 37, 371–392.
- Johnsen, Ø.; Dæhlen, O.G.; Østreng, G.; Skrøppa, T. Daylength and temperature during seed production interactively affect adaptive performance of Picea abies progenies. New Phytol. 2005, 168, 589–596.
- Rossi, S.; Isabel, N. The timing of bud break in warming conditions: Variation among seven sympatric conifer species from Eastern Canada. Int. J. Biometeorol. 2017, 61, 1983–1991.
- Tikhonova, I.V. Changes in the sex structure of pine populations related to temperature anomalies. Russ. J. Ecol. 2007, 38, 306–310.
- Guo, C.C.; Shen, Y.B.; Shi, F.H. Effect of temperature, light, and storage time on the seed germination of Pinus bungeana Zucc. ex Endl.: The role of seed-covering layers and abscisic acid changes. Forests 2020, 11, 300.
- Josef, U.; Ingwers, M.W.; Anne, M.G.M.; Teskey, R.O. Increase in leaf temperature opens stomata and decouples net photosynthesis from stomatal conductance in Pinus taeda and Populus deltoides x nigra. J. Exp. Bot. 2017, 68, 1757–1767.
- Atkin, O.K.; Tjoelker, M.G. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci. 2003, 8, 343–351.
- Han, X.Y.; Turgeon, R.; Schulz, A.; Liesche, J.; Epron, D. Environmental conditions, not sugar export efficiency, Limit the length of conifer leaves. Tree Physiol. 2018, 39, 312–319.
- Rozendaal, D.M.A.; Hurtado, V.H.; Poorter, L. Plasticity in leaf traits of 38 tropical tree species in response to light; Relationships with light demand and adult stature. Funct. Ecol. 2006, 20, 207–216.
- Salgado-Luarte, C.; Gianoli, E. Herbivory may modify functional responses to shade in seedlings of a light-demanding tree species. Funct. Ecol. 2011, 25, 492–499.
- Sachin, R.S.; Nicolas, D.; Rosario, G.G.M. Transcriptome analysis of shade avoidance and shade tolerance in conifers. Planta 2019, 250, 299–318.
- Calfapietra, C.; Tulva, I.; Eensalu, E.; Perez, M.; Angelis, P.D.; Scarascia-Mugnozza, G.; Kull, O. Canopy profiles of photosynthetic parameters under elevated CO2 and N fertilization in a poplar plantation. Environ. Pollut. 2005, 137, 525–535.
- Almqvist, C.; Jansson, G. Effects of pruning and stand density on cone and pollen production in an experimental Pinus sylvestris seed orchard. Silva. Fenn. 2015, 49, 1243.
- Vanderschaaf, C.L. Estimating understory vegetation response to multi-nutrient fertilization in Douglas-fir and ponderosa pine stands. J. For. Res. 2008, 13, 43–51.
- Drake, J.E.; Stoy, P.C.; Jackson, R.B.; DeLucia, E.H. Fine-root respiration in a loblolly pine (Pinus taeda L.) forest exposed to elevated CO2 and N fertilization. Plant Cell Environ. 2008, 31, 1663–1672.
- Noguchi, K.; Nagakura, J.; Kaneko, S. Biomass and morphology of fine roots of sugi (Cryptomeria japonica) after 3 years of nitrogen fertilization. Front. Plant Sci. 2013, 4, 347.
- Dobbertin, M.; Eilmann, B.; Bleuler, P.; Giuggiola, A.; Pannatier, E.G.; Landolt, W.; Schleppi, P. Effect of irrigation on needle morphology, shoot and stem growth in a drought-exposed Pinus sylvestris forest. Tree Physiol. 2010, 30, 346–360.
- Fox, T.R.; Lee, A.H.; Albaugh, T.J.; Rafael, R.; Carlson, C.A. Tree nutrition and forest fertilization of pine plantations in the southern United States. South. J. Appl. For. 2007, 31, 5–11.
- Timmer, V.R.; Miller, B.D. Effects of contrasting fertilization and moisture regimes on biomass, nutrients, and water relations of container grown red pine seedlings. New For. 1991, 5, 335–348.
- Peng, X.X.; Guo, Z.; Zhang, Y.J.; Li, J. Simulation of long-term yield and soil water consumption in apple orchards on the Loess Plateau, China, in response to fertilization. Sci. Rep. 2017, 7, 5444.
- King, N.T.; Seiler, J.R.; Fox, T.R.; Johnsen, K.H. Post-fertilization physiology and growth performance of loblolly pine clones. Tree Physiol. 2008, 28, 703–711.
- Chen, H.; Yang, Z.Q.; Tan, J.H.; Feng, Y.H.; Jia, J.; Tang, G.Q.; Jun, O. Molecular cloning PmFT1 gene and its effects on floral development of Pinus msaaoniana. Genom. Appl. Biol. 2015, 34, 806–812.
- Chen, H.; Li, M.J.; Zhong, Y.F.; Yang, Z.Q.; Hang, Y.L. Cloning and expression analysis of PmEMF2 of Pinus massoniana. Guangxi For. Sci. 2015, 44, 225–231.
- Bao, S.; Hua, C.; Shen, L.; Yu, H. New insights into gibberellin signaling in regulating flowering in Arabidopsis. J. Integr. Plant Biol. 2020, 62, 118–131.
- Yan, J.P.; Mao, D.; Liu, X.M.; Wang, L.L.; Xu, F.; Wang, G.Y.; Zhang, W.W.; Liao, Y.L. Isolation and functional characterization of a circadian-regulated CONSTANS homolog (GbCO) from Ginkgo biloba. Plant Cell Rep. 2017, 36, 1387–1399.
- Wang, J.J. Functional Analysis of Flowering-Related LFAFY, UFO and MADS-Box Genes in Metasequoia glyptostroboides; Beijing Forestry University: Beijing, China, 2019.
- Ye, L.X.; Zhang, J.X.; Hou, X.J.; Qiu, M.Q.; Wang, W.F.; Zhang, J.X.; Hu, C.G.; Zhang, J.Z. A MADS-box gene CiMADS43 is involved in citrus flowering and leaf development through interaction with CiAGL9. Int. J. Mol. Sci. 2021, 22, 5205.
- Song, G.Q.; Han, X. K-Domain technology: Constitutive expression of a blueberry keratin-Like domain mimics expression of multiple MADS-box genes in enhancing maize grain yield. Front. Plant Sci. 2021, 12, 844.
- Mouradov, A.T.; Glassick, B.; Hamdorf, R.D. Molecular control of early cone development in Pinus radiata. Protoplasma 1999, 208, 3–12.
- Uddenberg, D.; Reimegård, J.; Clapham, D.; Almqvist, C.; Arnold, S.; Emanuelsson, O.; Sundström, J.F. Early cone setting in Picea abies acrocona is associated with increased transcriptional activity of a MADS-box transcription factor. Plant Physiol. 2013, 161, 813–823.
- Xiao, F.; Yang, X.M.; Zhao, Y.; Fan, F.H. Transcriptome analysis of Pinus massoniana Lamb. microstrobili during sexual reversal. Open Life Sci. 2018, 13, 97–106.
- Neale, D.B.; Devey, M.E.; Je Rmstad, K.D.; Ahuja, M.R.; Alosi, M.C.; Marshall, K.A. Use of DNA markers in forest tree improvement research. New For. 1992, 6, 391–407.
- Dario, G.; Orzenil, B.S.J.; Rafael, T.R.; Eduardo, P.C.; Barbara, S.F.M.; Biyue, T.; Fikret, I.; Blaise, R.; Yousry, A.E.K. Quantitative genetics and genomics converge to accelerate forest tree breeding. Front. Plant Sci. 2018, 9, 1693.
- Robledo-Arnuncio, J.J.; Alia, R.; Gil, L. Increased selfing and correlated paternity in a small population of a predominantly outcrossing conifer Pinus sylvestris. Mol. Ecol. 2010, 13, 2567–2577.
- Klekowski, E.J. Genetic load and its causes in long-lived plants. Trees 1988, 2, 195–203.
- Kärkkäinen, K.; Savolainen, O. The degree of early inbreeding depression determines the selfing rate at the seed stage: Model and results from Pinus sylvestris (Scots pine). Heredity 1993, 71, 160–166.
- Chautá-Mellizo, A.; Campbell, S.A.; Bonilla, M.A.; Thaler, J.S.; Poveda, K. Effects of natural and artificial pollination on fruit and offspring quality. Basic Appl. Ecol. 2012, 13, 524–532.
- Bester, C.; van der Merwe, L.H.C.; Malema, J.L. Controlled pollination in Pinus Patula: Constraints and possible solutions. South. Afr. For. J. 2000, 189, 109–112.
- Song, J.; Blaise, R.; Tony, K.; Lai, B.S.; Jiří, K.; El-Kassaby, Y.A. Temporal quantification of mating system parameters in a coastal Douglas-fr seed orchard under manipulated pollination environment. Sci. Rep. 2018, 8, 11593.
- Eriksson, U.; Yazdani, R.; Wilhelmsson, L.; Danell, O. Success rate of supplemental mass pollination in a seed orchard of Pinus sylvestris L. Scand. J. For. Res. 1994, 9, 60–67.
- Sun, W.; Yu, D.; Dong, M.; Zhao, J.; Wang, X.; Zhang, H.; Zhang, J. Evaluation of efficiency of controlled pollination based parentage analysis in a Larix gmelinii var. principis-rupprechtii Mayr. seed orchard. PLoS ONE 2017, 12, e0176483.
- Torimaru, T.; Wennstro¨m, U.; Andersson, B.; Almqvist, C.; Wang, X.R. Reduction of pollen contamination in Scots pine seed orchard crop by tent isolation. Scand. J. For. Res. 2013, 28, 715–723.
- Han, S.U.; Kang, K.S.; Kim, C.S.; Kim, T.S.; Song, J.H. Effect of top-pruning in a clonal seed orchard of Pinus koraiensis. Ann. For. Res. 2013, 51, 155–156.
- Wang, F.; Li, S.; Li, J.; Guan, C.; Wang, H. Effects of dwarfing treatment of clonal seed orchard on seed and seed quality of Pinus sylvestris var. Mongolica. J. Northeast. For. Univ. 2017, 45, 26–28.
- Yang, P.H.; Fan, J.F.; Liu, Y.H.; Han, C.J.; Xie, J.F.; Sun, W.P. Effects of pruning on blossom of seed trees in clonal seed orchard of Pinus tabulaeformis. Shanxi For. Sci. Technol. 2010, 3, 12–14.
- Sun, W.S. Study on Management Techniques of Korean Pine Seed Orchard for High Genetic Quality and Ample Production of Seeds; Beijing Forestry University: Beijing, China, 2006.
- Chen, H.; Ou, J.; Li, H.X.; Guo, F.; Yang, Z.Q. Blossom and cone setting of second-generation clonal orchard of dwarf Pinus massoniana L. J. West China For. Sci. 2020, 49, 9–15.
- Tan, X.M.; Jin, G.Q.; Zhang, Y.; Qin, G.F.; Chu, D.Y.; Zhou, Z.C. Genetic variation of flowering and fruiting in dwarfed second-generation clonal seed orchard of Pinus massoniana. J. Northeast. For. Univ. 2011, 39, 39–42.
- Du, C.Q.; Xu, Y.Z.; Quan, Y.S.; Hu, L.X. Pruning effects on old seed tree in seed orchard of Larix kaempferi. Hubei For. Sci. Technol. 2012, 50, 74–77.
- Hale, S.E. The effect of thinning intensity on the below-canopy light environment in a Sitka spruce plantation. For. Ecol. Manag. 2003, 179, 341–349.
- Blanco, J.A.; Castillo, I.F.J. Thinning affects nutrient resorption and nutrient-use efficiency in two Pinus sylvestris stands in the Pyrenees. Ecol. Appl. 2009, 19, 682–698.
- Park, J.; Kim, T.; Moon, M.; Cho, S.; Ryu, D.; Kim, H.S. Effects of thinning intensities on tree water use, growth, and resultant water use efficiency of 50-year-old Pinus koraiensis forest over four years. For. Ecol. Manag. 2018, 408, 121–128.
- Sohn, J.A.; Saha, S.; Bauhus, J. Potential of forest thinning to mitigate drought stress: A meta-analysis. For. Ecol. Manag. 2016, 380, 261–273.
- Nguyen, T.T.; Tai, D.T.; Zhang, P.; Razaq, M.; Shen, H.L. Effect of thinning intensity on tree growth and temporal variation of seed and cone production in a Pinus koraiensis plantation. J. For. Res. 2019, 30, 835–845.
- Moreno-Fernández, D.; Caellas, I.; Calama, R.; Gordo, J.; Sánchez-González, M. Thinning increases cone production of stone pine (Pinus pinea L.) stands in the Northern Plateau (Spain). Ann. For. Sci. 2013, 70, 761–768.
- Liang, D.Y.; Ding, C.J.; Zhao, G.H.; Leng, W.W.; Zhang, M.; Zhao, X.Y.; Qu, G.Z. Variation and selection analysis of Pinus koraiensis clones in northeast China. J. For. Res. 2018, 29, 611–622.
- Zhang, H.; Zhang, Y.; Zhang, D.W.; Dong, L.H.; Liu, K.J.; Wang, Y.; Yang, C.P.; Chiang, V.L.; Tigabu, M.; Zhao, X.Y. Progeny performance and selection of superior trees within families in Larix olgensis. Euphytica 2020, 216, 212–222.
- Stoehr, M.; Hollefreund, C.; Webber, J.; Hewson, C.; Ross, S. Effects of crown-pruning on seed and pollen cone production in two lodgepole pine seed orchards in British Columbia. New For. 1995, 10, 133–143.
- Proe, M.F.; Mead, D.J.; Byrne, D. Effect of pruning on nitrogen dynamics within crowns of Pinus radiata. Tree Physiol. 2000, 20, 653–661.
- Nuorteva, H. Increased boron concentrations of Scots pine foliage induced by green pruning. Can. J. For. Res. 2002, 32, 1434–1440.
- Hevia, A.; álvarez-González, J.G.; Majada, J. Comparison of pruning effects on tree growth, productivity and dominance of two major timber conifer species. For. Ecol. Manag. 2016, 374, 82–92.
- Wang, M. Effects of several pruning measures on growth and yield of Pinus sylvestris var. Mongolica on sandy land. Prot. For. Sci. Technol. 2017, 48, 9–10.
- Wang, Y. Effects of thinning and pruning on growth and bearing of artificial Pinus koraiensis. Gansu Agric. Sci. Technol. 2016, 44–47.
- Zhang, L.Y.; Xu, S.T.; Du, Y.H. Effect of pruning and pollination on the seed orchard of Pinus sylvestris. Liaoning For. Sci. Technol. 1999, 14–15+17. Available online: https://xueshu.baidu.com/usercenter/paper/show?paperid=ee62cc6aa176004ca29aaf2efb7bdee6&site=xueshu_se (accessed on 15 February 2021).
- Bose, A.K.; Aaron, W.; Christian, K.; Wagner, R.G.; Eric, T.; Burkhart, H.E. Tree-level growth and survival following commercial thinning of four major softwood species in North America. For. Ecol. Manag. 2018, 427, 355–364.
- Trentini, C.P.; Campanello, P.I.; Villagra, M.; Ritter, L.; Ares, A.; Goldstein, G. Thinning of loblolly pine plantations in subtropical Argentina: Impact on microclimate and understory vegetation. For. Ecol. Manag. 2017, 384, 236–247.
- Wilson; Brayton, F. Effect of girdling on cambial activity in white pine. Can. J. Bot. 1968, 46, 141–146.
- Jean-Christophe, D.; Pruyn, M.L. Bole girdling affects metabolic properties and root, trunk and branch hydraulics of young ponderosa pine trees. Tree Physiol. 2008, 28, 1493–1504.
- Wilson, B.F.; Gartner, B.L. Effects of phloem girdling in conifers on apical control of branches, growth allocation and air in wood. Tree Physiol. 2002, 22, 347–353.
- Binkley, D.; Stape, J.L.; Takahashi, E.N.; Ryan, M.G. Tree-girdling to separate root and heterotrophic respiration in two Eucalyptus stands in Brazil. Oecologia 2006, 148, 447–454.
- Lee, W.Y.; Lee, J.S.; Lee, J.H.; Noh, E.W.; Park, E.J. Enhanced seed production and metabolic alterations in Larix leptolepis by girdling. For. Ecol. Manag. 2011, 261, 1957–1961.
- Ma, S.C.; Li, F.M.; Xu, B.C.; Huang, Z.B. Effects of root pruning on the growth and water use efficiency of winter wheat. Plant Growth Regul. 2009, 57, 233–241.
- Sharma, R.B. Plant-water relations in wheat as influenced by root pruning. Plant Soil 1987, 98, 429–432.
- Stupendick, J.T.; Shepherd, K.R. Root regeneration of root-pruned Pinus radiata seedlings. II. Effects of root-pruning on photosynthesis and translocation. N. Z. J. For. Sci. 1980, 10, 148–158.
- Hogberg, K.A.; Eriksson, U. Effects on root pruning and stem injections with gibberellin A4/7 on flowering and cone harvest in three Picea abies seed orchards. Scand. J. For. Res. 1994, 9, 323–328.
