Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lily Guo and Version 3 by Conner Chen.
Since the discovery of the human T-cell leukemia virus-1 (HTLV-1), cellular and animal models have provided invaluable contributions in the knowledge of viral infection, transmission and progression of HTLV-associated diseases. HTLV-1 is the causative agent of the aggressive adult T-cell leukemia/lymphoma and inflammatory diseases such as the HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP).
In this entry, authors recapitulate the most effective animal models applied to investigate the pathogenesis of HTLV-1-associated diseases such as transgenic and humanized mice, rabbit and monkey models.
- HTLV
- humanized mice
- ATL
1. Introduction
Human T-cell leukemia virus type 1 (HTLV-1), isolated in the early 1980s from T cell lines derived from patients with cutaneous T-cell lymphoma and human adult T-cell leukemia, was the first human retrovirus to be discovered [1][2]. Since then, four HTLV types have been isolated in humans and have been phylogenetically associated with the simian STLV viruses [3][4]. In contrast to the HIV retrovirus, no efficient therapy is yet available to avoid the onset of the most alarming diseases caused by HTLV-1. In vivo, HTLV-1 infects mainly CD4+ T cells, the key cells in the triggering and establishment of the adaptive immune response. Besides being the etiological agent of adult T-cell leukemia/lymphoma (ATL), in 3–5% of infected subjects, HTLV-1 causes immune hypersensitivity conditions like arthritis, uveitis, and most importantly, the HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP), a fatal chronic inflammatory neurological disorder [5][6][7][8]. Most infected people, however, remain asymptomatic, highlighting the role of the immune system in the control of infection [9][10][11]. Worldwide, more than 20 million subjects are infected by HTLV and, despite advances in treatment, patients with aggressive ATL generally have a poor prognosis [12][13].
HTLV-1 persistent infection is likely associated with the ability of the virus to evade the host’s immune response. Immune evasion might correlate with high proviral load and thus to disease outcome. HTLV-1 infection occurs exclusively through cell-to-cell contact, and the number of infected cells in vivo significantly impacts on viral spreading [14]. After primary infection, the clonal expansion of infected cells, rather than the novo infection of cells, represents the main route for HTLV-1 to establish persistent and chronic infection [15] (Figure 1).
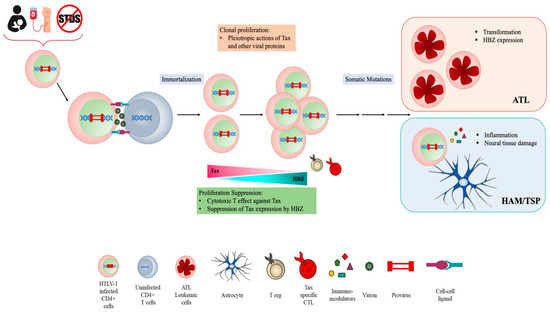
Figure 1. HTLV cell infection and transmission.
The proviral genome is integrated into the host genome and contains, in addition to structural proteins Gag, Pol and Env, a unique pX region coding for several regulatory and nonstructural proteins Tax, Rex, p12, p13, p30 and HTLV-1 basic zipper protein (HBZ), which is encoded by the antisense viral transcript. Among them, Tax and HBZ are thought to play key roles in HTLV-1 infection and oncogenesis. Transgenic mice expressing Tax or HBZ develop neoplastic diseases, indicating that they function as oncogenes [16][17][18][19]. Tax is a potent activator of viral transcription and exerts pleiotropic effects on cell signaling deregulating different cellular pathways thus mainly contributing to HTLV-1 induced neoplastic transformation. However, the frequent loss of Tax expression from ATL cells suggests that the viral protein is mainly involved in the onset of leukemic transformation. By contrast, HBZ is ubiquitously expressed, playing a crucial role in the maintenance of oncogenic process and disease progression. Furthermore, recent reports from our laboratory have demonstrated that HBZ subcellular localization could be a prognostic marker of HTLV-1-disease progression, as HBZ is expressed solely in the cytoplasm of asymptomatic carriers (AC) and HAM/TSP subjects, while in tumor cells isolated from leukemic patients, it is also present in the nucleus [20][21][22]. HBZ antagonizes many of the activities of Tax and suppresses Tax-induced viral transcription, thus the interaction between Tax and HBZ may significantly affect the outcome of HTLV-1 infection [23][24][25].
Although intensive studies in recent years have contributed to shedding light on the mechanisms of viral replication and host response, several aspects of HTLV-1 pathogenesis remain poorly understood, including the intimate molecular mechanism(s) of tumorigenesis, the progression of HTLV-1 leukemia toward the aggressive form, and the possibility of enhancing the host response to avoid or at least delay disease onset.
2. Animal Models
Animal models, including mice, rats, rabbits, squirrel monkeys, baboons, macaques, and even fruit flies, although not the natural hosts of HTLV infection, may help in elucidating some aspects of HTLV infection, persistence, host immune response, and diseases-associated developments [26][27][28][29][30]. In the following sections, we will discuss the most recent advances in the knowledge of HTLV infection and pathogenesis derived by studies in animal models.
2.1. Mouse Models
Although immunocompetent murine cells are not productively infected with HTLV-1, xenograft and transgenic mice are widely used for the study of HTLV-1 infection and related diseases. Starting from the late 1990s, when the C3H/HeJ and BALB/c strains were used to establish persistent infection injecting HTLV-1-producing MT-2 cell intraperitoneally in neonatal mice, several HTLV immunocompromised mouse models have been further developed [31][32][33][34]. The development of SCID mice, unable to perform VDJ recombination of B- and T-cell receptors because of a nonsense mutation in the PRKDC (Protein Kinase, DNA-Activated, Catalytic Subunit protein kinase) gene, has generated animals with severe combined immunodeficiency (SCID). These mice allow the engraftment of human cells. By introducing additional genetic mutations, several other types of SCID immunocompromised mouse strains become available, i.e., NOD-SCID mice, in which SCID mutation is present in a non-obese diabetic (NOD) genetic background mouse that shows NK cell dysfunction, low cytokine production, and T- and B-cell deregulation; NSG and NOG mice, in which different mutations in the interleukin-2 receptor common subunit γ (IL2R-γC) leading to a complete loss of T, B, and NK cells, are introduced into the NOD/SCID background; and BALB/c mice deficient in IL2R-γC and the recombinase-activating gene 2 (Rag2) (BRG), which are impaired in T- and B-cell differentiation and have high levels of NK-cell activity [35].
SCID xenograft mouse models can reproduce some features of HTLV-1 disease, such as multiple organ engraftments with ATL cells, expression of parathyroid hormone-related protein (PTHrP), a mediator of hypercalcemia in ATL patients, and increased levels of IL2 Rα and β-2 microglobulin [36][37][38]. These xenograft mouse models have contributed to the recapitulation of splenomegaly and lymphoma similar to ATL pathologic features [39]. Several studies have reported the successful engraftments in NOG mice of HTLV-1-transformed cell lines, ATL cells, and PBMCs from asymptomatic HTLV-1 carriers [40][41][42]. Engrafted SCID mice have also been used to assess the tumorigenic potential of ATL cell lines [43]. In NOG mice, a highly tumorigenic ATL cell was selected by serial xenotransplantation of patient leukemic cells and used to study features of ATL such as the involvement of carbonic anhydrase IX (CA9), a membrane-associated enzyme that regulates cellular pH. It was found that CA9 is upregulated and promotes tumorigenicity of ATL-derived cells [44]. A highly penetrant in vivo model of HTLV-1-induced T-cell lymphoma was established by intraperitoneally engrafting immune-compromised NOD/SCID mice with tumorigenic HTLV-1-transformed SLB1 and MET-1 lymphoma T cell lines. In this model, a cooperative role was found between the viral p30II latency regulatory factor and the cellular TP53-induced glycolysis and apoptosis regulator (TIGAR) in cancer progression, highlighting TIGAR involvement in tumor lymphocyte infiltration [45]. NOD/SCID mice injected with leukemic cells (MET-1) from a patient with ATL were proposed as preclinical in vivo murine models of ATL [46]. In this model, the ATL therapeutic efficacy of selected compounds has been reported. Among other treatments, the efficacy of daclizumab, a monoclonal antibody against the IL-2R-α (CD25), combined with depsipeptide, a member of the cyclic peptide class of HDAC inhibitors, was tested by analyzing the survival of the leukemia-bearing mice and the levels of soluble IL-2R-α and β2μ levels. Both depsipeptide and daclizumab led to inhibition of tumor growth and prolonged the survival of mice with leukemia suggesting its potential use in the treatment of ATL patients [38].
NOD/SCID mice have recently been used to evaluate a new therapeutic agent for ATL. In this study, NOD/SCID mice were injected with S1T cells, an HTLV-1-infected CD4 + T cell line derived from an ATL patient, and treated with dorsomorphin, an inhibitor of the bone morphogenetic protein (BMP) and AMP-activated protein kinase (AMPK) pathway. The administration of dorsomorphin to NOD/SCID mice proved to be efficient in reducing tumor growth [47]. In another mouse model, the efficacy of monoclonal antibodies in ATL therapy was investigated targeting the matricellular molecule OPN, which is known to participate in cancer processes by interaction with integrins. NSG mice inoculated with ATL cells present increased plasma levels of OPN, and when treated with a monoclonal antibody against OPN tumor growth, invasion and metastasis were inhibited [48]. More recently, the same group examined the antitumor effects of 2′-deoxy-2′-methylidenecytidine (DMDC) and its derivative 2′-deoxy-2′-methylidene-5-fluorocytidine FDMDC in NOG mice inoculated subcutaneously with an ATL-derived cell line. They observed that NOG mice bearing ATL tumor treated with the two compounds resulted in significant inhibition of tumor growth suggesting that nucleosides may be proposed as therapeutic agents in ATL [49].
Antitumor effects of autologous Tax-specific cytotoxic T cell (CTS) have also been tested in NOG mice bearing human primary ATL cells. Tax-CTL treatment led to Tax-specific CTL infiltration in the tumor site, recognition and blocking of the proliferation of autologous ATL cells and prolongation of mouse survival [50], although the reproducibility of this finding is not constant [51].
2.1.1. Humanized Mouse Models
Humanized mouse models derived from mouse xenotransplanted with human cells or engineered to express human genes may be used to study human-specific function in physiological and pathological conditions, most of them related to the human immune system [52]. In HTLV, humanized mouse models are mostly applied for studying the tropism and proliferation of HTLV-infected T cells, but also to elucidate the mechanism of in vivo development of ATL. Humanized mice infected with HTLV-1 may develop ATL, but they are not always consistent in reproducing the human immune responses against HTLV-1 [39][53]. An interesting model was generated by transplanting CD133+ human stem cells into the bone marrow cavity of NOD/Shi-scid/IL-2Rγc null (NOG) mice. These mice, named IBMI-huNOG mice, recapitulate distinct ATL-like symptoms, such as hyperproliferation of CD3+ T cells, clonal proliferation of CD25+ /CD4+ T cells, formation of flower cells in the peripheral blood, hepatosplenomegaly, inflammatory hypercytokinemia, and an adaptive immune response against HTLV-1 [54]. Humanized mice have also been employed to study HAM/TSP neuropathogenesis in an in vivo model. Balb/c-Rag1-hu −/− γc −/− (Rag1) and Bone Marrow Liver Thymic (BLT) humanized mice (hu-mice) were engrafted with human CD34+ hematopoietic stem cells and were able to reconstitute human macrophages, dendritic cells, T cells, and B cells [55]. Both models may be susceptible to HTLV-1 infection presenting Tax expression in the spleen and CNS. They also show myelin disruption resembling HTLV-1-associated neuropathogenesis.
Recently, humanized mice that cannot mount an adaptive immune response were obtained by injecting human umbilical-cord stem cells into the livers of immunodeficient NSG mice, and these were applied in the study of T cell tropism and lymphoproliferation of HTLV [56]. In these models, a different tropism of HTLV-1 compared to HTLV-2 was confirmed. HTLV-1 infection is associated with the preferential proliferation of CD4+ T cells, whereas CD8+ T proliferation is associated with HTLV-2. Notably, both viruses are lymphomagenic in mice, in contrast to human leukemia–lymphoma induction, which is typically associated only with HTLV-1 infection, suggesting that the adaptive immune response is critical in conditioning the lymphoma development. A relevant limit in applying the humanized mouse models is the development of graft-versus-host diseases (GVHD), which may cause the early death of mice or inefficiency in recapitulating, within the short lifetime of the mice, the complexity of events that occur in humans over decades of persistent virus prior to ATL development. This limitation was highlighted in a recent study using two humanized mouse models [57]. The authors investigated the role of p8 and p12 regulatory proteins in HTLV-1 infectivity and pathogenicity. p8 and p12, expressed by the open reading frames of the viral genome (orf-I), are required for persistent infection of primary human peripheral blood mononuclear cells in vitro and macaques in vivo [58][59], but are not required in rabbit models of HTLV-1 infection [60][61]. Using NSG-1d mice originated by NOD/SCID/γc −/− c-kit+ engrafted with human tissues and NSG mice implanted with human fetal liver, thymus tissue and stem cells (BLT mice), the authors demonstrated that these humanized mice were highly susceptible to HTLV-1 infection with the rapid polyclonal proliferation of CD4+ CD25+ T cells, similarly to the events in the healthy carrier stage of HTLV infection, although they did not reproduce the monoclonal origin of ATL, as happens in humans [57]. As proposed by the authors, these models may be valid for studying the early phase of HTLV-1 infection and proving interventions that may reduce the CD4+ proliferation induced by the virus.
In addition to the numerous studies aimed at dissecting the molecular function of the Tax and HBZ viral protein in in vitro cellular model interesting contributions towards interpreting their role in vivo in the lymphoproliferative process have been derived using humanized mouse models. Recently, the contribution of the Tax PDZ binding motif (PBM) to T-cell proliferation was analyzed in humanized mice carrying a human hemato-lymphoid system. It was shown that Tax-PBM enhanced HTLV-1-mediated T-cell proliferation compared to a PBM-deleted mutant, and that this domain is required for T-cell proliferation. Furthermore, comparative transcriptome analyses of T cells derived by humanized mice infected with wt and mutant Tax showed that the absence of PBM is associated with the deregulation of genes involved in T-cell signaling and proliferation, apoptosis induction, and cytoskeletal organization [62]. Taking advantage of humanized mice, the role of HBZ in altering the expression of the receptor activator of NF-κB ligand (RANKL), a regulator of osteoclast differentiation, was evaluated in vivo. In this HTLV-1-infected humanized mouse model, treatment with denosumab, a monoclonal antibody against human RANKL, resulted in reduced bone loss [63].
2.1.2. Transgenic Mouse Models
Transgenic mice have been generated mostly to analyze the oncogenic potential of Tax and HBZ viral protein. Indeed, Tax expression in transgenic mice is sufficient to induce tumors, confirming the in vivo oncogenic potential of Tax [27][64]. An interesting transgenic mouse model was developed by introducing the firefly luciferase gene driven by the HTLV-1 LTR (LTR-LUC) in transgenic Tax mice. The double transgenic Tax-Luc mice develop lymphoma, splenomegaly, hypercalcemia, osteolytic bone selections, and persistent activation of neutrophils [65]. The same team demonstrated that IL-15-deficient Tax-LUC mice developed an aggressive lymphoma and an increased expression of IL-α, thus suggesting IL- 15 and anti IL-1α as potential targets for ATL therapies [66].
To restrict Tax expression to the thymus, Tax transgenic mice have been generated using lymphocyte-specific protein–tyrosine kinase (lck) promoters. These Lck-Tax mouse models develop lymphoma and leukemia after a long latency period of almost 18 months and present most of the characteristics of acute ATL patients [67][68]. Tax transgenic models have also been used to test in vivo the efficacy of ATL therapy [69]. SCID mice injected with spleen cells from Tax transgenic mice developed ATL-like tumors. Treatment of these mice with arsenic/IFN-α or synthetic retinoid ST11926 compound resulted in a significant increase in animal survival [70][71]. In addition, normal syngenic mice injected with ATL cells from Tax-transgenic mice showed inefficient Tax-specific T-cell induction and ATL cells elimination [72].
As for Tax, the in vivo role of HBZ has been studied in HBZ transgenic (HBZ-Tg) mice. HBZ is the only regulatory/accessory gene encoded by HTLV-1 to be expressed in all ATL patients and necessary for the proliferation of ATL cells [23]. Mice expressing HBZ under the Granzyme B promoter (Gzmb-HBZ) developed lymphoproliferative disease and hypercalcemia [73]. HBZ transgenic models in which HBZ expression is restricted to CD4+ are preferentially used to study the inflammatory process correlated with HTLV-1–mediated pathogenesis. These HBZ-Tg mice develop systemic inflammation and T-cell lymphoma [74], and show higher levels of the immunosuppressive cytokine IL-10 and dysfunctional Treg cells [23][75]. In an interesting HBZ-Tg-based model, it was recently demonstrated that HBZ plays a pivotal role in dysregulating the cytokine signaling modulating the IL-10/JAK/STAT signaling pathway. As expected, in HBZ-Tg the loss of IL-6 and expression of IL 10 accelerates inflammation and lymphomagenesis [25]. HBZ-Tg-derived T-cell lymphoma has also been used to establish an HBZ-induced T cell line, named Ht48, which has been used to test an HBZ-targeted HTLV-1 vaccine. This model identified a candidate peptide (HBZ157-176) for vaccine development by using rVV-vaccinated mice [76].
PBMC-humanized NSG mice and HBZ-transgenic (Tg) mice, which develop systemic inflammation, were recently used to validate the efficacy of administration of pentosan polysulfate (PPS), a semisynthetic glycosaminoglycan, to counteract HTLV-1 infection and pathological sequelae. PPS blocked HTLV-1 infection in huPBMC NSG and suppressed the development of dermatitis and lung damage in HBZ-Tg mice, supporting the therapeutic use of PPS in the treatment of HTLV-1-induced inflammatory diseases [77].
Tax-transgenic (Tax-Tg) and HBZ Tg mouse models have contributed to identifying functional ATL stem cells (ATLSC) and determining that c-kit, a common surface marker of ATLSCs, is a key regulator of ATL disease initiation and progression [78]. Unexpected results were obtained using a double transgenic mouse model expressing both Tax and HBZ in CD4+ cells. These mice developed T-cell lymphoma but not ATL-like leukemia, suggesting that the balancing effect of Tax/HBZ expression is critical for oncogenic outcome [79]. Mouse models of acute-type ATL can be rapidly generated by transplanting in vitro-induced T cells that have been retrovirally transduced with HBZ. In this model, it is possible to study the cooperative action of HBZ and host factors in contributing to ATL development [80].
2.2. Rat Models
Rat models have been useful in the study of HAM/TPS pathology. HTLV-infected Wistar-King-Apekman (WKA) rat strain develops spastic paraparesis and clinical symptoms similar to the humans with HAM/TPS [81]. Rats have also been used to study mother-to-child transmission (MTCT) of HTLV-1 Recently an MTCT model was developed using orally HTLV-1-infected rats that did not have antibody responses against viral antigens. In this model, rats inoculated with ILT-M1, an IL-2-dependent HTLV-1-infected T cell line derived from an HAM/TSP patient, transmitted HTLV-1 to their offspring at a high rate (50–100%), and the rate of transmission correlated with the PVL of the infected mother rats [82]. This model has been also proposed for studying the neutralizing potential of antibodies against HTLV-1 envelope gp-46 (LAT27) through antibody-dependent cellular cytotoxicity in MTCT [83][84].
The role of host factors in supporting viral infection has also been investigated in rat models. Human CRM1 (hCRM1) protein, a member of the importin β family, acts as a cofactor of Rex-dependent viral mRNA transport. Transgenic CRMI1 rats intraperitoneally inoculated with HTLV-1-infected cells exhibited a much higher HTLV-1 viral production than wild type rats, and presented more extensive invasion of the thymus by HTLV-1, supporting the in vitro evidence of the key role of CRM1 in HTLV-1 infection [85]. Rat models were also used to test the effect of vaccines based on HTLV-1 Tax-specific cytotoxic T lymphocyte immunity response, the oncolytic potential of vaccinia viruses (VVs) and the ability of siRNA Tax downregulated HTLV-1-infected cells to develop tumors in T-cell-deficient nude rats [86][87][88]. These studies confirm the significant roles of Tax in activating cytotoxic host immune response to the virus and in the survival of infected cells in vivo.
2.3. Rabbit Models
Rabbits are well established and reproducible models to study HTLV-1 transmission, immune responses, and viral determinants required for HTLV infection. Rabbits can be infected with HTLV, but do not develop HTLV-associated diseases; nevertheless, they produce a persistent infection and represent a useful animal model for studying the early steps of infection [29][89][90]. New Zealand White (NZW) rabbits injected with an HTLV-1 carrying a PBM-deleted form of Tax-1 showed that this domain was important for the establishment and maintenance of persistent infection [91]. A similar model was used to demonstrate in vivo that HBZ enhances infectivity and persistence and that the HBZ leucine zipper domain is critical for HBZ functional activity, whereas HBZ is dispensable for immortalization/transformation of primary T lymphocytes in cell culture [92]. In rabbits, APH-2 studies have demonstrated that compared to HBZ, APH is not required for viral persistence [93]. In addition, rabbit models have been successfully applied to demonstrate that the HTLV-1 accessory proteins p12, p13 and p30 are necessary to establish the infection and maintain viral loads in vivo [60][94][95].
Recently the NZW rabbit model was also used to study epigenetic regulation of HTLV-1 gene expression in vivo, demonstrating that the CCCTC binding site present in the overlapping p12 and HBZ sequences of the HTLV-1 genome is dispensable for persistent infection [96]. Particularly worthy of note are studies in rabbit models that have contributed to better defining the differences in the HTLV-1 and HTLV-2 tropisms. It has been possible to determine that at early steps of infection, at the entry step, the tropism is almost the same, represented by CD4+ and CD8+ T cells, although, consistent with reports in humans, HTLV-1 establishes a more robust infection in both CD4+ and CD8+ T cells, compared to HTLV-2 [97].
2.4. Non-Human Primate Models
Non-human primates are susceptible to HTLV-1 infection and develop HTLV-1-associated diseases, including leukemia. Squirrel monkeys, cynomolgus monkeys, rhesus macaques, and pig-tailed macaques have been used to study HTLV immune response, viral persistence, and ATL-like disease. In squirrel monkey Saimiri sciureus injected with HTLV-1-immortalized PBMCs, the spleen and lymph nodes were shown to serve as major reservoirs for HTLV-1 [98]. p12, p30, and HBZ have been found to be essential for establishing and maintaining HTLV-1 infection in macaques, but not in rabbits [58]. Furthermore, by inoculating macaques intravenously with lethally γ-irradiated B-cell lines producing mutated viral clones, it was shown that p12 and p8 are necessary for efficient viral persistence and spread [59]. The non-human primate models have contributed to recapitulating the initial steps of viral infections, including viral genome reverse transcription and persistent clonal expansion of infected cells [99]. Squirrel monkeys, as well as macaque rhesus, have also been used to evaluate the immunogenicity of experimental vaccines against HTLV-1 [76][100]. However, due to the high cost and restrictive regulations related to their application in experimental research, non-human primate models remain of very limited use in the study of HTLV-1.
2.5. Transgenic Fly Model
A Tax and HBZ transgenic Drosophila melanogaster fly model was recently proposed as a suitable model for studying HTLV-I transformation, persistence, and epigenetic modification. The in vivo fly model demonstrated that Tax activates the chromatin polycomb repressive complex 2 (PRC2), which acts on the regulation of the expression of genes involved in cell survival, proliferation, or apoptosis. In this model, HBZ does not induce transformation or NF-κB activation, but its expression abolishes Tax-mediated PRC2 activation in flies expressing both Tax and HBZ [30][101].
3. HTLV-1-Related Virus and Animal Models of Leukemogenesis
3.1. HTLV-1/BLV Models
Bovine leukemia virus (BLV) is a retrovirus closely related to HTLV-1 that causes B-cell lymphoma in ~5% of infected animals and has been proposed as a model for investigating the transmission, latency and pathogenesis of both BLV and HTLV [89][102]. In addition to cattle, BLV may infect sheep, and both species can develop leukemia and lymphoma. Sheep experimentally infected with BLV represent an interesting model for studying leukemia/lymphoma, as they systematically develop leukemia/lymphoma in a shorter period of ∼20 months. In this model, it is possible to monitor all stages of the viral-induced disease, from infection, through asymptomatic stages, to terminal leukemia, recapitulating the development of HTLV-1-associated human malignancy. BLV sheep models have contributed to defining the viral and host determinants for viral persistence and latency and to exploring the efficacy of potential cancer treatment and viral vaccine [103][104][105]. Recently, comparative analyses of HTLV-1/BLV proviral integration sites in the host genomes were performed from the primary tumors and asymptomatic stages of the infection using high-throughput sequencing mapping and RNAseq [106]. This study demonstrated that HTLV/BLV proviruses are integrated close to cancer driver genes, the expression of which may be cis-perturbed, contributing to malignant progression in the polyclonal expansion of the infected cells. Proteome analysis of sheep lymphocytes in the course of BLV-induced leukemia identified novel potential protein markers of disease progression such as spleen trypsin inhibitor, CXCL4/PF-4, thrombospondin, vasodilator-stimulated phosphoprotein, and the fibrinogen alpha chain that are worthy of further investigation in HTLV-induced leukemia [107]. Defining the genetic and epigenetics factors that characterize the sheep BLV leukemia also offers the opportunity to test antiviral gene target therapies.
3.2. STLV Models
STLV-1 naturally infects non-human primates such as the Japanese rhesus macaque, Mandrillus sphinx, and Papio anubis and, like HTLV-1, causes ATL adult T-cell leukemia and lymphoma. [108]. Compared to HTLV-1 infection, Japanese monkeys infected by STLV-1 present similar host immune responses to viral protein and similar clonality of virus-infected T cells, representing a valid model for studying persistent infection and for developing immune-based therapy and prophylaxis [108]. Administration of anti-CCR4 antibodies to STLV-1-infected Japanese macaques resulted in a reduced proviral load in vivo, which is consistent with its efficacy in patient ATL treatment [109]. Furthermore, a long-lasting decrease in the number of STLV-1-infected cells in vivo was observed when Japanese macaques were treated with the humanized anti-CCR4 monoclonal antibody mogamulizumab, which enhances T-cell responses to viral antigens and suppresses CCR4+ Treg cells [110]. Recently, the effect of monoclonal antibodies on CD8 and CD16 was also explored in Japanese macaques infected with STLV-1; although not conclusive, the results suggested that depletion of CD8+ cells was able to modify the clonal proliferation of the infected cells [111].
In Papio papio baboons naturally infected with STLV-1, it was observed that the combined treatment with valproate, an inhibitor of histone deacetylases, and azidothymidine, an inhibitor of reverse transcriptase, caused a strong decrease in the proviral load and an increase in the STLV-1 specific cytotoxic T-cell population [112]. Due to the similarity with the human immune system, STLV-1-infected baboons have been proposed as a model for testing HTLV-1 vaccines based on immunogenic Tax epitopes. In this model, distinct Tax epitope-rich regions have been shown to be targeted by STLV-1-specific CD8+ T cells [113].
References
- Poiesz, B.J.; Ruscetti, F.W.; Gazdar, A.F.; Bunn, P.A.; Minna, J.D.; Gallo, R.C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 1980, 77, 7415–7419.
- Yoshida, M.; Miyoshi, I.; Hinuma, Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. USA 1982, 79, 2031–2035.
- Mahieux, R.; Gessain, A. The human HTLV-3 and HTLV-4 retroviruses: New members of the HTLV family. Pathol. Biol. 2009, 57, 161–166.
- Afonso, P.V. Molecular epidemiology, genetic variability and evolution of HTLV-1 with special emphasis on African genotypes. Retrovirology 2019, 16, 39.
- Gessain, A.; Vernant, J.C.; Maurs, L.; Barin, F.; Gout, O.; Calender, A.; De Thé, G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet 1985, 326, 407–410.
- Osame, M.; Usuku, K.; Izumo, S.; Ijichi, N.; Amitani, H.; Igata, A.; Matsumoto, M.; Tara, M. HTLV-1 associated myelopathy. A new clinical entity. Lancet 1986, 327, 1031–1032.
- Matsuoka, M.; Jeang, K.-T. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat. Rev. Cancer 2007, 7, 270–280.
- Taylor, G.P.; Matsuoka, M. Natural history of adult T-cell leukemia/lymphoma and approaches to therapy. Oncogene 2005, 24, 6047–6057.
- Pilotti, E.; Bianchi, V.; De Maria, A.; Bozzano, F.; Romanelli, M.G.; Bertazzoni, U.; Casoli, C. HTLV-1/-2 and HIV-1 co-infections: Retroviral interference on host immune status. Front. Microbiol. 2013, 4, 372.
- Giam, C.-Z. HTLV-1 Replication and Adult T Cell Leukemia Development. In Viruses and Human Cancer; Springer: Cham, Switzerland, 2021; pp. 209–243.
- Yasunaga, J. Strategies of Human T-Cell Leukemia Virus Type 1 for Persistent Infection: Implications for Leukemogenesis of Adult T-Cell Leukemia-Lymphoma. Front. Microbiol. 2020, 11, 979.
- Marino-Merlo, F.; Balestrieri, E.; Matteucci, C.; Mastino, A.; Grelli, S.; Macchi, B. Antiretroviral Therapy in HTLV-1 Infection: An Updated Overview. Pathogens 2020, 9, 342.
- El Hajj, H.; Tsukasaki, K.; Cheminant, M.; Bazarbachi, A.; Watanabe, T.; Hermine, O. Novel Treatments of Adult T Cell Leukemia Lymphoma. Front. Microbiol. 2020, 11, 1062.
- Igakura, T.; Stinchcombe, J.C.; Goon, P.K.C.; Taylor, G.P.; Weber, J.N.; Griffiths, G.M.; Tanaka, Y.; Osame, M.; Bangham, C.R.M. Spread of HTLV-I Between Lymphocytes by Virus-Induced Polarization of the Cytoskeleton. Science 2003, 299, 1713–1716.
- Nosaka, K.; Matsuoka, M. Adult T-cell leukemia-lymphoma as a viral disease: Subtypes based on viral aspects. Cancer Sci. 2021, 112, 1688–1694.
- Cavallari, I.; Rende, F.; Bender, C.; Romanelli, M.G.; D’Agostino, D.M.; Ciminale, V. Fine tuning of the temporal expression of HTLV-1 and HTLV-2. Front. Microbiol. 2013, 4, 235.
- D’Agostino, D.M.; Cavallari, I.; Romanelli, M.G.; Ciminale, V. Post-transcriptional Regulation of HTLV Gene Expression: Rex to the Rescue. Front. Microbiol. 2019, 10, 1958.
- Grossman, W.J.; Kimata, J.T.; Wong, F.H.; Zutter, M.; Ley, T.J.; Ratner, L. Development of leukemia in mice transgenic for the tax gene of human T-cell leukemia virus type I. Proc. Natl. Acad. Sci. USA 1995, 92, 1057–1061.
- Satou, Y.; Yasunaga, J.; Yoshida, M.; Matsuoka, M. HTLV-I basic leucine zipper factor gene mRNA supports proliferation of adult T cell leukemia cells. Proc. Natl. Acad. Sci. USA 2006, 103, 720–725.
- Baratella, M.; Forlani, G.; Raval, G.U.; Tedeschi, A.; Gout, O.; Gessain, A.; Tosi, G.; Accolla, R.S. Cytoplasmic Localization of HTLV-1 HBZ Protein: A Biomarker of HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis (HAM/TSP). PLoS Negl. Trop. Dis. 2017, 11, e0005285.
- Forlani, G.; Baratella, M.; Tedeschi, A.; Pique, C.; Jacobson, S.; Accolla, R.S. HTLV-1 HBZ Protein Resides Exclusively in the Cytoplasm of Infected Cells in Asymptomatic Carriers and HAM/TSP Patients. Front. Microbiol. 2019, 10, 819.
- Forlani, G.; Shallak, M.; Tedeschi, A.; Cavallari, I.; Marçais, A.; Hermine, O.; Accolla, R.S. Dual cytoplasmic and nuclear localization of HTLV-1-encoded HBZ protein is a unique feature of adult T cell leukemia. Haematologica 2021.
- Satou, Y.; Yasunaga, J.-I.; Zhao, T.; Yoshida, M.; Miyazato, P.; Takai, K.; Shimizu, K.; Ohshima, K.; Green, P.L.; Ohkura, N.; et al. HTLV-1 bZIP factor induces T-cell lymphoma and systemic inflammation in vivo. PLoS Pathog. 2011, 7, e1001274.
- Arnold, J.; Zimmerman, B.; Li, M.; Lairmore, M.D.; Green, P.L. Human T-cell leukemia virus type-1 antisense-encoded gene, Hbz, promotes T-lymphocyte proliferation. Blood 2008, 112, 3788–3797.
- Higuchi, Y.; Yasunaga, J.-I.; Mitagami, Y.; Tsukamoto, H.; Nakashima, K.; Ohshima, K.; Matsuoka, M. HTLV-1 induces T cell malignancy and inflammation by viral antisense factor-mediated modulation of the cytokine signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 13740–13749.
- Panfil, A.R.; Al-Saleem, J.J.; Green, P.L. Animal Models Utilized in HTLV-1 Research. Virol. Res. Treat. 2013, 4, 49–59.
- Niewiesk, S. Animals Models of Human T Cell Leukemia Virus Type I Leukemogenesis. ILAR J. 2016, 57, 3–11.
- Marsden, M.D.; Zack, J.A. Studies of retroviral infection in humanized mice. Virology 2015, 479–480, 297–309.
- Dodon, M.D.; Villaudy, J.; Gazzolo, L.; Haines, R.; Lairmore, M. What we are learning on HTLV-1 pathogenesis from animal models. Front. Microbiol. 2012, 3, 320.
- Akkouche, A.; Moodad, S.; Hleihel, R.; Skayneh, H.; Chambeyron, S.; El Hajj, H.; Bazarbachi, A. In vivo antagonistic role of the Human T-Cell Leukemia Virus Type 1 regulatory proteins Tax and HBZ. PLoS Pathog. 2021, 17, e1009219.
- Fang, J.; Kushida, S.; Feng, R.; Tanaka, M.; Kawamura, T.; Abe, H.; Maeda, N.; Onobori, M.; Hori, M.; Uchida, K.; et al. Transmission of human T-cell leukemia virus type 1 to mice. J. Virol. 1998, 72, 3952–3957.
- Kushida, S.; Maeda, N.; Fang, J.; Uchida, K.; Miwa, M. Establishment of HTLV-1 carrier mice by injection with HTLV-1-producing T cells. Leukemia 1997, 11, 260–262.
- Zimmerman, B.; Niewiesk, S.; Lairmore, M.D. Mouse models of human T lymphotropic virus type-1-associated adult T-cell leukemia/lymphoma. Vet. Pathol. 2010, 47, 677–689.
- Moodad, S.; Akkouche, A.; Hleihel, R.; Darwiche, N.; El-Sabban, M.; Bazarbachi, A.; El Hajj, H. Mouse Models That Enhanced Our Understanding of Adult T Cell Leukemia. Front. Microbiol. 2018, 9, 558.
- Mian, S.A.; Anjos-Afonso, F.; Bonnet, D. Advances in Human Immune System Mouse Models for Studying Human Hematopoiesis and Cancer Immunotherapy. Front. Immunol. 2020, 11, 619236.
- Dewan, M.Z.; Takamatsu, N.; Hidaka, T.; Hatakeyama, K.; Nakahata, S.; Fujisawa, J.; Katano, H.; Yamamoto, N.; Morishita, K. Critical role for TSLC1 expression in the growth and organ infiltration of adult T-cell leukemia cells in vivo. J. Virol. 2008, 82, 11958–11963.
- Richard, V.; Lairmore, M.D.; Green, P.L.; Feuer, G.; Erbe, R.S.; Albrecht, B.; D’Souza, C.; Keller, E.T.; Dai, J.; Rosol, T.J. Humoral hypercalcemia of malignancy: Severe combined immunodeficient/beige mouse model of adult T-cell lymphoma independent of human T-cell lymphotropic virus type-1 tax expression. Am. J. Pathol. 2001, 158, 2219–2228.
- Chen, J.; Zhang, M.; Ju, W.; Waldmann, T.A. Effective treatment of a murine model of adult T-cell leukemia using depsipeptide and its combination with unmodified daclizumab directed toward CD25. Blood 2009, 113, 1287–1293.
- Villaudy, J.; Wencker, M.; Gadot, N.; Gillet, N.A.; Scoazec, J.-Y.; Gazzolo, L.; Manz, M.G.; Bangham, C.R.M.; Dodon, M.D. HTLV-1 propels thymic human T cell development in “human immune system” Rag2−/− gamma c−/− mice. PLoS Pathog. 2011, 7, e1002231.
- Miyazato, P.; Yasunaga, J.; Taniguchi, Y.; Koyanagi, Y.; Mitsuya, H.; Matsuoka, M. De novo human T-cell leukemia virus type 1 infection of human lymphocytes in NOD-SCID, common gamma-chain knockout mice. J. Virol. 2006, 80, 10683–10691.
- Kawano, N.; Ishikawa, F.; Shimoda, K.; Yasukawa, M.; Nagafuji, K.; Miyamoto, T.; Baba, E.; Tanaka, T.; Yamasaki, S.; Gondo, H.; et al. Efficient engraftment of primary adult T-cell leukemia cells in newborn NOD/SCID/beta2-microglobulin(null) mice. Leukemia 2005, 19, 1384–1390.
- Takajo, I.; Umeki, K.; Morishita, K.; Yamamoto, I.; Kubuki, Y.; Hatakeyama, K.; Kataoka, H.; Okayama, A. Engraftment of peripheral blood mononuclear cells from human T-lymphotropic virus type 1 carriers in NOD/SCID/gammac(null) (NOG) mice. Int. J. Cancer 2007, 121, 2205–2211.
- Imada, K.; Takaori-Kondo, A.; Akagi, T.; Shimotohno, K.; Sugamura, K.; Hattori, T.; Yamabe, H.; Okuma, M.; Uchiyama, T. Tumorigenicity of human T-cell leukemia virus type I-infected cell lines in severe combined immunodeficient mice and characterization of the cells proliferating in vivo. Blood 1995, 86, 2350–2357.
- Nasu, K.; Yamaguchi, K.; Takanashi, T.; Tamai, K.; Sato, I.; Ine, S.; Sasaki, O.; Satoh, K.; Tanaka, N.; Tanaka, Y.; et al. Crucial role of carbonic anhydrase IX in tumorigenicity of xenotransplanted adult T-cell leukemia-derived cells. Cancer Sci. 2017, 108, 435–443.
- Hutchison, T.; Malu, A.; Yapindi, L.; Bergeson, R.; Peck, K.; Romeo, M.; Harrod, C.; Pope, J.; Smitherman, L.; Gwinn, W.; et al. The TP53-Induced Glycolysis and Apoptosis Regulator mediates cooperation between HTLV-1 p30II and the retroviral oncoproteins Tax and HBZ and is highly expressed in an in vivo xenograft model of HTLV-1-induced lymphoma. Virology 2018, 520, 39–58.
- Phillips, K.E.; Herring, B.; Wilson, L.A.; Rickford, M.S.; Zhang, M.; Goldman, C.K.; Tso, J.Y.; Waldmann, T.A. IL-2Ralpha-Directed monoclonal antibodies provide effective therapy in a murine model of adult T-cell leukemia by a mechanism other than blockade of IL-2/IL-2Ralpha interaction. Cancer Res. 2000, 60, 6977–6984.
- Aikawa, A.; Kozako, T.; Uchida, Y.; Yoshimitsu, M.; Ishitsuka, K.; Ohsugi, T.; Honda, S.-I. Cell death induced by dorsomorphin in adult T-cell leukemia/lymphoma is AMPK-independent. FEBS J. 2020, 287, 4005–4015.
- Maeda, N.; Ohashi, T.; Chagan-Yasutan, H.; Hattori, T.; Takahashi, Y.; Harigae, H.; Hasegawa, H.; Yamada, Y.; Fujii, M.; Maenaka, K.; et al. Osteopontin-integrin interaction as a novel molecular target for antibody-mediated immunotherapy in adult T-cell leukemia. Retrovirology 2015, 12, 99.
- Maeda, N.; Matsuda, A.; Otsuguro, S.; Takahashi, M.; Fujii, M.; Maenaka, K. Antitumor Effect of Sugar-Modified Cytosine Nucleosides on Growth of Adult T-Cell Leukemia Cells in Mice. Vaccines 2020, 8, 658.
- Masaki, A.; Ishida, T.; Suzuki, S.; Ito, A.; Mori, F.; Sato, F.; Narita, T.; Yamada, T.; Ri, M.; Kusumoto, S.; et al. Autologous Tax-specific CTL therapy in a primary adult T cell leukemia/lymphoma cell-bearing NOD/Shi-scid, IL-2Rγnull mouse model. J. Immunol. Baltim. Md 1950 2013, 191, 135–144.
- Kawamura, K.; Tanaka, Y.; Nakasone, H.; Ishihara, Y.; Kako, S.; Kobayashi, S.; Tanaka, Y.; Ohmori, T.; Uchimaru, K.; Okamoto, S.; et al. Development of a Unique T Cell Receptor Gene-Transferred Tax-Redirected T Cell Immunotherapy for Adult T Cell Leukemia. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2020, 26, 1377–1385.
- Walsh, N.C.; Kenney, L.L.; Jangalwe, S.; Aryee, K.-E.; Greiner, D.L.; Brehm, M.A.; Shultz, L.D. Humanized Mouse Models of Clinical Disease. Annu. Rev. Pathol. 2017, 12, 187–215.
- Pérès, E.; Bagdassarian, E.; This, S.; Villaudy, J.; Rigal, D.; Gazzolo, L.; Duc Dodon, M. From Immunodeficiency to Humanization: The Contribution of Mouse Models to Explore HTLV-1 Leukemogenesis. Viruses 2015, 7, 6371–6386.
- Tezuka, K.; Xun, R.; Tei, M.; Ueno, T.; Tanaka, M.; Takenouchi, N.; Fujisawa, J. An animal model of adult T-cell leukemia: Humanized mice with HTLV-1-specific immunity. Blood 2014, 123, 346–355.
- Ginwala, R.; Caruso, B.; Khan, Z.K.; Pattekar, A.; Chew, G.M.; Corley, M.J.; Loonawat, R.; Jacobson, S.; Sreedhar, S.; Ndhlovu, L.C.; et al. HTLV-1 Infection and Neuropathogenesis in the Context of Rag1−/−γc−/− (RAG1-Hu) and BLT Mice. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2017, 12, 504–520.
- Huey, D.D.; Bolon, B.; La Perle, K.M.D.; Kannian, P.; Jacobson, S.; Ratner, L.; Green, P.L.; Niewiesk, S. Role of Wild-type and Recombinant Human T-cell Leukemia Viruses in Lymphoproliferative Disease in Humanized NSG Mice. Comp. Med. 2018, 68, 4–14.
- Galli, V.; Nixon, C.C.; Strbo, N.; Artesi, M.; de Castro-Amarante, M.F.; McKinnon, K.; Fujikawa, D.; Omsland, M.; Washington-Parks, R.; Romero, L.; et al. Essential Role of Human T Cell Leukemia Virus Type 1 orf-I in Lethal Proliferation of CD4+ Cells in Humanized Mice. J. Virol. 2019, 93, e00565-19.
- Valeri, V.W.; Hryniewicz, A.; Andresen, V.; Jones, K.; Fenizia, C.; Bialuk, I.; Chung, H.K.; Fukumoto, R.; Parks, R.W.; Ferrari, M.G.; et al. Requirement of the human T-cell leukemia virus p12 and p30 products for infectivity of human dendritic cells and macaques but not rabbits. Blood 2010, 116, 3809–3817.
- Pise-Masison, C.A.; de Castro-Amarante, M.F.; Enose-Akahata, Y.; Buchmann, R.C.; Fenizia, C.; Washington Parks, R.; Edwards, D.; Fiocchi, M.; Alcantara, L.C.; Bialuk, I.; et al. Co-dependence of HTLV-1 p12 and p8 functions in virus persistence. PLoS Pathog. 2014, 10, e1004454.
- Bartoe, J.T.; Albrecht, B.; Collins, N.D.; Robek, M.D.; Ratner, L.; Green, P.L.; Lairmore, M.D. Functional role of pX open reading frame II of human T-lymphotropic virus type 1 in maintenance of viral loads in vivo. J. Virol. 2000, 74, 1094–1100.
- Collins, N.D.; Newbound, G.C.; Albrecht, B.; Beard, J.L.; Ratner, L.; Lairmore, M.D. Selective ablation of human T-cell lymphotropic virus type 1 p12I reduces viral infectivity in vivo. Blood 1998, 91, 4701–4707.
- Pérès, E.; Blin, J.; Ricci, E.P.; Artesi, M.; Hahaut, V.; Van den Broeke, A.; Corbin, A.; Gazzolo, L.; Ratner, L.; Jalinot, P.; et al. PDZ domain-binding motif of Tax sustains T-cell proliferation in HTLV-1-infected humanized mice. PLoS Pathog. 2018, 14, e1006933.
- Xiang, J.; Rauch, D.A.; Huey, D.D.; Panfil, A.R.; Cheng, X.; Esser, A.K.; Su, X.; Harding, J.C.; Xu, Y.; Fox, G.C.; et al. HTLV-1 viral oncogene HBZ drives bone destruction in adult T cell leukemia. JCI Insight 2019, 4, 128713.
- Ohsugi, T. A transgenic mouse model of human T cell leukemia virus type 1-associated diseases. Front. Microbiol. 2013, 4, 49.
- Rauch, D.; Gross, S.; Harding, J.; Bokhari, S.; Niewiesk, S.; Lairmore, M.; Piwnica-Worms, D.; Ratner, L. T-cell activation promotes tumorigenesis in inflammation-associated cancer. Retrovirology 2009, 6, 116.
- Rauch, D.A.; Harding, J.C.; Ratner, L. IL-15 deficient tax mice reveal a role for IL-1α in tumor immunity. PLoS ONE 2014, 9, e85028.
- Hasegawa, H.; Sawa, H.; Lewis, M.J.; Orba, Y.; Sheehy, N.; Yamamoto, Y.; Ichinohe, T.; Tsunetsugu-Yokota, Y.; Katano, H.; Takahashi, H.; et al. Thymus-derived leukemia-lymphoma in mice transgenic for the Tax gene of human T-lymphotropic virus type I. Nat. Med. 2006, 12, 466–472.
- Ohsugi, T.; Kumasaka, T.; Okada, S.; Urano, T. The Tax protein of HTLV-1 promotes oncogenesis in not only immature T cells but also mature T cells. Nat. Med. 2007, 13, 527–528.
- Hasegawa, H.; Sano, K.; Ainai, A.; Suzuki, T. Application of HTLV-1 tax transgenic mice for therapeutic intervention. Adv. Biol. Regul. 2018, 68, 10–12.
- El Hajj, H.; Khalil, B.; Ghandour, B.; Nasr, R.; Shahine, S.; Ghantous, A.; Abdel-Samad, R.; Sinjab, A.; Hasegawa, H.; Jabbour, M.; et al. Preclinical efficacy of the synthetic retinoid ST1926 for treating adult T-cell leukemia/lymphoma. Blood 2014, 124, 2072–2080.
- El Hajj, H.; El-Sabban, M.; Hasegawa, H.; Zaatari, G.; Ablain, J.; Saab, S.T.; Janin, A.; Mahfouz, R.; Nasr, R.; Kfoury, Y.; et al. Therapy-induced selective loss of leukemia-initiating activity in murine adult T cell leukemia. J. Exp. Med. 2010, 207, 2785–2792.
- Nakamura-Hoshi, M.; Suzuki, T.; Ainai, A.; Hasegawa, H.; Ishii, H.; Matano, T. Inefficient Tax-Specific T-Cell Responses in Mice after Syngeneic Transplantation with tax-Transgenic Mouse-Derived Adult T-Cell Leukemia Cells. Jpn. J. Infect. Dis. 2020, 73, 221–225.
- Esser, A.K.; Rauch, D.A.; Xiang, J.; Harding, J.C.; Kohart, N.A.; Ross, M.H.; Su, X.; Wu, K.; Huey, D.; Xu, Y.; et al. HTLV-1 viral oncogene HBZ induces osteolytic bone disease in transgenic mice. Oncotarget 2017, 8, 69250–69263.
- Mitagami, Y.; Yasunaga, J.-I.; Kinosada, H.; Ohshima, K.; Matsuoka, M. Interferon-γ Promotes Inflammation and Development of T-Cell Lymphoma in HTLV-1 bZIP Factor Transgenic Mice. PLoS Pathog. 2015, 11, e1005120.
- Yasuma, K.; Yasunaga, J.; Takemoto, K.; Sugata, K.; Mitobe, Y.; Takenouchi, N.; Nakagawa, M.; Suzuki, Y.; Matsuoka, M. HTLV-1 bZIP Factor Impairs Anti-viral Immunity by Inducing Co-inhibitory Molecule, T Cell Immunoglobulin and ITIM Domain (TIGIT). PLoS Pathog. 2016, 12, e1005372.
- Sugata, K.; Yasunaga, J.-I.; Mitobe, Y.; Miura, M.; Miyazato, P.; Kohara, M.; Matsuoka, M. Protective effect of cytotoxic T lymphocytes targeting HTLV-1 bZIP factor. Blood 2015, 126, 1095–1105.
- Ma, G.; Yasunaga, J.-I.; Ohshima, K.; Matsumoto, T.; Matsuoka, M. Pentosan Polysulfate Demonstrates Anti-human T-Cell Leukemia Virus Type 1 Activities In Vitro and In Vivo. J. Virol. 2019, 93, e00413-19.
- Yamazaki, J.; Mizukami, T.; Takizawa, K.; Kuramitsu, M.; Momose, H.; Masumi, A.; Ami, Y.; Hasegawa, H.; Hall, W.W.; Tsujimoto, H.; et al. Identification of cancer stem cells in a Tax-transgenic (Tax-Tg) mouse model of adult T-cell leukemia/lymphoma. Blood 2009, 114, 2709–2720.
- Zhao, T.; Satou, Y.; Matsuoka, M. Development of T cell lymphoma in HTLV-1 bZIP factor and Tax double transgenic mice. Arch. Virol. 2014, 159, 1849–1856.
- Kasugai, Y.; Yoshida, N.; Ohshima, K.; Matsuo, K.; Seto, M.; Tsuzuki, S. New mouse model of acute adult T-cell leukemia generated by transplantation of AKT, BCLxL, and HBZ-transduced T cells. Cancer Sci. 2016, 107, 1072–1078.
- Miyatake, Y.; Ikeda, H.; Ishizu, A.; Baba, T.; Ichihashi, T.; Suzuki, A.; Tomaru, U.; Kasahara, M.; Yoshiki, T. Role of neuronal interferon-gamma in the development of myelopathy in rats infected with human T-cell leukemia virus type 1. Am. J. Pathol. 2006, 169, 189–199.
- Murakami, Y.; Hasegawa, A.; Ando, S.; Tanaka, R.; Masuda, T.; Tanaka, Y.; Kannagi, M. A novel mother-to-child human T-cell leukaemia virus type 1 (HTLV-1) transmission model for investigating the role of maternal anti-HTLV-1 antibodies using orally infected mother rats. J. Gen. Virol. 2017, 98, 835–846.
- Fujii, H.; Shimizu, M.; Miyagi, T.; Kunihiro, M.; Tanaka, R.; Takahashi, Y.; Tanaka, Y. A Potential of an Anti-HTLV-I gp46 Neutralizing Monoclonal Antibody (LAT-27) for Passive Immunization against Both Horizontal and Mother-to-Child Vertical Infection with Human T Cell Leukemia Virus Type-I. Viruses 2016, 8, 41.
- Tanaka, Y.; Zeng, L.; Shiraki, H.; Shida, H.; Tozawa, H. Identification of a neutralization epitope on the envelope gp46 antigen of human T cell leukemia virus type I and induction of neutralizing antibody by peptide immunization. J. Immunol. 1991, 147, 354–360.
- Takayanagi, R.; Ohashi, T.; Yamashita, E.; Kurosaki, Y.; Tanaka, K.; Hakata, Y.; Komoda, Y.; Ikeda, S.; Tsunetsugu-Yokota, Y.; Tanaka, Y.; et al. Enhanced replication of human T-cell leukemia virus type 1 in T cells from transgenic rats expressing human CRM1 that is regulated in a natural manner. J. Virol. 2007, 81, 5908–5918.
- Ohashi, T.; Hanabuchi, S.; Kato, H.; Tateno, H.; Takemura, F.; Tsukahara, T.; Koya, Y.; Hasegawa, A.; Masuda, T.; Kannagi, M. Prevention of adult T-cell leukemia-like lymphoproliferative disease in rats by adoptively transferred T cells from a donor immunized with human T-cell leukemia virus type 1 Tax-coding DNA vaccine. J. Virol. 2000, 74, 9610–9616.
- Ohashi, T.; Nakamura, T.; Kidokoro, M.; Zhang, X.; Shida, H. Combined cytolytic effects of a vaccinia virus encoding a single chain trimer of MHC-I with a Tax-epitope and Tax-specific CTLs on HTLV-I-infected cells in a rat model. BioMed Res. Int. 2014, 2014, 902478.
- Nomura, M.; Ohashi, T.; Nishikawa, K.; Nishitsuji, H.; Kurihara, K.; Hasegawa, A.; Furuta, R.A.; Fujisawa, J.; Tanaka, Y.; Hanabuchi, S.; et al. Repression of tax expression is associated both with resistance of human T-cell leukemia virus type 1-infected T cells to killing by tax-specific cytotoxic T lymphocytes and with impaired tumorigenicity in a rat model. J. Virol. 2004, 78, 3827–3836.
- Lairmore, M.D. Animal models of bovine leukemia virus and human T-lymphotrophic virus type-1: Insights in transmission and pathogenesis. Annu. Rev. Anim. Biosci. 2014, 2, 189–208.
- Miyoshi, I.; Yoshimoto, S.; Fujishita, M.; Kubonishi, I.; Taguchi, H.; Ohtsuki, Y. Infectious transmission of human T-cell leukemia virus to animals. Princess Takamatsu Symp. 1984, 15, 121–127.
- Xie, L.; Yamamoto, B.; Haoudi, A.; Semmes, O.J.; Green, P.L. PDZ binding motif of HTLV-1 Tax promotes virus-mediated T-cell proliferation in vitro and persistence in vivo. Blood 2006, 107, 1980–1988.
- Arnold, J.; Yamamoto, B.; Li, M.; Phipps, A.J.; Younis, I.; Lairmore, M.D.; Green, P.L. Enhancement of infectivity and persistence in vivo by HBZ, a natural antisense coded protein of HTLV-1. Blood 2006, 107, 3976–3982.
- Yin, H.; Kannian, P.; Dissinger, N.; Haines, R.; Niewiesk, S.; Green, P.L. Human T-cell leukemia virus type 2 antisense viral protein 2 is dispensable for in vitro immortalization but functions to repress early virus replication in vivo. J. Virol. 2012, 86, 8412–8421.
- Silverman, L.R.; Phipps, A.J.; Montgomery, A.; Ratner, L.; Lairmore, M.D. Human T-cell lymphotropic virus type 1 open reading frame II-encoded p30II is required for in vivo replication: Evidence of in vivo reversion. J. Virol. 2004, 78, 3837–3845.
- Hiraragi, H.; Kim, S.-J.; Phipps, A.J.; Silic-Benussi, M.; Ciminale, V.; Ratner, L.; Green, P.L.; Lairmore, M.D. Human T-lymphotropic virus type 1 mitochondrion-localizing protein p13(II) is required for viral infectivity in vivo. J. Virol. 2006, 80, 3469–3476.
- Martinez, M.P.; Cheng, X.; Joseph, A.; Al-Saleem, J.; Panfil, A.R.; Palettas, M.; Dirksen, W.P.; Ratner, L.; Green, P.L. HTLV-1 CTCF-binding site is dispensable for in vitro immortalization and persistent infection in vivo. Retrovirology 2019, 16, 44.
- Kannian, P.; Yin, H.; Doueiri, R.; Lairmore, M.D.; Fernandez, S.; Green, P.L. Distinct transformation tropism exhibited by human T lymphotropic virus type 1 (HTLV-1) and HTLV-2 is the result of postinfection T cell clonal expansion. J. Virol. 2012, 86, 3757–3766.
- Kazanji, M.; Ureta-Vidal, A.; Ozden, S.; Tangy, F.; de Thoisy, B.; Fiette, L.; Talarmin, A.; Gessain, A.; de Thé, G. Lymphoid organs as a major reservoir for human T-cell leukemia virus type 1 in experimentally infected squirrel monkeys (Saimiri sciureus): Provirus expression, persistence, and humoral and cellular immune responses. J. Virol. 2000, 74, 4860–4867.
- Mortreux, F.; Kazanji, M.; Gabet, A.S.; de Thoisy, B.; Wattel, E. Two-step nature of human T-cell leukemia virus type 1 replication in experimentally infected squirrel monkeys (Saimiri sciureus). J. Virol. 2001, 75, 1083–1089.
- Kazanji, M.; Tartaglia, J.; Franchini, G.; de Thoisy, B.; Talarmin, A.; Contamin, H.; Gessain, A.; de Thé, G. Immunogenicity and protective efficacy of recombinant human T-cell leukemia/lymphoma virus type 1 NYVAC and naked DNA vaccine candidates in squirrel monkeys (Saimiri sciureus). J. Virol. 2001, 75, 5939–5948.
- Shirinian, M.; Kambris, Z.; Hamadeh, L.; Grabbe, C.; Journo, C.; Mahieux, R.; Bazarbachi, A. A Transgenic Drosophila melanogaster Model To Study Human T-Lymphotropic Virus Oncoprotein Tax-1-Driven Transformation In Vivo. J. Virol. 2015, 89, 8092–8095.
- Pluta, A.; Jaworski, J.P.; Douville, R.N. Regulation of Expression and Latency in BLV and HTLV. Viruses 2020, 12, 1079.
- Aida, Y.; Murakami, H.; Takahashi, M.; Takeshima, S.-N. Mechanisms of pathogenesis induced by bovine leukemia virus as a model for human T-cell leukemia virus. Front. Microbiol. 2013, 4, 328.
- Barez, P.-Y.; de Brogniez, A.; Carpentier, A.; Gazon, H.; Gillet, N.; Gutiérrez, G.; Hamaidia, M.; Jacques, J.-R.; Perike, S.; Neelature Sriramareddy, S.; et al. Recent Advances in BLV Research. Viruses 2015, 7, 6080–6088.
- Hajj, H.E.; Nasr, R.; Kfoury, Y.; Dassouki, Z.; Nasser, R.; Kchour, G.; Hermine, O.; de Thé, H.; Bazarbachi, A. Animal models on HTLV-1 and related viruses: What did we learn? Front. Microbiol. 2012, 3, 333.
- Rosewick, N.; Durkin, K.; Artesi, M.; Marçais, A.; Hahaut, V.; Griebel, P.; Arsic, N.; Avettand-Fenoel, V.; Burny, A.; Charlier, C.; et al. Cis-perturbation of cancer drivers by the HTLV-1/BLV proviruses is an early determinant of leukemogenesis. Nat. Commun. 2017, 8, 15264.
- Reichert, M. Proteome analysis of sheep B lymphocytes in the course of bovine leukemia virus-induced leukemia. Exp. Biol. Med. 2017, 242, 1363–1375.
- Jégado, B.; Kashanchi, F.; Dutartre, H.; Mahieux, R. STLV-1 as a model for studying HTLV-1 infection. Retrovirology 2019, 16, 41.
- Miura, M.; Yasunaga, J.; Tanabe, J.; Sugata, K.; Zhao, T.; Ma, G.; Miyazato, P.; Ohshima, K.; Kaneko, A.; Watanabe, A.; et al. Characterization of simian T-cell leukemia virus type 1 in naturally infected Japanese macaques as a model of HTLV-1 infection. Retrovirology 2013, 10, 118.
- Sugata, K.; Yasunaga, J.-I.; Miura, M.; Akari, H.; Utsunomiya, A.; Nosaka, K.; Watanabe, Y.; Suzushima, H.; Koh, K.-R.; Nakagawa, M.; et al. Enhancement of anti-STLV-1/HTLV-1 immune responses through multimodal effects of anti-CCR4 antibody. Sci. Rep. 2016, 6, 27150.
- Izaki, M.; Yasunaga, J.-I.; Nosaka, K.; Sugata, K.; Utsunomiya, H.; Suehiro, Y.; Shichijo, T.; Yamada, A.; Sugawara, Y.; Hibi, T.; et al. In vivo dynamics and adaptation of HTLV-1-infected clones under different clinical conditions. PLoS Pathog. 2021, 17, e1009271.
- Afonso, P.V.; Mekaouche, M.; Mortreux, F.; Toulza, F.; Moriceau, A.; Wattel, E.; Gessain, A.; Bangham, C.R.M.; Dubreuil, G.; Plumelle, Y.; et al. Highly active antiretroviral treatment against STLV-1 infection combining reverse transcriptase and HDAC inhibitors. Blood 2010, 116, 3802–3808.
- Castro, I.; Giret, T.M.; Magnani, D.M.; Maxwell, H.S.; Umland, O.; Perry, J.K.; Pecotte, J.K.; Brasky, K.M.; Barber, G.N.; Desrosiers, R.C.; et al. Cellular Immune Responses against Simian T-Lymphotropic Virus Type 1 Target Tax in Infected Baboons. J. Virol. 2016, 90, 5280–5291.
More
