In the targeted therapy, nanoparticles (NPs) with specific properties, nanomedicine, are designed to specifically transport therapeutic agents to tumor sites and to release under controlled conditions. This strategy could potentially overcome the limitations of conventional methods and improve the cancer treatment outcomes by distinguishing malignant cells from non-malignant cells and selectively kill malignant cells. Bio-distribution, biocompatibility, biodegradability, and systemic clearance are the general challenges of using NPs in the targeted therapy. An effective NP-based drug delivery system should predict and control the fate of NPs in the biological environment. To develop and achieve a sound and efficient NPs-based system, we need to enhance our understanding of the nano-bio-interaction (NBI) happening between nanomaterials and a complex heterogeneous biological environment. At the cellular level, the NBI occurs at the interface of NPs surface and cell membrane. The interaction behavior of NPs is highly dependent on the physical and chemical properties of NPs.
- nano-bio-interaction
- nanoparticle
- mechanobiological properties
- cancer cells
- cell mechanics
- migratory index
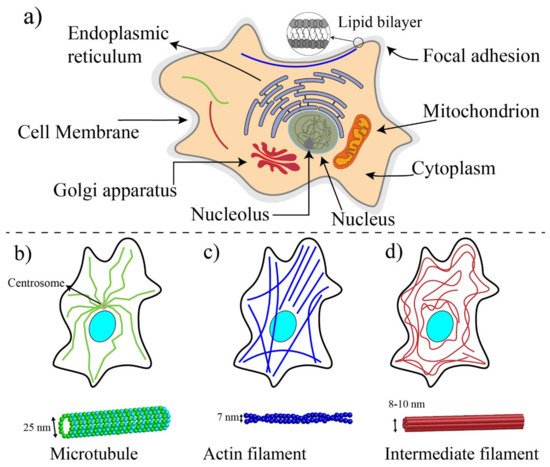
1. Basic Components of Cells and Biomechanics
2. Techniques for Mechanobiological Characterizations
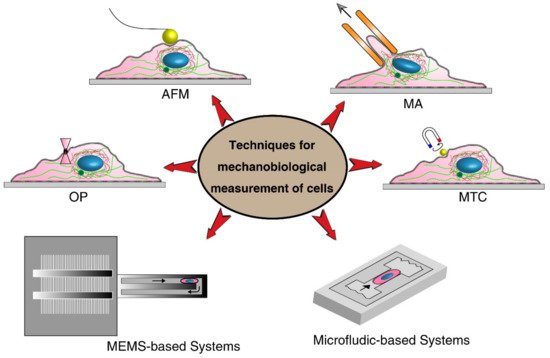
| Techniques | Cell Type | Mechanical Stimuli | Important Parameters | Advantages | Limitations | |
|---|---|---|---|---|---|---|
| Classical Techniques | Atomic Force Microscopy (AFM) | MCF7 [28][104]; Human bladder [20][96] |
Cantilever micro indention | Tip deflection, Young’s modulus | High-resolution measurement; Provids both structural and mechanical information for local, whole, and interior measurements [29][21][23,97] | Low throughput; Mechanical hitting of AFM tip may affect cell activities and position of probe; Requires a high-resolution microscope |
| Micropipette aspiration (MA) | Human cartilage [22][98]; Colon cancer cells [30][105] |
Negative force | Young’s modulus | Low-cost and well-established method | Limited spatial resolution; Low throughput; For suspended cells only | |
| Magnetic twisting cytometry (MTC) | Melanoma [24][100]; MCF7 [31][106] |
Force is applied by magnetic beads | Stiffness and Young’s modulus | Inducing little heat and photodamages compared to optical tweezer [32][10] | Resolution limitation; Inducing non-uniform stress; Beads are localized randomly on cell; Attachment angle affects the displacement | |
| Optical tweezers (OP) | RBC [23][33][99,107] | Laser-induced surface force | Deformation index | Without physical contact | Only for suspended cells; Damaging consequence of optical heating on cells; Limited magnitude of forces |
|
| Parallel plate | Epithelial ovarian cancer [29][23]; MCF7 [31][34][106,108] |
Shear stress | Aspect ratio | Homogeneity of the applied shear stress; Simplicity; Ability to study cell population | Need bulky devices; Large amount of reagents; Difficult to visualize deformation | |
| Microfluidic Techniques | Fluid-induced deformation | PBMCs [26][102] | Fluid shear stress |
Deformation index, size | High throughput; Simultaneously, other chemical assays can be done; The measurment can be done continuously; Contactless deformation; Applicable for both suspended and adhered cells | Needing expensive high-speed camera for imaging |
| Constriction-induced deformation | K562 [35][109]; MDA-MB-231 [36][110] |
Mechanical squeezing | Passage time, entry times, stiffness | Wide-ranging applications in cell deformation; Applicable for different geometry structures; Adjustable dimension for different cell types |
Clogging and channel blockage; Possible effects of friction between cell and channel’s wall on measurements; Ignoring the effects of membrane rigidity and viscosity | |
| Aspiration-induced deformation | Neutrophils [37][24] | Negative pressure | Young’s modulus, cortical tension | Straightforward method; Well-established mathematical model | Leaking problem; Rectangle-like cross-section of microfluidic channels; Time-consuming process; Requiring high-vacuum pressure | |
| Optical stretcher | MCF7 [31][106]; MCF-7, MCF-10, MDA-MB-231 [38][111]; Red blood cells [23][99]; Melanoma cells [39][112] |
Optically-induced surface forces | Deformation index, cell elasticity | No physical contact; Relatively high-throughput measurements | Alignment problem; Optical heating; Thermal damage |
|
| Electrical-induced deformation | MCF-10A, MCF-7 [40][113] | Electroporation-induced swelling | Deformation index, size of cells | Fast heat dissipation; Better resolution; Automation and parallelization of test with reduced amount of samples |
High energy consumption and high voltage | |
| MEMS Techniques | Suspended microcantilever | Circulating tumor cells [41][114]; Fibroblast [25][101] | External actuator | Frequency of cantilever, passage time, transit time | All-inclusive systems; Parallel analysis; Better quality factor; Automation | Fabrication is expensive; Non-transparent channels; High stiffness of silicon; calibration process |
| MEMS resonator | MCF7 [42][115] | External actuator | Frequency of cantilever | High throughput | Expensive fabrication; Requiring external electrical system; Only for adherent cells | |
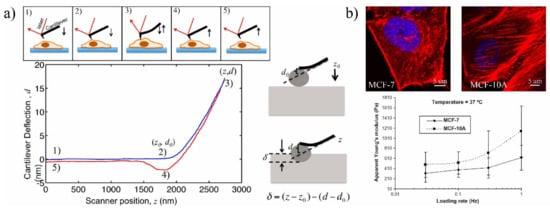
2.1. Classical Methods
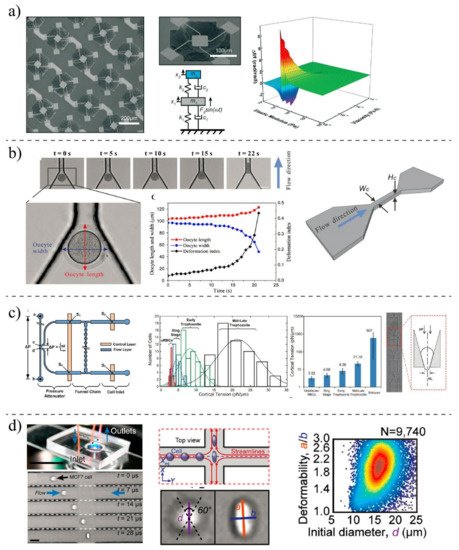
2.2. MEMS- and Microfluidic-Based Techniques
3. Impacts of Nanoparticles on Structural Elements and Morphology of Cells
| Author | Cell Type | NPs Type | Methods | Cytoskeleton Changes |
|---|---|---|---|---|
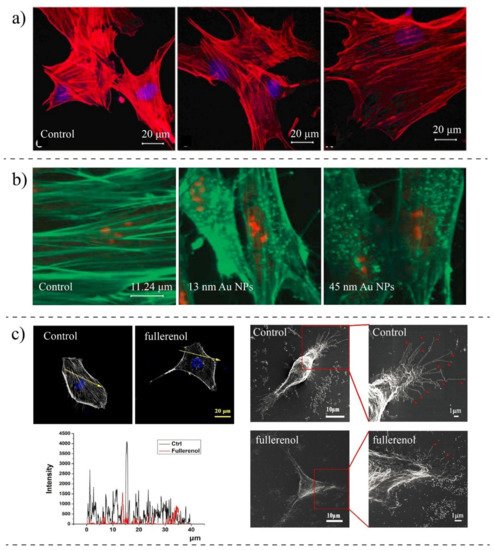
| Pernodet et al., 2007 [94][162] | CF-31 (human dermal fibroblast) | Gold NPs (13 nm) | TEM, Confocal Imaging, Migration Assay | Modification in actin networks; NPs impaired motility and adhesion |
| Pi et al., 2013 [95][163] | MCF-7 (breast cancer) | Selenium NPs | AFM, Confocal Microscopy | The organization of F-actin is changed, and they are aggregated; Actin concentration is reduced |
| Choudhury 2013 [82][150] | A549 (lung cancer) | Citrate-capped Gold NPs (20–60 nm) | Raman, FTIR, TEM, Darkfield Microscopy, UV-Visible Spectroscopy | Inhibiting the polarization of MT; MT structures are damaged, affecting the dynamic equilibrium |
| Qin et al., 2018 [83][151] | MDA-MB-231 (breast cancer) | Fullerenol NPs | SEM, Fluorescence Imaging, AFM, Scratch Assay | The concentration of actin is reduced, the migration speed is reduced, disturbing actin assembly |
| Hot et al., 2012 [76][145] | HeLa (cervical cancer) | Single-wall carbon nanotube (1 ± 0.3 nm) | Fluorescence Imaging Microscopy | NPs cause cells to have shorter F-actin; Traction force is reduced; NPs do not affect G-actin and myosin II |
| Huang et al., 2010 [72][141] | A375 (melanoma) | Silica NPs (MSNs) | TEM, Confocal Microscopy, Western Blot | The actin structure is disorganized and disrupted with NPs; Cell migration is reduced |
| Patra et al., 2007 [91][159] | A549 (lung cancer) | Gold NPs | Confocal Microscopy | The morphology is changed; Treated cells are rounded compared to non-treated |
| Pisanic et al., 2007 [80][149] | PC12M (brain) | Fe2O3 NPs | TEM, Western Blot, Fluorescent Microscopy | Reduction in the formation of actin microfilaments; They are less organized; NPs diminish the ability for differentiation |
| Wu et al., 2012 [93][161] | HAEC (aortic endothelial cells) | Diesel exhaust particles (DEPs) | AFM, Fluorescent Imaging | Cells became degraded; Cellular cytoskeletal structures were impaired |
| Wen et al., 2013 [86][154] | Acting and tubulin proteins (cell-free system) | Silver NPs | TEM, Hyperspectral Imaging, | Inducing changes in the secondary structures; Silver NPs tend to bind actin vs. tubulin |
| Cooper et al., 2015 [78][147] | B35 (neuroblastoma) | Silver NPs | Immunocytochemistry | NPs induce F-actin inclusion, disrupting the actin function |
| Rasel et al., 2015 [89][157] | Osteoblast cells | Boron nitride NPs | AFM, TEM, X-Ray | They do not affect the morphology of cells |
| Liu et al., 2017 [77][146] | HUVEC (Endothelial cells) | Gold NPs-coated with PEG (20 nm) | Fluorescent Microscopy, Traction Force Microscopy | NPs re-arranged actin filaments; Inhibition of Rock activity reduced the polymerization of actin; Reducing the focal adhesion |
| Vieira et al., 2017 [96][164] | CCD1072Sk (Normal cells-skin) | Gold NPs and silver NPs | Immunofluorescence Imaging, Cytofluorometry | NPs impair the F-actin;Cytoskeletal reorganization; Cells lose the cell polarization (without losing their viability) |
| Ali et al., 2017 [90][158] | HSC-3 (tongue cancer) | Gold nanorods coated with PEG and REG | Western Blot, DIC Microscopy, Scratch Assay | The cytoskeletal proteins are rearranged; Cytoskeletal protrusions (filopodia and lamellipoda) are reduced |
| Beaudet et al., 2017 [97][48] | HeLa (cervical cancer) | AuNPs, Swarna Bhasma | Fluorescent Imaging | Larger particles disrupted the microtubules networks |
| Ibrahim et al., 2018 [75][144] | SaOS-2 (bone cancer) | TiO2 spherical NPs | Hyperspectral Imaging, Fluorescent Imaging, Western Blot | The actin and microtubule cytoskeletal networks are disorganized |
| Kralovec et al., 2020 [74][143] | A549 (lung cancer) | Fe3O4@SiO2 | Fluorescent Imaging, Western Blot | Severe disruption of the actin filament and microtubules |
| Kota et al., 2021 [98][165] | VSMCs (vascular smooth muscle cells) | ZIF-8 NPs | AFM, Fluorescent Imaging, Polymerization Assay | Morphological changes and cytoskeletal disorganization were observed; NPs caused changes in actin filaments at basal and apical surfaces. |
