Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Peter Tang and Version 2 by Chyn Boon Wong.
Bifidobacterium breve M-16V is a commonly used probiotic strain in infants. M-16V has been demonstrated to offer potential in protecting infants from developing devastating necrotising enterocolitis (NEC) and allergic diseases.
- Bifidobacterium breve M-16V
- infant health
- clinical efficacy
- probiotics
- gut microbiota
1. Introduction
Gut microbiota has become an important aspect of human health. Gut microbes regulate host intestinal, immunological, and metabolic activities through their wide array of modulatory capabilities and enzymatic armoury[1]. Recent advances in microbial research have revealed the importance of the early gut microbiome for neonatal health development and disease pathologies[2]. Aberrations of infant gut microbiota—a state of altered microbial composition and functionality—are associated with adverse health-related consequences including asthma[3], necrotising enterocolitis (NEC)[4], eczema[5], and inflammatory bowel disease[6] in the neonatal stage or later in life.
Microbial ecosystem is established during the first three years of life for which a host-microbe symbiotic interaction that mutually benefits both is initiated[7]. It has been implicated that a number of extrinsic factors, such as gestational age, delivery mode, and feeding types, are affecting the process of microbial colonisation in newborns[7][8]. Initial neonatal gut microbial colonisation represents a crucial window of opportunity for shaping a healthy gastrointestinal tract and immune system[9], and positive modulation of gut microbiota during this critical period could be an effective preventative approach against immune-mediated and microbiome-related disease pathologies. Consequently, probiotics intervention is receiving significant attention as a non-invasive attempt to optimize the infant microbiota as a means to improve health or prevent disease.
Probiotics are defined as “live microorganisms, which when administered in adequate amounts, confer a health benefit on the host”[10]. Studies over the last decade have demonstrated that probiotics supplementation could promote gut microbial colonisation and prevent or treat diseases in infants[11]. These reports have led to a massive application of probiotics in a range of products including foods, infant formula, dietary supplements, and pharmaceutical products for promoting infant health. Nevertheless, many of the marketed probiotic products encompass limited well-consolidated regulatory oversight and a lack of human substantiation of efficacy[12]. Moreover, the safety and effects of probiotics in the vulnerable preterm population remain relatively limited and inconsistent[13]. Therefore, a detailed review of the scientific basis of a specific probiotic strain has emerged as an important aspect for an optimised selection of suitable probiotic candidates for use in infants.
2. Probiotics for Infant Health
Probiotics intervention has gained overwhelming popularity over the last two decades as a potential nutritional supplementation approach to promote and maintain a healthy gut milieu and protect against dysbiosis in early life[14]. Accumulating evidence suggests that manipulation of the microbiota with the use of probiotics at an early stage may lead to appropriate microbial colonisation and could have long-lasting impacts on child and adult health[15]. Probiotics that have been commonly given to neonates and infants include species of Bifidobacterium and Lactobacillus. Among them, Bifidobacterium is thought to be a keystone taxon in infant gut microbiota that plays a vital role in regulating immunological and physiological functions[16].
Bifidobacterial species have been isolated from the gastrointestinal tract of humans and animals as well as a few that have been isolated from the human vagina, oral cavity, breast milk, sewage, and foods, and could be categorised into two major groups; bifidobacterial species of human origins as human-residential bifidobacteria (HRB), whereas other species which are the natural inhabitants of animals or environment as non-HRB[17]. It has been demonstrated that bifidobacterial species of different residential origins display different levels of adaptability and functionality in the infant gut[17]. Of note, B. longum subsp. infantis (B. infantis), B. longum subsp. longum (B. longum), B. bifidum, and B. breve, which are frequently isolated from infants' intestines and are referred to as infant-type HRB[17][18], have a large repertoire of genes for the utilisation of human milk oligosaccharides (HMOs)[19][20]. Studies have reported that infant-type HRB species are capable of utilising HMOs with different metabolic pathways and degrees of degradation, highly compatible with human breast milk, and tolerant to lysozyme[19][21], demonstrating how well adapted they are to the transmission routes and growth conditions in the infant's gut. Infant-type HRB species are the exclusive members of healthy breastfed infants[22][23], while formula-fed infants are also colonised with species that are commonly isolated from adult intestines (adult-type HRB) such as B. adolescentis and B. pseudocatenulatum[24], implying the specific strains of infant-type HRB could be better probiotic candidates for infant use.
Several studies have demonstrated the use of infant-type HRB, including the strains of B. breve[25], B. longum[26], B. infantis[27], and B. bifidum[28] as probiotics for therapeutic purposes in neonates and infants. Administration of infant-type HRB probiotic strains in the first stage of life may result in the prevention of NEC and reduction in the risk as well as treatment of infectious and atopic disease[11][29]. Despite the promise, questions and concerns have been raised about the safety and clinical efficacy of probiotics administration, especially if the product is destined for use in infants. It is increasingly apparent that not all probiotics are equally safe, and the effects demonstrated with one strain cannot be extrapolated to another strain, even if they belong to the same species[30]. Of note, among many infant-type HRB probiotic strains that have been studied, M-16V possesses a proven track record of safety and a number of beneficial attributes that make it an attractive probiotic candidate for infant use. The following paragraphs will review the safety and specific health benefits of M-16V in infants within the field that seek to provide rigorous preclinical characterisation and substantial clinical evidence of M-16V for successful probiotics selection.
3. Bifidobacterium breve M-16V as Infant Probiotic
3.1. Origin and Characteristics
M-16V was originated from the gut of an infant in 1963 and was first commercially available in Japan in 1976 with the launch of Vinelac dietary supplement. In 1982, M-16V was added to a growing-up powdered formula called Yochien-Jidai in Japan and has since been incorporated in several other products including term and preterm infant formula.
M-16V is a non-motile, non-spore-forming, rod-shaped anaerobic Gram-positive bacterium. It was identified as B. breve based on morphological, physiological, and genetic characteristics. M-16V is highly accessible to the human gastrointestinal tract with strong adherence activity[31]. In addition, lyophilised powder of M-16V manufactured by Morinaga Milk Industry Co., Ltd. possesses excellent stability during storage and high survivability in finished products such as powdered formula until consumption[32].
3.2. Safety
M-16V is well-evaluated for safety and has met the safety standards regulated by the Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO) guidelines for the evaluation of microbes for probiotics use in foods[33]. In 2013, M-16V attained not only FDA-Notified Generally Recognized as Safe (GRAS) status for food uses (GRN No., 453), but also GRAS status for infants (GRN No., 454). In addition, in 2016, M-16V has been included in the list of authorised probiotic strains for infant food in China, in which M-16V is the only infant-type HRB strain among the nine strains in the list[34]. To date, there has been broad use of M-16V in low birth weight infants to reduce the risk of preterm birth complications in more than 120 neonatal intensive care units (NICUs) in affiliated hospitals in Japan, Australia, New Zealand, and Singapore[35][36][37].
Comprehensive safety evaluation of M-16V, which includes functional, genomic, and in vivo analyses, demonstrated that M-16V is a non-pathogenic, non-toxigenic, non-haemolytic, and non-antibiotic resistant probiotic bacterium that does not contain any plasmids and does not display harmful metabolic activities[31][38][39]. M-16V produces L-lactic acid but no D-lactic acid. In addition, M-16V was reported to possess conjugated bile salt hydrolytic activity[31]. M-16V was able to hydrolyse conjugated bile acids taurocholic and glycocholic acid to the primary bile acid (cholic acid) and glycochenodeoxycholic and taurochenodeoxycholic acid to chenodeoxycholic acid, while the production of hepatotoxic and carcinogenic secondary bile acids (deoxycholic and lithocholic acid) was not detected upon complete biotransformation of bile salts[40]. These results resolve the concern about the safety of administering a secondary bile acids-producing bacterium.
Studies on acute and chronic toxicological features of M-16V revealed that both single and repeated oral administration of M-16V did not cause death and any toxic symptoms in a rat model[38]. For instance, groups of 10 male and 10 female three-week-old Crj:CD (SD) rats were orally administered with a single dose of M-16V at 6000 mg/kg (1.4 × 1012 CFU/kg) or 3000 mg/kg (6.9 × 1011 CFU/kg) and examined for acute toxic symptoms for 14 days. There were no gross abnormalities or histopathological findings attributable to the treatment in all organs throughout the test period, although slightly lower body weight was observed in male rats administered a high dose of M-16V as compared to the control on days 8 and 10. Furthermore, oral administration of M-16V with a 90-day repeated dose (2.3 × 1011 CFU/kg/day) to five-week-old Crj:CD (SD) IGS rats revealed no adverse effects attributed to M-16V during the study period. M-16V induced no significant histopathological changes in all organs examined. These findings demonstrate the absence of acute and chronic toxicity by consumption of M-16V. Additional in vitro tests showed that M-16V did not possess mucin degradation ability[41]. Taken together, these studies support that M-16V is safe for use as a probiotic in humans.
4. Effects of M-16V on Premature Birth Complications
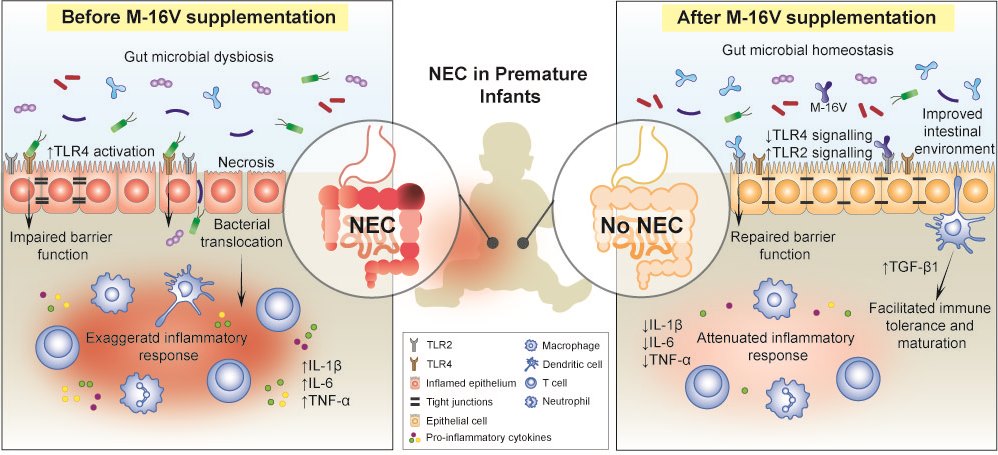
Prematurity, prolonged hospitalisation, immunodeficiency, antibiotics use, and delayed enteral feeding are challenging ways to begin life for preterm infants[42]. Premature infants are at elevated risk to develop multiple health comorbidities; one of which is the devastating necrotising enterocolitis (NEC)[43]. It is a major cause of morbidity and mortality in extremely preterm infants that is associated with severe sepsis and intestinal perforation[44]. Although the exact aetiology and pathogenesis of NEC remain elusive, perturbation of the gut microbiota, leading to a hyperinflammatory response, appears to be a key factor that predisposes neonates to NEC[45]. Premature infants often present with an immature gut and exhibit delayed gut colonisation with beneficial commensal bacteria such as Bifidobacterium and Bacteroides, where instead they are more susceptible to colonisation by Enterobacteriaceae and Enterococcus [46] Moreover, the use of antibiotics in premature and low birth weight infants disturbs the colonisation patterns of Bifidobacterium and shifts the gut microbial composition toward a high abundance of Proteobacteria, with a decrease in the overall diversity of the infant’s gut microbiota[47][48][49]. To this end, the neonatal period has; therefore, emerged as an opportune time for preventive M-16V probiotics intervention to promote bifidobacterial colonisation, facilitate the development of the gut mucosal immune system and improve infant health. It is evident that M-16V may potentially reduce the risk of developing NEC in premature infants by promoting bifidobacterial colonisation, modulating the expressions of toll-like receptors (TLRs) and inflammatory responses, and aiding in the development of mucosal immunity.

5. Effects of M-16V on Allergic Disorders
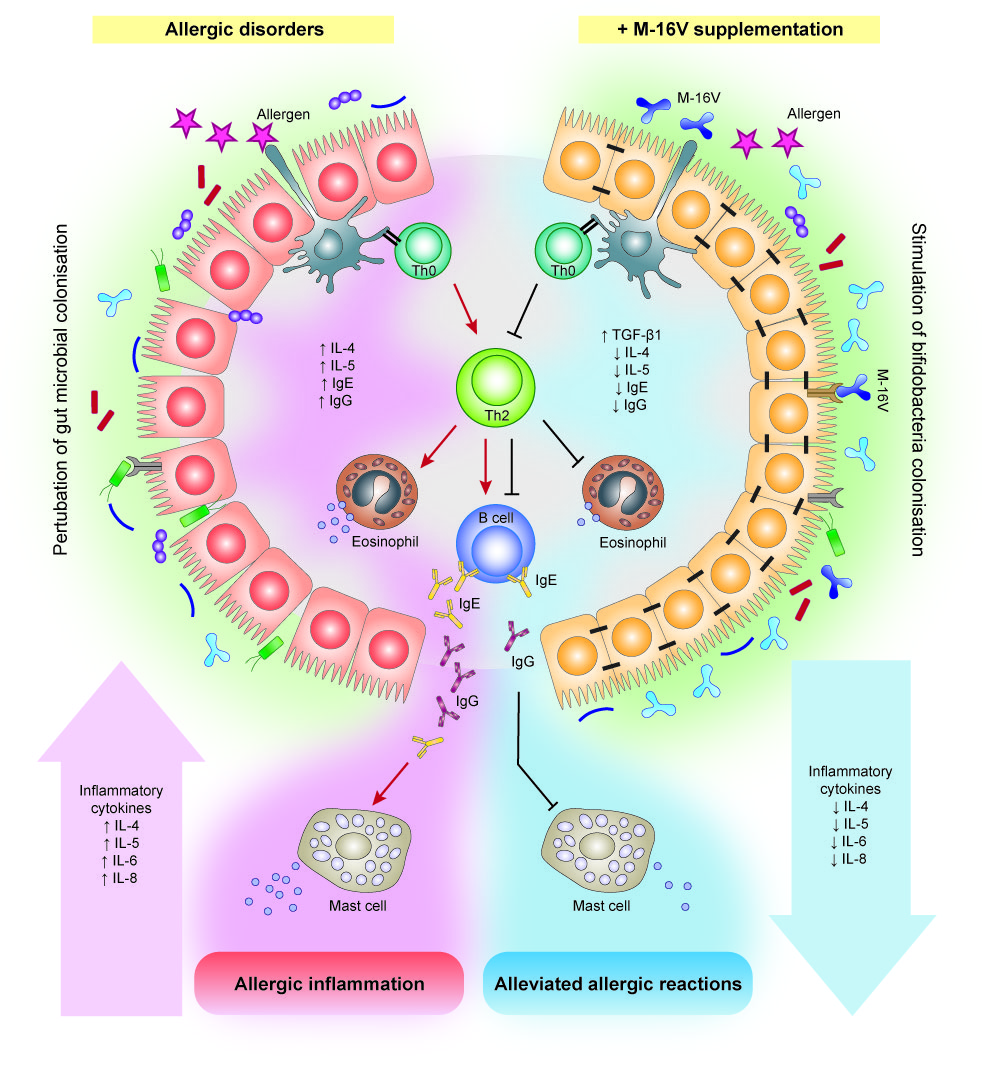
The prevalence of allergic diseases in infants has increased strikingly worldwide in the past few decades[50]. While the pathogenesis of allergic diseases is likely to be multifactorial, deviations in gut colonisation during early life are possible major factors promoting abnormal postnatal immune maturation[51]. The hygiene hypothesis suggests that insufficient or aberrant microbial stimulation during the critical neonatal period may lead to an exaggerated adaptive immune response and reduced tolerance[52]. Although compelling evidence for microbiota associations with allergic disease and related conditions is emerging, a causal relationship between specific bacterial taxa and the development of allergy remains unclear. Several studies have reported differences in gut microbiota composition and lower abundance of bifidobacteria and lactobacilli in the infant’s gut precede the onset of allergic manifestations[53][54]. In addition, multiple cohort studies suggested that high abundance of Escherichia coli or Clostridium difficile was associated with the development of eczema or atopy[55][56], while a low gut microbial diversity and an elevated Enterobacteriaceae to Bacteroidaceae (E/B ratio) in early infancy may contribute to the development of food allergy[57]. In this instance, a notable higher abundance of Firmicutes particularly Clostridium spp., Blautia spp., and a lower abundance of Actinobacteria in the early gut microbiota has also been described to contribute to the development of allergic diseases such as food allergy in infants[58], and type 1 diabetes in children[59]. On this basis, modulation of gut microbiota during early life through M-16V intervention has emerged as a potential measure to prevent allergic disorders in infants. Several interventional studies suggest that M-16V could promote bifidobacterial colonisation and prevent or reduce the severity of allergic diseases, including atopic dermatitis (eczema), food allergy, allergic rhinitis, and asthma [60][61][62].
References
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N.; Role of the normal gut microbiota.. World J. Gastroenterol. 2015, 21, 8787, 10.3748/wjg.v21.i29.8787.
- Arrieta, M.-C.; Stiemsma, L.T.; Amenyogbe, N.; Brown, E.M.; Finlay, B.; The intestinal microbiome in early life: Health and disease.. Front. Immunol. 2014, 5, 427.
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Björkstén, B.; Engstrand, L.; Jenmalm, M.C.; Low gut microbiota diversity in early infancy precedes asthma at school age.. Clin. Exp. Allergy 2014, 44, 842-850.
- Cassir, N.; Benamar, S.; Khalil, J.B.; Croce, O.; Saint-Faust, M.; Jacquot, A.; Million, M.; Azza, S.; Armstrong, N.; Henry, M.; et al. Clostridium butyricum strains and dysbiosis linked to necrotizing enterocolitis in preterm neonates.. Clin. Infect. Dis. 2015, 61, 1107-1115.
- Fujimura, K.E.; Sitarik, A.R.; Havstad, S.; Lin, D.L.; Levan, S.; Fadrosh, D.; Panzer, A.R.; LaMere, B.; Rackaityte, E.; Lukacs, N.W.; et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation.. Nat. Med. 2016, 22, 1187.
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in new-onset Crohn’s disease.. Cell Host Microbe 2014, 15, 382-392.
- Rodríguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C.; et al. The composition of the gut microbiota throughout life, with an emphasis on early life.. Microb. Ecol. Health Dis. 2015, 26, 26050.
- Chong, C.; Bloomfield, F.; O’Sullivan, J.; Factors affecting gastrointestinal microbiome development in neonates.. Nutrients 2018, 10, 274.
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D.; et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences.. Cell 2014, 158, 705-721.
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic.. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506.
- Chang, H.-Y.; Chen, J.-H.; Chang, J.-H.; Lin, H.-C.; Lin, C.-Y.; Peng, C.-C.; Multiple strains probiotics appear to be the most effective probiotics in the prevention of necrotizing enterocolitis and mortality: An updated meta-analysis.. PLoS ONE 2017, 12, e0171579.
- de Simone, C.; The Unregulated Probiotic Market.. Clin. Gastroenterol. Hepatol. 2018, 17, 809–817.
- Quin, C.; Estaki, M.; Vollman, D.M.; Barnett, J.A.; Gill, S.K.; Gibson, D.L.; Probiotic supplementation and associated infant gut microbiome and health: A cautionary retrospective clinical comparison.. Sci. Rep. 2018, 8, 8283.
- Gritz, E.C.; Bhandari, V.; The human neonatal gut microbiome: A brief review.. Front. Pediatr. 2015, 3, 17.
- Chassard, C.; de Wouters, T.; Lacroix, C.; Probiotics tailored to the infant: A window of opportunity.. Curr. Opin. Biotechnol. 2014, 26, 141-147.
- Laforest-Lapointe, I.; Arrieta, M.-C.; Patterns of early-life gut microbial colonization during human immune development: An ecological perspective. Front. Immunol. 2017, 8, 788.
- Wong, C.B.; Sugahara, H.; Odamaki, T.; Xiao, J.-z.; Different physiological properties of human-residential and non-human-residential bifidobacteria in human health.. Benef. Microbes. 2018, 9, 111-122.
- Turroni, F.; Van Sinderen, D.; Ventura, M.; Genomics and ecological overview of the genus Bifidobacterium.. Int. J. Food Microbiol. 2011, 149, 37–44.
- Katayama, T.; Host-derived glycans serve as selected nutrients for the gut microbe: Human milk oligosaccharides and bifidobacteria.. Biosci. Biotechnol. Biochem. 2016, 80, 621-632.
- Odamaki, T.; Horigome, A.; Sugahara, H.; Hashikura, N.; Minami, J.; Xiao, J.-z.; Abe, F.; Comparative genomics revealed genetic diversity and species/strain-level differences in carbohydrate metabolism of three probiotic bifidobacterial species.. Int. J. Genomics 2015, 2015, 1.
- Minami, J.; Odamaki, T.; Hashikura, N.; Abe, F.; Xiao, J.Z.; Lysozyme in breast milk is a selection factor for bifidobacterial colonisation in the infant intestine.. Benef. Microbes. 2016, 7, 53-60.
- Roger, L.C.; Costabile, A.; Holland, D.T.; Hoyles, L.; McCartney, A.L.; Examination of faecal Bifidobacterium populations in breast-and formula-fed infants during the first 18 months of life.. Microbiology 2010, 156, 3329–3341.
- Sakata, S.; Tonooka, T.; Ishizeki, S.; Takada, M.; Sakamoto, M.; Fukuyama, M.; Benno, Y.; Cultureindependent analysis of fecal microbiota in infants, with special reference to Bifidobacterium species.. FEMS Microbiol. Lett. 2005, 243, 417–423.
- Turroni, F.; Foroni, E.; Pizzetti, P.; Giubellini, V.; Ribbera, A.; Merusi, P.; Cagnasso, P.; Bizzarri, B.; de’Angelis, G.L.; Shanahan, F.; et al. Exploring the diversity of the bifidobacterial population in the human intestinal tract.. Appl. Environ. Microbiol. 2009, 75, 1534–1545.
- Bozzi Cionci, N.; Baffoni, L.; Gaggìa, F.; Di Gioia, D.; Therapeutic Microbiology: The Role of Bifidobacterium breve as Food Supplement for the Prevention/Treatment of Paediatric Diseases.. Nutrients 2018, 10, 1723.
- Ishizeki, S.; Sugita, M.; Takata, M.; Yaeshima, T.; Effect of administration of bifidobacteria on intestinal microbiota in low-birth-weight infants and transition of administered bifidobacteria: A comparison between one-species and three-species administration.. Anaerobe 2013, 23, 38–44.
- Liu, M.-Y.; Yang, Z.-Y.; Dai, W.-K.; Huang, J.-Q.; Li, Y.-H.; Zhang, J.; Qiu, C.-Z.; Wei, C.; Zhou, Q.; Sun, X.; et al. Protective effect of Bifidobacterium infantis CGMCC313-2 on ovalbumin-induced airway asthma and β- lactoglobulin-induced intestinal food allergy mouse models.. World J. Gastroenterol. 2017, 23, 2149.
- Yeşilova, Y.; Çalka, Ö.; Akdeniz, N.; Berktaş, M.; Effect of probiotics on the treatment of children with atopic dermatitis.. Ann. Dermatol. 2012, 24, 189-193.
- Cuello-Garcia, C.A.; Brożek, J.L.; Fiocchi, A.; Pawankar, R.; Yepes-Nuñez, J.J.; Terracciano, L.; Gandhi, S.; Agarwal, A.; Zhang, Y.; Schünemann, H.J.; et al. Probiotics for the prevention of allergy: A systematic review and meta-analysis of randomized controlled trials.. J. Allergy Clin. Immunol. 2015, 136, 952–961.
- McFarland, L.V.; Evans, C.T.; Goldstein, E.J.; Strain-specificity and disease-specificity of probiotic efficacy: A systematic review and meta-analysis.. Front. Med. 2018, 5, 1.
- Toscano, M.; De Vecchi, E.; Gabrieli, A.; Zuccotti, G.V.; Drago, L.; Probiotic characteristics and in vitro compatibility of a combination of Bifidobacterium breve M-16 V, Bifidobacterium longum subsp. infantis M-63 and Bifidobacterium longum subsp. longum BB536.. Ann. Microbiol. 2015, 65, 1079–1086.
- Abe, F.; Miyauchi, H.; Uchijima, A.; Yaeshima, T.; Iwatsuki, K.; Stability of bifidobacteria in powdered formula.. Int. J. Food Sci. Technol. 2009, 44, 718–724.
- Guidelines for the evaluation of probiotics in food. . World Health Organization. Retrieved 2021-9-10
- List of authorised probiotic strains for infant’s food in China . Foodmate.net. Retrieved 2021-9-10
- Patole, S.; Keil, A.D.; Chang, A.; Nathan, E.; Doherty, D.; Simmer, K.; Esvaran, M.; Conway, P.; Effect of Bifidobacterium breve M-16V supplementation on fecal bifidobacteria in preterm neonates-a randomised double blind placebo controlled trial.. PLoS ONE 2014, 9, e89511.
- Patole, S.K.; Rao, S.C.; Keil, A.D.; Nathan, E.A.; Doherty, D.A.; Simmer, K.N.; Benefits of Bifidobacterium breve M-16V supplementation in preterm neonates-a retrospective cohort study.. PLoS ONE 2016, 11, e0150775.
- Satoh, Y.; Shinohara, K.; Umezaki, H.; Shoji, H.; Satoh, H.; Ohtsuka, Y.; Shiga, S.; Nagata, S.; Shimizu, T.; Yamashiro, Y.; et al. Bifidobacteria prevents necrotizing enterocolitis and infection in preterm infants.. Int. J. Probiotics Prebiotics 2007, 2, 49.
- Abe, F.; Yaeshima, T.; Iwatsuki, K.; Safety evaluation of two probiotic bifidobacterial strains, Bifidobacterium breve M-16V and Bifidobacterium infantis M-63, by oral toxicity tests using rats.. Biosci. Microflora. 2009, 28, 7–15.
- Xiao, J.-z.; Takahashi, S.; Odamaki, T.; Yaeshima, T.; Iwatsuki, K.; Antibiotic susceptibility of bifidobacterial strains distributed in the Japanese market.. Biosci. Biotechnol. Biochem. 2010, 74, 336-342.
- Grill, J.P.; Manginot-Dürr, C.; Schneider, F.; Ballongue, J.; Bifidobacteria and probiotic effects: Action of Bifidobacterium species on conjugated bile salts.. Curr. Microbiol. 1995, 31, 23–27.
- Abe, F.; Muto, M.; Yaeshima, T.; Iwatsuki, K.; Aihara, H.; Ohashi, Y.; Fujisawa, T.; Safety evaluation of probiotic bifidobacteria by analysis of mucin degradation activity and translocation ability.. Anaerobe 2010, 16, 131–136.
- Shane, A.L.; Deshpande, G.C.; Merenstein, D.; Improved neonatal outcomes with probiotics.. JAMA Pediatr. 2013, 167, 885–886.
- Berdon, W.E.; Grossman, H.; Baker, D.H.; Mizrahi, A.; Barlow, O.; Blanc, W.A.; Necrotizing enterocolitis in the premature infant.. Radiology 1964, 83, 879–887.
- Linder, N.; Hammel, N.; Hernandez, A.; Fridman, E.; Dlugy, E.; Herscovici, T.; Klinger, G.; Intestinal perforation in very-low-birth-weight infants with necrotizing enterocolitis.. J. Pediatr. Surg. 2013, 48, 562– 567.
- Groer, M.W.; Luciano, A.A.; Dishaw, L.J.; Ashmeade, T.L.; Miller, E.; Gilbert, J.A.; Development of the preterm infant gut microbiome: A research priority.. Microbiome 2014, 2, 38.
- Arboleya, S.; Binetti, A.; Salazar, N.; Fernández, N.; Solís, G.; Hernandez-Barranco, A.; Margolles, A.; de los Reyes-Gavilan, C.G.; Gueimonde, M.; Establishment and development of intestinal microbiota in preterm neonates.. FEMS Microbiol. Ecol. 2012, 79, 763–772.
- Fricke, W.F.; The more the merrier? Reduced fecal microbiota diversity in preterm infants treated with antibiotics.. J. Pediatr. 2014, 165, 8–10.
- Greenwood, C.; Morrow, A.L.; Lagomarcino, A.J.; Altaye, M.; Taft, D.H.; Yu, Z.; Newburg, D.S.; Ward, D.V.; Schibler, K.R.; Early empiric antibiotic use in preterm infants is associated with lower bacterial diversity and higher relative abundance of Enterobacter.. J. Pediatr. 2014, 165, 23–29.
- Tanaka, S.; Kobayashi, T.; Songjinda, P.; Tateyama, A.; Tsubouchi, M.; Kiyohara, C.; Shirakawa, T.; Sonomoto, K.; Nakayama, J.; Influence of antibiotic exposure in the early postnatal period on the development of intestinal microbiota.. FEMS Immunol. Med. Microbiol. 2009, 56, 80–87.
- Pawankar, R.; Allergic diseases and asthma: A global public health concern and a call to action.. World Allergy Organ J. 2014, 7, 12.
- West, C.E.; Jenmalm, M.C.; Prescott, S.L.; The gut microbiota and its role in the development of allergic disease: A wider perspective.. Clin. Exp. Allergy 2015, 45, 43–53.
- Noverr, M.C.; Huffnagle, G.B.; The ‘microflora hypothesis’ of allergic diseases.. Clin. Exp. Allergy 2005, 35, 1511–1520.
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Björkstén, B.; Engstrand, L.; Jenmalm, M.C.; Low diversity of the gut microbiota in infants with atopic eczema. J. Allergy Clin. Immunol. 2012, 129, 434–440.
- Kalliomäki, M.; Kirjavainen, P.; Eerola, E.; Kero, P.; Salminen, S.; Isolauri, E.; Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing.. J. Allergy Clin. Immunol. 2001, 107, 129–134.
- Melli, L.; do Carmo-Rodrigues, M.S.; Araújo-Filho, H.B.; Solé, D.; De Morais, M.B.; Intestinal microbiota and allergic diseases: A systematic review.. Allergol. Immunopathol. 2016, 44, 177–188.
- Penders, J.; Thijs, C.; van den Brandt, P.A.; Kummeling, I.; Snijders, B.; Stelma, F.; Adams, H.; van Ree, R.; Stobberingh, E.E.; Gut microbiota composition and development of atopic manifestations in infancy: The KOALA Birth Cohort Study.. Gut 2007, 56, 661–667.
- Dong, P.; Feng, J.-j.; Yan, D.-y.; Lyu, Y.-j.; Xu, X.; Early-life gut microbiome and cow’s milk allergy-a prospective case-control 6-month follow-up study.. Saudi. J. Biol. Sci. 2018, 25, 875–880.
- Ling, Z.; Li, Z.; Liu, X.; Cheng, Y.; Luo, Y.; Tong, X.; Yuan, L.; Wang, Y.; Sun, J.; Li, L.; et al. Altered fecal microbiota composition for food allergy in infants.. Appl. Environ. Microbiol. 2014, 80, 2546–2554.
- Qi, C.-J.; Zhang, Q.; Yu, M.; Xu, J.-P.; Zheng, J.; Wang, T.; Xiao, X.-H.; Imbalance of fecal microbiota at newly diagnosed type 1 diabetes in Chinese children.. Chin. Med. J. 2016, , 129, 1298.
- Hattori, K.; Yamamoto, A.; Sasai, M.; Taniuchi, S.; Kojima, T.; Kobayashi, Y.; Iwamoto, H.; Namba, K.; Yaeshima, T.; Effects of administration of bifidobacteria on fecal microflora and clinical symptoms in infants with atopic dermatitis. Arerugi 2003, 52, 20-30.
- Taniuchi, S.; Hattori, K.; Yamamoto, A.; Sasai, M.; Hatano, Y.; Kojima, T.; Kobayashi, Y.; Iwamoto, H.; Yaeshima, T.; Administration of Bifidobacterium to infants with atopic dermatitis: Changes in fecal microflora and clinical symptoms. J. Appl. Res. Clin. Exp. Therapeut. 2005, 5, 387.
- Enomoto, T.; Sowa, M.; Nishimori, K.; Shimazu, S.; Yoshida, A.; Yamada, K.; Furukawa, F.; Nakagawa, T.; Yanagisawa, N.; Iwabuchi, N.; et al. Effects of bifidobacterial supplementation to pregnant women and infants in the prevention of allergy development in infants and on fecal microbiota.. Allergol. Int. 2014, 63, 575-585.
More
