Telehealth has become a viable option for glaucoma screening and glaucoma monitoring due to advances in technology. The ability to measure intraocular pressure without an anesthetic and to take optic nerve photographs without pharmacologic pupillary dilation using portable equipment have allowed glaucoma screening programs to generate enough data for assessment. At home, patients can perform visual acuity testing, web-based visual field testing, rebound tonometry, and video visits with the physician to monitor for glaucomatous progression.
- telehealth
- glaucoma
- screening
- monitoring
1. Introduction
Glaucoma is a progressive disease of the optic nerve and a leading cause of irreversible vision loss. Globally, in 2013, the prevalence of glaucoma was 3.54% among people aged 40–80, affecting 64.3 million [1]. It was estimated that by 2040, this number will increase to 111.8 million [1]. The demand for ophthalmologists to take care of glaucoma patients is expected to exceed the supply. In 2018, the Association of American Medical Colleges (AAMC) forecasted that there will be worsening shortages of physicians in the United States, with an estimated shortfall of 33,800 to 72,700 specialists by 2030 [2]. The report did not state what the estimated shortfall of ophthalmologists will be per se, but the trend is expected to be similar. The reasons for this shortfall include the stagnant number of ophthalmology residency and glaucoma fellowship positions, the increasing number of retiring ophthalmologists, and the aging population. In order to ensure adequate care for the increasing population of glaucoma patients, each ophthalmologist will have to accommodate a greater number of patients, eventually leading to overbooked clinic schedules, long wait times for patients, and crowded waiting rooms. The increasingly long wait times for the next available appointment can be detrimental to patient care. New strategies, such as the use of telehealth, will be increasingly important to limit clinic visits to patients who absolutely need to be seen, without compromising the care of patients with a stable disease.
Telehealth, as defined by Merriam-Webster, is health care provided remotely to a patient in a separate location using a two-way voice and visual communication. A computer or smartphone is needed to establish this communication. Because of the coronavirus disease 2019 (COVID-19) pandemic, the use of telehealth has accelerated due to patients’ fear of contracting COVID-19 and the reduced number of in-person appointments given. Telehealth has also provided a convenient way for people living in rural regions to access their doctors.
There are three main purposes of telehealth in the field of glaucoma. One, is to screen for patients who have glaucoma, or are glaucoma suspects (i.e., those who have optic nerve appearances suspicious but not definitive for glaucoma). Two, for those newly diagnosed with glaucoma, to determine the severity of the disease and treatment plan. Three, for those diagnosed in the past, to monitor for disease progression and change management as needed. Each purpose requires a different set of equipment, as discussed below.
2. Setups for Telehealth Programs
As discussed previously, telehealth can serve three purposes: (1) screen for glaucoma, (2) evaluate the severity of glaucoma to determine the treatment plan, and (3) to monitor disease progression. How each of these purposes can be achieved should depend on the equipment/facilities available, the patient population (the prevalence of certain types of glaucoma can vary), and the socioeconomic and/or geographic barriers to face-to-face ophthalmologic care.
Rather than screening the community for glaucoma, a telemedicine program [93][3] in Northern Alberta served as a glaucoma consult service. Patients seen by an optometrist, ophthalmologist, or family physician were referred to the program if they had risk factors for glaucoma or suspicious-looking optic discs or visual field test. At each office, a tonometer, corneal pachymeter, visual field machine, and a retinal camera were available for use by technicians. A glaucoma specialist at the University of Alberta then reviewed the data remotely and gave recommendations for management and follow-up.
In addition to screening for glaucoma, telemedicine can be used to monitor for the development of glaucoma. The Kaiser Permanente Eye Monitoring Center conducted a 2-year telemedicine program [94][4] to monitor low-risk glaucoma suspects. Each year, a technician checked the visual acuity, measured the IOP using a handheld applanation tonometer, and took OCT RNFL images at a local ophthalmology clinic. Different from other telemedicine programs, the data were sent to a trained technician first, rather than a glaucoma specialist. If there was a decline in visual acuity of at least two lines, an IOP elevation ≥ 5 mm Hg, or a significant change in the RNFL thickness in the superotemporal or inferotemporal region (defined as ≥10-micron reduction or transition into the abnormal red range), the technician would send the patient data to a glaucoma specialist for review remotely. Of the 225 glaucoma suspects enrolled in the program, five were referred for examination by an ophthalmologist due to concern for progression on OCT. Of those five patients, two were started on glaucoma medications. This program demonstrated that telemedicine is a viable option for monitoring glaucoma suspects and can capture the small number of patients who develop glaucoma and need treatment.
A review of these glaucoma telehealth programs shows that a variety of setups can be used to screen for glaucoma and monitor for disease progression. At the bare minimum, a technician should record the patient’s medical history, visual acuity, IOP, and take a fundus photograph. Technological advances have allowed IOP measurements without the use of topical anesthetic and fundus imaging without pharmacologic pupillary dilation. SAP to detect visual field scotomas and OCT to detect structural nerve fiber layer loss can provide additional valuable data, but these bulky machines are unlikely available outside of the ophthalmology clinic setting. As deep learning artificial intelligence technology matures, fundus imaging may be all that is needed to accurately predict RNFL thickness and visual field loss. Artificial intelligence will play a significant role in reducing the amount of equipment required for glaucoma screening and monitoring through telehealth. A summary of the components of a glaucoma telehealth examination is listed in Table 1 . Even at its current state, without reliance on artificial intelligence, telehealth has shown to be cost-effective. An analysis of remote glaucoma screening in rural Alberta, Canada revealed that teleglaucoma costs an average of CAD 867 per patient, which was dramatically less than the average CAD 4420 per patient for in-person screening [97][5]. In order to control healthcare costs while providing access to care, especially in rural regions, telehealth will become an important tool in the screening and monitoring of chronic diseases such as glaucoma.
| Utility | Disadvantages | |
|---|---|---|
| Visual Acuity | Changes can be due to a new central scotoma, refractive error, cataract, and other ocular pathologies. | Glaucoma typically presents with peripheral visual field loss which visual acuity does not assess. Only very advanced glaucoma affects visual acuity. |
| Intraocular Pressure (IOP) | A very important parameter to assess the efficacy of treatment and a major risk factor for disease progression. | Goldmann applanation, the gold standard for measuring IOP, is only performed in clinic. Portable tonometers can significantly differ from Goldmann measurements for IOPs outside the normal range. |
| Anterior Segment Photography | In lieu of the slit lamp examination, the camera can capture abnormalities of the external and anterior parts of the eye. | The camera may miss subtle pathologies such as a pigment deposition on the corneal endothelium or iris neovascularization. In addition, it cannot capture the anterior chamber cell and flare. |
| Iridocorneal Angle Imaging | Identifies eyes with anatomic narrow angles at risk for acute angle closure glaucoma. | Angle camera devices and UBM require instillation of topical anesthetic. |
| Fundus Photography | Captures images of the optic nerve head and macula. Progressive cupping of the optic nerve is a sign of uncontrolled glaucoma. | Although many cameras do not require pharmacologic dilation, the brightness and resolution of the images may be affected by pupil size. |
| Ocular Coherence Tomography (OCT) | Measures retinal nerve fiber layer thickness using near-infrared light. A reference database is available for comparison. An abnormally thin nerve fiber layer or progressive thinning is a sign of uncontrolled glaucoma. | The device is not portable and is only available in clinic. |
| Visual Field | Testing is important to detect early peripheral visual field loss and to monitor for expansion of the scotoma. The amount of visual field loss determines the severity of disease and plays a role in setting the target IOP. | Traditional standard automated perimetry is not portable and is only available in clinic. Visual field monitoring at home can only be performed using a web-based program on a computer, a tablet, or with virtual reality glasses. |
| Artificial Intelligence (Deep Learning) | By self-learning via an artificial neural network, the technology has demonstrated remarkable accuracy in diagnosing glaucoma and monitoring for disease progression using fundus images, OCT, and visual field. | It is still under development and not available to the public yet. |
3. How the Coronavirus Pandemic Shaped Telehealth
In December 2019, a novel respiratory illness COVID-19 emerged in Wuhan, China. Because of the disease’s highly contagious nature, it quickly spread globally, and a pandemic was declared by the World Health Organization on 11 March 2020. Governments worldwide imposed lockdowns to stop the spread of disease, as COVID-19 overwhelmed hospital systems with vast numbers of people requiring ventilators. In the United States, state governments issued stay-at-home orders and social distancing guidelines. People were asked to work from home and to avoid venturing outside except for essential activities. On 18 March 2020, the American Academy of Ophthalmology (AAO) recommended the cessation of elective surgery and routine clinic visits to protect patients from catching COVID-19 and to conserve personal protective equipment (PPE).
Because many ophthalmology practices closed their offices, telehealth through video visits became a necessary way for patients to see their doctors. In the United States, the Centers for Medicare and Medicaid Services (CMS) relaxed the requirements to bill for telehealth visits; thus, allowing practices to be reimbursed for remote patient care. Many practices implemented a telemedicine program for the first time and had to develop protocols to address patient needs virtually. Saleem et al. [98][6] depicted a workflow diagram as a reference to implement an ophthalmology telemedicine program. Essentially, the front desk staff reaches out to patients who had their appointments canceled and offers them a telephone or video visit for non-urgent problems. If the patient describes an issue that appears to be an emergency, the physician is contacted to determine whether the problem can be addressed remotely or the patient must be examined in person.
A major hurdle in managing glaucoma patients through video visits is that glaucoma, for the most part, is an asymptomatic disease, unless there is a substantial increase in IOP causing eye pain or rapid visual field loss causing noticeable constriction in vision. A video visit does not allow for IOP measurement, visual field testing, or the visualization of the optic nerve. A crude method for the patient to estimate IOP is via finger palpation on the eye through the eyelid. A more accurate way than digital palpation is for the patient to use the iCare HOME rebound tonometer on him/herself. The tonometer is easy to use, comfortable, and requires no topical anesthetic. Because the device is expensive, companies such as MyEYES (myeyes.net) and Enlivened (enlivened.com) offer rentals for a fee. Patients are taught how to use the device and borrow it for one or more weeks. The downside, however, is that the IOP readings are not displayed; the patient must return the device to the office to extract the IOP diurnal curve. An alternative to using the iCare HOME is to wear the Sensimed Triggerfish ® Contact Lens, which makes automated corneoscleral dimensional measurements for 24 h. However, the patient is required to have a circular antenna taped around the eye, wear a recording device hanging from the neck, and return to the office the next day to extract the diurnal curve. A new contact lens being developed in South Korea allows for convenient IOP monitoring using a smartphone. Implantable devices such as the Eyemate ® and Injectsense can provide IOP monitoring as well. If there is concern for visual field progression, the patient can use the computer-based Peristat Online Perimetry, the tablet-based Melbourne Rapid Fields program, or virtual reality perimetry to generate a visual field report and send it to the physician.
As pandemic lockdown restrictions loosened, ophthalmology practices reopened with the implementation of new protocols for the safety of the patients and staff members. Vinod et al. [99][7] described methods that practices used to enforce social distancing and enhance safety, such as limiting the number of appointments, rearranging chairs in the waiting rooms, asking patients to remain in their cars outside the clinic until they are called, mandating everyone to wear masks, and installing large breath shields on slit lamps. However, some patients are still uncomfortable with in-person examinations and prefer telehealth until the pandemic ends.
4. Conclusions
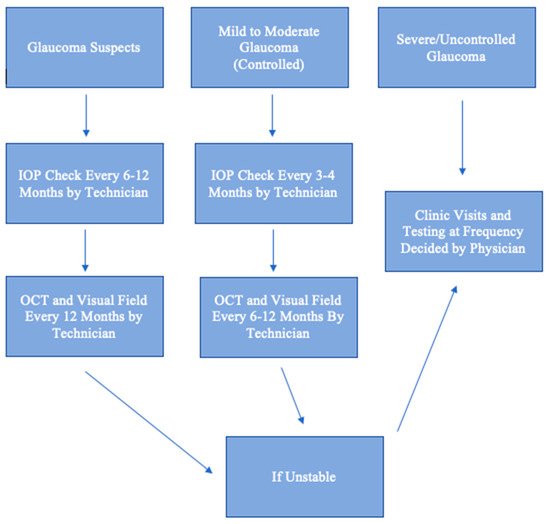
As demand for glaucoma care increases, there will be a need for telehealth. Just as radiologists review scans remotely, ophthalmologists can review results and risk-stratify patients. A glaucoma suspect can be monitored remotely, provided that one has access to an OCT or visual field machine yearly. A patient with well-controlled mild to moderate glaucoma can also be monitored remotely if one has IOP measurements performed regularly and that an in-person dilated examination is performed annually. A patient with uncontrolled or severe glaucoma should have face-to-face visits, as there is much less room for error and a high likelihood of needing laser or surgical procedures. This algorithm for remote monitoring is illustrated in Figure 1 . Essentially, face-to-face examinations can be limited to confirmation of diagnosis, management of patients with uncontrolled or severe glaucoma, and patients with new, concerning ocular symptoms. The telehealth approach is cost-effective and can increase patient satisfaction by decreasing waiting time during visits. Telehealth is particularly beneficial for patients in rural areas who have limited access to care and in the setting of a pandemic, when social distancing is enforced and the number of appointments is severely limited to reduce disease spread. Deep learning artificial intelligence will play an increasing role in the diagnosis and management of glaucoma using data extracted from telehealth.
References
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology 2014, 121, 2081–2090.
- Dall, T.; Reynolds, R.; Chakrabarti, R.; Jones, K.; Iacobucci, W. The Complexities of Physician Supply and Demand: Projections From 2018 to 2033. Assoc. Am. Med Coll. 2020, 1–92.
- Verma, S.; Arora, S.; Kassam, F.; Edwards, M.C.; Damji, K.F. Northern Alberta Remote Teleglaucoma Program: Clinical Outcomes and Patient Disposition. Can. J. Ophthalmol. 2014, 49, 135–140.
- Modjtahedi, B.S.; Chu, K.; Luong, T.Q.; Hsu, C.; Mattox, C.; Lee, P.P.; Nakla, M.L.; Fong, D.S. Two-Year Outcomes of a Pilot Glaucoma Suspect Telemedicine Monitoring Program. Clin. Ophthalmol. 2018, 12, 2095–2102.
- Thomas, S.; Hodge, W.; Malvankar-Mehta, M. The Cost-Effectiveness Analysis of Teleglaucoma Screening Device. PLoS ONE 2015, 10, e0137913.
- Saleem, S.M.; Pasquale, L.R.; Sidoti, P.A.; Tsai, J.C. Virtual Ophthalmology: Telemedicine in a COVID-19 Era. Am. J. Ophthalmol. 2020, 216, 237–242.
- Vinod, K.; Sidoti, P.A. Glaucoma Care during the Coronavirus Disease 2019 Pandemic. Curr. Opin. Ophthalmol. 2021, 32, 75–82.
